Abstract
Several dehydration protocols were evaluated for their ability to cryopreserve intact seeds and excised embryonic axes of Mimusops elengi and Manilkara zapota (Sapotaceae). Both interspecific and intraspecific variations in cryotolerance were found. M. zapota embryonic axes were more tolerant of cryopreservation than those of M. elengi, and showed higher desiccation tolerance, higher post-thawing survival and development, and a much wider range of moisture contents for cryopreservation. Maximum development rates were 94% and 27% for M. zapota and M. elengi, respectively. Intact seeds of both species tolerated desiccation to low moisture levels, but were sensitive to liquid nitrogen exposure, and cryopreserved seeds failed to germinate. Assessment of developing embryos excised from cryopreserved seeds associated nonviability of cotyledons and plumules with germination failure. Other structures survived at variable rates; most hypocotyls and radicles (up to 76% and 98% for M. elengi and M. zapota, respectively) were viable. The different cryotolerance between hypocotyls and cotyledons is a critical cause for failure in cryopreservation, contributing to the difficulty in developing protocols for such intermediate oily seeds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Sapotaceae family includes approximately 800 species of tropical-adapted evergreen trees and shrubs in 65 genera. These plants are of high socio-economic importance because they produce edible fruits, luxurious wood, and folk medicine (Malik et al. 2012). Most Sapotaceae species produce recalcitrant/intermediate seeds, including two widely planted fruit trees, Mimusops elengi and Manilkara zapota (Mai-Hong et al. 2006; Liu et al. 2008). The poor long-term viability of such seed prevents conventional preservation methods in gene banks, causing difficulties for forestry production and germplasm conservation. As an example, seeds of M. elengi lost viability within 6 mo under hermetic storage at room temperature with 13 ± 2% moisture content (Kaul 1979). Furthermore, its seeds are sensitive to storage at low temperature (Mai-Hong et al. 2006; National Gene Bank for Medicinal and Aromatic Plants 2008; Luo et al. 2012).
As conventional methods for long-term storage of intermediate seeds are not suitable, the development of cryopreservation methods for such species may enable long-term germplasm conservation (Hor et al. 2005). Because relatively few species produce intermediate seeds (∼2% of the world flora; Tweddle et al. 2003), these species are less extensively studied, and no methods for cryopreservation of M. elengi or M. zapota have been developed.
In this study, we investigated the responses of whole seeds, their components, and embryonic axes to several cryopreservation protocols as a step towards developing suitable methods for cryopreservation of M. elengi and M. zapota.
Materials and Methods
Plant material.
Mature fruits of these two species were collected from introduced trees growing in Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (21° 41′ N, 101° 25′ E) in 2011 and 2012, with M. elengi in April and M. zapota in early June, respectively, when fruits began to fall. Seeds were extracted, cleaned manually in tap water, air-dried for half a d, and stored in polyethylene bags at 15°C for up to 1 wk (except as mentioned otherwise below) prior to use in experiments.
Moisture content determination.
Moisture content was expressed on a dry weight basis as gram of water per gram of dry mass, calculated based on loss of weight after oven drying at 103 ± 2°C for 17 h. Moisture contents of different seed constituents were measured separately as required. Measurements were based on eight replicates, each containing an intact seed, kernel, endosperm or testa, or containing five embryos or embryonic axes.
Seed desiccation and subzero temperature storage.
Two methods of desiccation were investigated for seeds harvested in 2011. In the fast desiccation (FD) method, intact seeds were buried in silica gel desiccant for the duration of the desiccation treatment. In the slow desiccation (SD) method, seeds were first placed in a monolayer under 50% relative humidity (RH) and 15°C for 2 wk, and then put into sandwich boxes, each carrying 130 seeds, over 10–50 g silica gel for 1–5 d for further desiccation. Samples were taken regularly to assess viability and moisture content, and as materials for subsequent treatments. For M. zapota, all experiments used only fresh seeds (stored for up to 1 wk). For M. elengi, experiments used fresh seeds (treatments FD1 and SD1) or stored seeds (stored for 1 mo; treatments FD2 and FD2).
A third method of desiccation was investigated using seeds harvested in 2012. This equilibrium desiccation experiment followed the methods of Hor et al. (2005). Seeds were equilibrated for 5 wk in enclosed chambers over one of ten saturated salt solutions (KOH, K-acetate, K2CO3, NH4NO3, NaCl, NH4Cl, (NH4)2SO4, KCl, BaCl2, or KNO3). The relative humidities provided by these treatments ranged from 8% to 95%. Fifty seeds from each equilibration hydration treatment were used to determine seed and seed constituent moisture contents.
From each method of desiccation, seeds at various moisture levels were obtained. Seed samples at each moisture level were divided into two (or three, see below) 120-seed subsamples; one subsample was used to assess viability following desiccation. The other subsample was cryopreserved by placing seeds into 100 ml polypropylene tubes, hanging them over LN on a cord for 1 h, and then immersing them in LN, according to Wen et al. (2010). They were withdrawn from LN after 1 d and thawed at ambient conditions. For the FD treatment of M. zapota seeds and SD1 and FD1 treatments of M. elengi seeds, a third subsample was taken, sealed in laminated aluminum foil packets, and held at −20°C in the seed bank for 2 mo prior to assessing seed viability.
Viability of desiccated seeds and cryopreserved seeds was assessed based on the responses of 120 seeds from each treatment. Rehydration was accomplished by exposing seeds to water-saturated air at 35°C for 24 h according to Wen et al. (2010), sowing seeds on 1% plain agar, i.e., 1% agar in de-ionized water, in Petri dishes, with 20 seeds each in six Petri dishes, and incubating at 30°C. Seedling development was used as the germination criterion. Embryos excised from cryopreserved seeds were cultured on media and viability assessed as described below.
Embryonic axes desiccation and cryopreservation.
Newly-harvested seeds were cleaned and crushed to extract kernels. The kernels were surface-sterilized under aseptic conditions with 75% ethanol for 90 s, followed by immersion in 10% commercial bleach for 30 min, and then rinsed five times with sterilized deionized water prior to removing the embryonic axes. Embryonic axes (with 1/4 cotyledon attached) were desiccated by the flash drying method (Wen et al. 2010). After desiccation, embryonic axes were fast cooled by direct LN immersion and rapid thawed in 40°C water.
Viability of excised embryonic axes was assessed after culture on MS medium (Murashige and Skoog 1962) containing 30 g l−1 sucrose, 0.1 mg l−1 2-naphthalene acetic acid and 0.1 mg l−1 6-benzylaminopurine, and solidified with 7 g l−1 agar. The medium was dispensed into 55-mm diameter Petri dishes after autoclaving at 121°C for 15 min. All cultures were maintained at 24 ± 2°C, with 40–50% RH and a photoperiod of 14 h light (66 μmol m−2 s−1) and 10 h dark for 6 mo. “Development” was scored as the percentage of embryos with hypocotyl and radicle growth during this period; “survival” was scored as the percentage of embryos showing any visible elongation.
Results
Water sorption properties of seeds and seed constituents.
Seeds of both species exhibited slight interannual variation in hundred-seed weight and initial seed moisture content. For M. elengi seeds, hundred-seed weight and initial seed moisture content were 71.82 g and 0.528 g/g, respectively, in 2011 and 75.77 g and 0.584 g/g in 2012. For M. zapota seeds, hundred-seed weight and initial seed moisture content were 69.23 g and 0.507 g/g in 2011 and 65.65 g and 0.443 g/g in 2012.
Seeds subjected to equilibration desiccation treatments were sampled at 21, 28, and 35 d of exposure to various levels of RH to determine when seed moistures reached equilibrium. The moisture contents of seeds subjected to RH levels of <75% showed no substantial changes after the third week, and only slight variation was detected among seed samples subjected to RH levels of >80% (Figs. 1a, b ). Thus, a 5-wk treatment was determined adequate to reach moisture equilibrium.
Changes in moisture content, and water sorption isotherms of Mimusops elengi seeds (a), Manilkara zapota seeds (b), Mimusops elengi seed constituents (c), and Manilkara zapota seed constituents (d) equilibrated to various moisture contents over saturated salt solutions. All values are expressed as means ± SE.
Equilibrium desiccation treatments resulted in equilibration of the seed samples to various discrete moisture contents. The resultant water sorption isotherms described the relationship between moisture contents and equilibrium relative humidity. The typical reverse sigmoidal shape of moisture sorption isotherms of whole seeds for either M. elengi or M. zapota was not observed (Fig. 1a, b ). This was not surprising because only one treatment at <20% RH was employed. The equilibrium moisture contents of M. elengi seeds were always higher than those of M. zapota seeds held at any given RH treatment (Fig. 1a, b ), suggesting different sorption characteristics for seed of these two species. For example, seed moisture contents resulting from equilibration in 22.5% RH were 0.062 and 0.052 g/g for M. elengi and M. zapota, respectively.
The sorption characteristics of different seed components also varied (Fig. 1c, d ). For both species, embryos always had lower moisture contents than the other seed constituents, suggesting that they are high in lipid content. Interspecific variation in this trait was noted with the moisture contents of M. zapota embryos being a little lower than those of M. elengi. At low RH, equilibration moisture contents of the testa were a little higher than the endosperm, and vice versa at high RH.
Seed desiccation tolerance and subzero temperature storage.
Both species showed tolerance to desiccation, regardless of the desiccation method used. M. zapota seeds were slightly more tolerant than M. elengi seeds. As expected, seeds of both species exhibited intermediate storage behavior, and seeds lost viability when stored at −20°C in a conventional seed bank for 2 mo or cryopreserved using a simplified slow-cooling procedure regardless of the method and level of desiccation (data not shown).
Since none of the desiccation treatments reduced M. zapota seed viability to 50%, a kernel moisture content corresponding to 80% germination was used as the critical moisture content to assess desiccation tolerance. Probit analysis indicated that this critical moisture content varied between 0.06 and 0.1 g/g for M. elengi seeds and was around 0.05 g/g for M. zapota seeds, depending on seed treatment and desiccation method used. Storage for 1 mo increased critical moisture content in M. elengi seeds (Fig. 2a ). The higher percentage germination exhibited by M. zapota seeds after equilibrium desiccation versus silica gel desiccation may have been caused by different desiccation methods and/or interannual variation in these seed lots (Fig. 2b ).
Embryonic axis desiccation tolerance and cryopreservation.
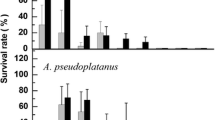
In contrast to the failure of whole seed cryopreservation, development and survival of cryopreserved axes was successful for both M. elengi and M. zapota (Figs. 3 and 4), although the latter species was more tolerant of cryopreservation. Slight desiccation was safe for M. elengi embryonic axes (Fig. 4a ). After 90 min desiccation to 0.1 g/g, M. elengi embryonic axes developed into normal seedlings; both plumules and radicals grew, but some cotyledons did not turn green (Fig. 4b ). No cotyledons survived desiccation to 0.05 g/g. At this moisture level, the outer layer of the radicals was also nonviable, with the roots developing from their inner tissue, indicating sensitivity to desiccation (Fig. 4c ). Further desiccation resulted in reduced development and survival of embryonic axes (Fig. 3a ). M. elengi embryonic axes survived LN exposure only from a very narrow moisture window between 0.025 and 0.1 g/g. Maximum development (27%) and maximum survival (72.5%) occurred at 0.038 g/g. In these embryonic axes, only hypocotyls and radicles showed development, but neither plumules nor cotyledons survived cryopreservation (Figs. 3a and 4d ).
Viability of Mimusops elengi (a) and Manilkara zapota (b) excised embryonic axes after desiccation and/or freeze-thaw treatment. Moisture contents were determined using 8 replicates of 5 embryonic axes, survival and development assessed using 4 × 10 embryos each. All values are expressed as means ± SE.
Desiccation treatments did not reduce development or survival of M. zapota embryonic axes, with almost all unfrozen axes recovering normal growth in media (Fig. 3b ). Compared to M. elengi, M. zapota embryos tolerated a wider range of moisture contents for cryopreservation. Cryostorage was lethal to fully hydrated axes and slight desiccation enabled development from a few frozen axes only. As moisture content decreased, development and survival increased quickly, with the maximum development (94%) and maximum survival (100%) occurring around 0.081 g/g. Plumules growth was observed neither, but different from M. elengi, cotyledon portions can turn green in cryopreserved embryonic axes in this species. Severe desiccation did not impair development of unfrozen M. zapota axes, but greatly reduced development and survival of cryopreserved axes (Fig. 3b ).
Plant regeneration from embryos in cryopreserved seeds.
Recovery of intact embryos from frozen seeds for these two species was difficult as most cotyledons were cracked and detached from the embryonic axes when excised. One to eight complete embryos from each treatment were cultured and observed. Hypocotyls and radicles showed development, but plumules did not grow and the cotyledons did not turn green (Figs. 5a, b ). Detached complete cotyledons from cryopreserved seeds were completely nonviable as greening was not observed when cultured in media. Therefore, only hypocotyl and radicle development and survival were used to assess viability of embryos excised from cryopreserved seeds. For M. elengi seeds, equilibration at 81% RH resulted in maximum development (Fig. 6a ). Embryos derived from seeds equilibrated to lower equilibrium relative humidity (eRH) levels showed reduced development, and at 22.5% eRH, no development was observed. Hypocotyl and radicle survival exhibited almost the same pattern in this species (Fig. 6a ).
Embryos derived from cryopreserved seeds of M. zapota responded differently than those derived from M. elengi. Progressively increasing levels of hypocotyl development were observed between 85% and 78% eRH, with maximum hypocotyl development (98%) occurring at 75% eRH. Further reductions in eRH resulted in only slight reductions in development, with nearly 80% hypocotyls developing from seed at 8% eRH; while hypocotyl and radicle survival in this species kept above 85% in the eRH range of 8–80% (Fig. 6b ).
Discussion
This study investigated parameters that affected the survival of cryopreserved seed, seed components, and excised embryos for M. elengi and M. zapota. Overall, M. zapota exhibited greater tolerance to desiccation and cryopreservation than did Mimusops elangi. However, whole seeds of either species that were subjected to cryopreservation failed to germinate. Development and survival after cryopreservation was observed only for embryonic axes that were excised and desiccated prior to cryopreservation, or from embryonic axes derived from cryopreserved seeds. Cotyledons, plumules, hypocotyls and radicles exhibited different responses to cryostorage within and between species in M. elengi and M. zapota seeds.
M. elengi seeds have previously been desiccated to 8–12% moisture content (fresh weight basis; Mai-Hong et al. 2006) and stored at temperatures ranging from 5°C to −196°C (Mai-Hong et al. 2006; National Gene Bank for Medicinal and Aromatic Plants 2008). In this report, we show that excised embryos show higher tolerance to cryopreservation, and present data that will assist in the development of cryopreservation protocols specific to both M. elengi and M. zapota.
Our results confirmed previous reports that both M. elengi (Mai-Hong et al. 2006) and M. zapota (Liu et al. 2008) produce intermediate seeds (Ellis et al. 1990). Such seeds often have relatively high levels of desiccation tolerance compared to other non-orthodox seeds, but do not have the very high desiccation tolerances of orthodox seeds (in equilibrium with 15% RH). In contrast with orthodox seeds, intermediate seeds show decreased seed longevity at low moisture contents as storage temperatures are decreased (Ellis et al. 1990; Dussert et al. 2006). The causes for sensitivity of intermediate seeds to LN exposure are largely unknown, and likely to involve complex interactions related to oxidative stress and/or imbibitional damage (Sacandé et al. 2000b; Dussert et al. 2003, 2006). The extent of this kind of damage could be influenced by the lipid composition of these seeds. Species that produce intermediate seeds tend to produce seeds with high oil content or unusual oil properties that may result in an elevated gel-to-liquid crystalline phase transition temperature of the membrane (Sacandé et al. 2000a, 2001; Crane et al. 2003). Seed lipid reserves may also be damaged. Hydration of seed containing crystallized triacylglycerols is lethal (Crane et al. 2006), with massive cellular disruption observed during early imbibition of Cuphea seeds containing crystallized triacylglycerols (Volk et al. 2006).
The moisture content of seeds prior to cryopreservation is critical for viability. Intermediate seeds usually have only a narrow moisture content range that allows tolerance to LN exposure. On the one hand, this is limited by desiccation sensitivity—the lower limit—and on the other by the occurrence of intracellualar ice formation—the higher limit (Dussert et al. 2001). Equilibration of seeds over saturated salt solutions has been used to control seed moisture contents prior to cryopreservation of intermediate seeds, such as coffee (Dussert and Engelmann 2006) and Citrus species (Hor et al. 2005). However, species-specific methods must be developed for successful cryopreservation of intermediate seeds, as seen in this and other studies (Malik et al. 2010).
The critical moisture content of seed kernels for 80% germination varied between 0.06 and 0.1 g/g for M. elengi and was around 0.05 g/g for M. zapota (Fig. 2); while the observed equilibrium moisture content of endosperms (equilibrated at 22.5% RH at 25°C) were 0.058 and 0.044 g/g in M. elengi and M. zapota, respectively. Therefore, in the water sorption isotherm, seeds of these two species can be classed with other species that can withstand removal of sorption type II water only. In addition, because of their sensitivity to subzero temperatures, these species conform to type II seeds (Pritchard 2004). Such seeds are expected to have a T g (glass-to-liquid transition temperature) of around 5°C. On the premise that a safe temperature for seed storage may be around T g −70°C; it is possible that such seeds could be stored around −70°C, with cryopreservation the most promising method for their long-term conservation.
Although cryopreservation of type II seeds of coffee (Dussert and Engelmann 2006), oil palm (Grout et al. 1983; Engelmann et al. 1995), neem (Berjak and Dumet 1996) and papaya (Ashmore et al. 2008) have been reported, cryopreservation of type II seeds remains difficult. Most type II seeds are from tropical plants and may therefore not be innately cryotolerant. The development of successful cryopreservation protocols is complicated by interspecific and intraspecific variation, variation among different parent plants, and variation in the responses of different constituents from the same individual seed (Abdelnour-Esquiel et al. 1992; Hong and Ellis 1995; Dussert et al. 1998; Wen and Song 2007a, b; Wen et al. 2010).
Variation in cryotolerance within different constituents of individual seeds is the most complex. For instance, we showed that germination failure of cryopreserved seeds was associated with nonviability of the cotyledon and plumule, while hypocotyls and radicles retained some developmental capacity. Previous reports of similar phenomena include survival of embryo axes but not of mature seed of hazelnut (Normah et al. 1994), and higher sensitivities of some organs to cryopreservation (Chin et al. 1988; Kuranuki and Yoshida 1996; Dussert et al. 1998, 2001; Vasquez et al. 2005).
References
Abdelnour-Esquivel A.; Villalobos V.; Engelmann F. Cryopreservation of zygotic embryos of Coffea spp. CryoLetters 13: 297–302; 1992.
Ashmore S. E.; Drew R. A.; O'Brien C.; Parisi A. Cryopreservation of papaya (Carica papaya L.) seed: overcoming dormancy and optimizing seed desiccation and storage conditions. Acta Hort 839: 229–235; 2008.
Berjak P.; Dumet D. Cryopreservation of seeds and isolated embryonic axes of neem (Azadirachta indica). CryoLetters 17: 99–105; 1996.
Chin H. F.; Krishnapillay B.; Alang Z. C. Cryopreservation of Veitchia and Howea palm embryos: non-development of the haustorium. CryoLetters 9: 372–379; 1988.
Crane J.; Kovach D.; Gardner C.; Walters C. Triacylglycerol phase and ‘intermediate’ seed storage physiology: a study of Cuphea carthagenensis. Planta 223: 1081–1089; 2006.
Crane J.; Miller A. L.; van Roekel J. W.; Walters C. Triacylglycerols determine the unusual storage physiology of Cuphea seed. Planta 217: 699–708; 2003.
Dussert S.; Chabrillange N.; Engelmann F.; Anthony F.; Louarn J.; Hamon S. Cryopreservation of seeds of four species (Coffea arabica, C. costatifructa, C. racemosa and C. sessiliflora): importance of moisture content and cooling rate. Seed Sci. Res. 8: 9–15; 1998.
Dussert S.; Chabrillange N.; Montillet J.; Agnel J.; Engelmann F.; Noirot M. Basis of coffee seed sensitivity to liquid nitrogen exposure: oxidative stress or imbibitional damage? Physiol. Plant. 119: 534–543; 2003.
Dussert S.; Chabrillange N.; Rocquelin G.; Engelmann F.; Lopez M.; Hamon S. Tolerance of coffee (Coffea spp.) seeds to ultra-low temperature exposure in relation to calorimetric properties of tissue water, lipid composition, and cooling procedure. Physiol. Plant. 112: 495–504; 2001.
Dussert S.; Davey M. W.; Laffargue A.; Doulbeau S.; Swennen R.; Etienne H. Oxidative stress, phospholipid loss and lipid hydrolysis during drying and storage of intermediate seeds. Physiol. Plant. 127: 192–204; 2006.
Dussert S.; Engelmann F. New determinants for tolerance of coffee (Coffea arabica L.) seeds to liquid nitrogen exposure. CryoLetters 27: 169–178; 2006.
Ellis R. H.; Hong T. D.; Roberts E. H. An intermediate category of seed storage behaviour? I. Coffee. J. Exp. Bot. 41: 1167–1174; 1990.
Engelmann F.; Chabrillange N.; Dussert S.; Duval Y. Cryopreservation of zygotic embryos and kernels of oil palm (Elaeis guineensis Jacq.). Seed Sci. Res. 5: 81–86; 1995.
Grout B. W. W.; Shelton K.; Pritchard H. W. Orthodox behaviour of oil palm seed and cryopreservation of the excised embryo for genetic conservation. Ann. Bot. 52: 381–384; 1983.
Hong T. D.; Ellis R. H. Interspecific variation in seed storage behaviour within two genera—Coffea and Citrus. Seed Sci. Technol. 23: 165–182; 1995.
Hor Y. L.; Kim Y. J.; Ugap A.; Chabrillange N.; Sinniah U. R.; Engelmann F.; Dussert S. Optimal hydration status for cryopreservation of intermediate oily seeds: Citrus as a case study. Ann. Bot. 95: 1153–1161; 2005.
Kaul M. L. H. The life-span of some Indian forest seeds. Beitr Trop Lanwirt 17: 283–286; 1979.
Kuranuki Y.; Yoshida S. Different responses of embryonic axes and cotyledons from tea (Camellia sinensis) seeds to desiccation and cryoexposure. Breed. Sci. 46: 149–154; 1996.
Liu K.; Eastwood R. J.; Flynn S.; Turner R. M.; Stuppy W. H. Seed information database (release 7.1, May 2008) http://www.kew.org/data/sid; 2008.
Luo Y. L.; Lan Q. Y.; Lu X.; Chen Z. X.; Tan Y. H. Storage behaviour and antioxidant activities of Mimusops elengi seeds subjected to different drying rates. Seed Sci. Technol. 40: 354–364; 2012.
Mai-Hong T.; Hong T. D.; Hien N. T.; Hai H. H.; Tung T. D.; Le-Tam V. T.; Ngoc-Tam B.; Ellis R. H. Seed development, maturation and storage behaviour of Mimusops elengi L. New Forest 32: 9–19; 2006.
Malik S. K.; Chaudhury R.; Dhariwal O. P.; Bhandari D. C. Genetic resources of tropical underutilized fruits in India. NBPGR, New Delhi, p 168; 2010.
Malik S. K.; Choudhary R.; Kumar S.; Dhariwal O. P.; Deswal R. P. S.; Chaudhury R. Socio-economic and horticultural potential of Khirni [Manilkara hexandra (Roxb.) Dubard]: a promising underutilized fruit species of India. Genet. Resour. Crop. Evol. 59: 1255–1265; 2012.
Murashige T.; Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497; 1962.
National Gene Bank for Medicinal and Aromatic Plants. Project Completion Report. Sponsored by the Department of Biotechnology, Government of India; 2008.
Normah M. N.; Reed B. M.; Yu X. Seed storage and cryoexposure behaviour in hazelnut (Corylus avellana cv. Barcelona). CryoLetters 15: 315–322; 1994.
Pritchard H. W. Classification of seed storage type for ex situ conservation in relation to temperature and moisture. In: Guerrant E. O.; Havens K.; Maunder M. (eds) Ex situ plant conservation. Supporting species survival in the wild. Island Press, Washington, D.C., pp 139–161; 2004.
Sacandé M.; Buitink J.; Hoekstra F. A. A study of water relations in neem (Azadirachta indica) seed that is characterized by complex storage behaviour. J. Exp. Bot. 51: 635–643; 2000a.
Sacandé M.; Golovina E. A.; van Aelst A. C.; Hoekstra F. A. Viabiltiy loss of neem (Azadirachta indica) seed associated with membrane phase behaviour. J. Exp. Bot. 52: 919–931; 2001.
Sacandé M.; Hoekstra F. A.; van Aelst A. C.; De Vos C. H. R. Is oxidative stress involved in the loss of neem (Azadirachta indica) seed viability? Seed Sci. Res. 10: 381–392; 2000b.
Tweddle J. C.; Dickie J. B.; Baskin C. C.; Baskin J. M. Ecological aspects of seed desiccation sensitivity. J. Ecol. 91: 294–304; 2003.
Vasquez N.; Salazar K.; Anthony F.; Chabrillange N.; Engelmann F.; Dussert S. Variability in response of seed to liquid nitrogen exposure in wild coffee (Coffea arabica L.). Seed Sci. Technol. 33: 293–301; 2005.
Volk G. M.; Crane J.; Caspersen A. M.; Hill L. M.; Gardner C.; Walters C. Massive cellular disruption occurs during early imbibition of Cuphea seeds containing crystallized triacylglycerols. Planta 224: 1415–1426; 2006.
Wen B.; Cai C. T.; Wang R. L.; Tan Y. H.; Lan Q. Y. Critical moisture content windows differ for the cryopreservation of pomelo (Citrus grandis) seeds and embryonic axes. CryoLetters 31: 29–39; 2010.
Wen B.; Song S. Q. Acquisition of cryotolerance in maize embryos during seed development. CryoLetters 28: 109–118; 2007a.
Wen B.; Song S. Q. Acquisition and loss of cryotolerance in Livistona chinensis embryos during seed development. CryoLetters 28: 291–302; 2007b.
Acknowledgments
We are grateful to Prof. Richard Corlett and Dr. Uromi Manage Goodale in our botanical garden for providing language help and the National Natural Science Foundation of China (31170626) and National Science and Technology Support Program (2012BAC01B05) for providing financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Rida Shibli
Rights and permissions
About this article
Cite this article
Wen, B., Wang, X., Tan, Y. et al. Differential responses of Mimusops elengi and Manilkara zapota seeds and embryos to cryopreservation. In Vitro Cell.Dev.Biol.-Plant 49, 717–723 (2013). https://doi.org/10.1007/s11627-013-9562-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-013-9562-4