Abstract
Arsenic (As) is a highly toxic environmental contaminant to which most living organisms are exposed. Plants have evolved several mechanisms to cope with this toxic metalloid; however, these mechanisms are only partially understood. The response of plants to As phytotoxicity is highly complex, with considerable variation among species. In this study, arsenate (As+5) effects on germination and early root development of tobacco (Nicotiana tabacum) seedlings were investigated. Also, As+5 tolerance and removal efficiency of tobacco hairy roots (HRs) and seedlings were assessed and compared. Total seed germination capacity was not affected by 10 to 200 μM As+5, while primary root length and root branching were reduced by As+5 concentrations that were at or above 100 μM. Both systems were able to tolerate As+5 concentrations of 10 μM since no growth inhibition was detected. For higher As+5 concentrations, phytotoxicity increased, but it was mitigated by higher phosphate (Pi) availability. Under the studied conditions, As+5 removal efficiency of HRs greatly exceeded that of seedlings. Further, tobacco HRs were able to accumulate As in their tissues. These results justify further investigations on As tolerance and detoxification mechanisms in tobacco, an easy-to-transform crop species with high biomass, which could allow evaluation of the possible application of wild type or alternatively transgenic tobacco plants for As phytoextraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a highly toxic metalloid that is present in soils, sediments, and water, in some cases, at high concentrations. It is disseminated in the environment not only through anthropogenic activities such as mining, metal manufacturing, use of As-containing pesticides, herbicides, and wood additives, but also through natural processes such as rock erosion, volcanic emissions, and thermal discharges (Zhu and Rosen 2009; Zhao et al. 2010).
Arsenic phytotoxicity is mainly attributed to the chemical properties of two prevalent inorganic species: arsenate (As+5) and arsenite (As+3). Since As+5 is an analog of phosphate (Pi), it interferes with essential cellular processes such as oxidative phosphorylation and ATP synthesis, whereas As+3 negatively affects the enzymatic activity of some proteins as it binds to their sulfhydryl groups. In higher plants, As+5 is taken up and translocated via the high-affinity Pi transporter system due to the chemical analogy with Pi (Meharg and Macnair 1990). Thus, phosphorous deficiency, frequently occurring in land soils (Vance et al. 2003), becomes a problem when it co-exists with As. Phosphorous deficiency exacerbates As phytotoxicity in plants and it even promotes higher As accumulation in their tissues. Thus, it is very important to evaluate the effect of external Pi supply on As phytotoxicity of different plant species.
Even though several physical and chemical technologies have been applied to remove As from soil and water, these technologies have shown limitations. However, the discovery of As-hyperaccumulating ferns (Francesconi et al. 2002) and As-hyperaccumulating aquatic plants (Robinson et al. 2006; Rahman et al. 2007) has provided the possibility of developing cost-effective and eco-friendly As phytoextraction treatments of soil and water (Grill et al. 2006). Plants for phytoextraction should possess essential traits like fast growth, high biomass, easy harvesting, and accumulation of heavy metals in their harvestable parts (Jabeen et al. 2009). Few plants fulfill all these criteria because natural hyperaccumulation is generally associated with a low biomass and slow growth rate. However, a rapidly growing non-accumulator plant could be modified and/or engineered so that it achieves most of the above-mentioned attributes. Many plant species are being investigated to determine their usefulness for phytoextraction, especially plants with high biomass yields and those that are not consumed by humans and animals. Thus, a species that gains importance for phytoextraction is tobacco, since it is a fast growing, high biomass plant, repulsive to herbivores, which has been shown to be able to uptake As from the soil and concentrate it in the leaves (Glebert et al. 2003; Lugon-Moulin et al. 2008). Thus, tobacco plants used for As phytoextraction may possibly be commercially valuable as it could still be used to produce smoking tobacco as a recent research suggests that As in tobacco would not be associated with cigarette smoker diseases (Marano et al. 2012).
In organic and inorganic phytoremediation research, in vitro plant tissue cultures have been recommended as interesting systems (Doran 2009; Talano et al. 2012). They have many experimental advantages, especially in studies examining the intrinsic metabolic capabilities of plant cells for pollutant detoxification, avoiding interference from microorganisms. Moreover, the results derived from tissue culture studies can be used to predict whole plant responses, improve the design, and thus, reduce the cost of subsequent conventional whole plant experiments (Doran 2009). The biological behavior of hairy roots (HRs) offers a high degree of authenticity and similarity to normal roots, since they are an organized tissue system, with structure and functions close to whole plant organs, and greater genotypic and phenotypic stability compared with dedifferentiated plant cultures (Flores 1987). In addition, pollutant biotransformation using HRs do not involve translocation to aerial parts, which allows to determine whether translocation is necessary for pollutant accumulation or metabolism. Furthermore, HR technology has been used as a strategy to obtain highly branched root systems with large surface area in some hyperaccumulator plants, hence increasing rhizofiltration efficiency (Nedelkoska and Doran 2000; Eapen et al. 2003).
HRs have been widely used for uptake, toxicity, and tolerance evaluation as well as for removal studies of some metals. For example, HRs from different plant species have been used for cadmium (Nedelkoska and Doran 2000; Wu et al. 2001; Boominathan and Doran 2003), nickel (Nedelkoska and Doran 2001), and uranium removal (Eapen et al. 2003). Regarding arsenic, Sato et al. (1991) reported the use of HRs of Rubia tinctorum for As removal from liquid medium. However, to our knowledge, there is no information about tolerance and removal of As using tobacco (Nicotiana tabacum) HRs.
In this study, experiments were conducted to determine tobacco usefulness for As phytoextraction. Thus, specific As-induced changes in the root system and As+5 tolerance in tobacco HRs and seedlings were investigated. Also, Pi-modulated response of tobacco to As phytotoxicity was assessed. Finally, removal assays were carried out using tobacco HRs and seedlings in order to compare and discuss the ability of both plant systems to cope with this metalloid.
Materials and Methods
Plant material.
In vitro seedlings and HR cultures of tobacco (Nicotiana tabacum cv. Wisconsin) were used. Tobacco seeds were surface disinfected with 35% (v/v) ethanol for 1 min and then with 30% (v/v) commercial bleach (Ayudin® Clorox Company, Oakland, ARG) solution for 10 min. After that, seeds were washed five times with sterile distilled water. For the development of seedlings, disinfected seeds were placed in Falcon tubes containing half-strength Murashige–Skoog (Murashige and Skoog 1962) plus RT vitamin complex (MSRT) liquid medium (Flocco et al. 1998) supplemented with 3% (w/v) sucrose. Tobacco seedlings developed in this hydroponic system under 16-h photoperiod (growth light 200 μmol/m2 s) at 25 ± 2°C, in an orbital shaker at 100 rpm.
HR cultures were previously obtained as described in Sosa Alderete et al. (2009) and grown in 50-mL Erlenmeyer flasks containing MSRT liquid medium with 3% (w/v) sucrose. They were sub-cultured periodically (every 20 d) in the same medium and maintained in an orbital shaker at 100 rpm, 25 ± 2°C, in the dark.
Tobacco seedling germination and early root development under As +5 treatment.
To evaluate As+5 effects on tobacco germination and early root development, tobacco seeds were germinated and grown under different concentrations of the metalloid. For that, 20 sterile seeds were placed in 85-mm Petri dishes containing MSRT solid medium (0.9% w/v agar) with 10-, 50-, 100-, and 200-μM sodium arsenate heptahydrate (Na2HAsO4 7H2O; Sigma-Aldrich, St. Louis, MO). The stock sodium arsenate solution was previously sterilized by filtration (pore size 0.2 μm) and the proper volume was added to reach the specific final concentration for each assay. After 10 d of growth, final fresh weight (FW), root length, and number of lateral roots of tobacco seedlings were registered. This assay was done in triplicate.
To analyze tobacco tolerance to As+5, five 5-d-old germinated seeds grown in half-strength MSRT liquid medium with sucrose (3%, w/v) were selected based on their homogeneous size and transferred to Falcon tubes containing 20 mL of the same medium supplemented with 50- or 100-μM Pi and 10- or 50-μM As+5. The seedling growth was performed under light, temperature, and agitation conditions as described previously. Final FW was registered after 25 d. The assay was done in triplicate.
As +5 removal by tobacco seedlings from culture medium and naturally contaminated water.
For As removal assays by seedlings, five seeds were grown in half-strength MSRT liquid medium for 15 d and then transferred to 100-mL Erlenmeyer flasks containing 20 mL of the same medium and supplemented with 10- or 50-μM As+5. After 25 d, residual As concentration was determined spectrophotometrically in post-removal solutions by a silver method, which is described later. Results were expressed as As removed (in milligrams per liter) per gram of FW, as indicated below. As a preliminary study, an As+5 removal assay of naturally contaminated water was carried out using 15-d-old tobacco seedlings. After 6-d incubation of tobacco seedlings with contaminated tap water from Buena Esperanza (San Luis, Argentina) containing 0.1 mg/L As, residual metalloid was determined using a commercial kit, described below.
HR growth and removal studies under As +5 treatment.
To evaluate the effect of As+5 and different phosphate concentration on HR growth, tolerance of tobacco HRs grown in MSRT liquid medium supplemented with As+5 was studied. HR cultures of around 25 d were previously incubated in a Pi-modified MSRT medium containing low Pi concentration (30-μM potassium phosphate) during 7 d, in order to induce Pi starvation. It is important to note that regular MS and MSRT medium contains around 1 mM of potassium phosphate. From these cultures, HR inocula of 200 mg were weighed under sterile conditions and transferred to 50-mL Erlenmeyer flasks with 20 mL Pi-modified MSRT medium containing variable potassium phosphate concentrations (50, 100, and 1,000 μM) and As+5 (5, 10, 50, and 100 μM). Control HR cultures were performed using MSRT medium with the different Pi concentrations and without As+5. HR cultures were incubated under agitation as described before and after 25 d, they were harvested and weighed to evaluate the effect of As+5 on HR growth. The results were expressed as final FW. Experiments were carried out in triplicate and repeated twice.
Superoxide dismutase and ascorbate peroxidase activity determination.
Superoxide dismutase (SOD) and ascorbate peroxidase (APx) activity were determined in HR tissue after 10- and 50-μM As+5 treatments during 25 d. For this purpose, HR tissue was frozen in liquid nitrogen, then ground with a pestle and mortar into a fine powder and kept at −80°C. These samples were homogenized with 50-mM potassium phosphate buffer pH 7.8 containing 0.5-mM ethylenediaminetetraacetic acid (EDTA) and using a ratio 1:10 tissue/buffer. A spatula of polyvinylpolypyrrolidone (PVPP) was added to the crude extract mixture. These homogenates were centrifuged at 15,000×g for 20 min. Briefly, SOD activity was measured at 560 nm by the riboflavin-nitroblue tetrazolium (NBT) method, according to Beauchamp and Fridovich (1973). The assay was based on the ability of SOD to inhibit the reduction of NBT into formazan blue by superoxide radicals generated photochemically. The reaction mix contained potassium phosphate buffer 50-mM pH 7.8, 0.1-mM EDTA, 0.75-mM NBT, and 130-mM methionine. A set of standards using riboflavin were evaluated, in which no sample was added and NBT was completely reduced. One unit of SOD was defined as the amount of enzyme required to inhibit the reduction rate of NBT by 50% at 25°C.
APx activity was determined using potassium phosphate buffer pH 7, 0.5 mM L-ascorbic acid and 0.2-mM H2O2 as substrates in 1-mL reaction mixture. Ascorbate disappearance was monitored at 290 nm (ε 290 nm, 2.8 mM−1 cm−1; Shalata et al. 2001). One unit (U) of APx activity was defined as the amount of enzyme that produced the disappearance of 1 mmol of substrate in 1 min of reaction under the conditions described. The enzymatic activity measurements were carried out using a Spectronic Genesys 5 (Milton Roy Company, Warminster, PA) thermostatized spectrophotometer.
As +5. removal by tobacco HRs from culture medium.
Removal assays were carried out using HRs previously subjected to a Pi starvation period as described. Inocula of 200 mg were incubated in 20-mL MSRT liquid medium containing 3% (w/v) sucrose, 100-μM Pi, and 10- or 50-μM As+5 in the dark, at 25 ± 2°C with agitation (100 rpm) as previously described. After 25 d, residual As concentration was determined spectrophotometrically in post-removal solutions. Removal results were expressed as removal efficiency (A), which is defined as the percentage of metalloid removed from solution under established experimental conditions. In order to compare As removal efficiency of both plant systems, the results were expressed as As removed per gram of final FW and they were obtained as follows (B):
-
(A)
Removal efficiency (%) = (Ci − Cf) × 100/Ci
-
(B)
As removed (in milligrams per liter)/gram FW = (Ci − Cf) × 1,000/Bt
where Ci is the initial As concentration, Cf is the final As concentration, and Bt is the total tissue biomass as final FW (in milligrams). HR tissue was oven dried to constant weight and then used to determine As accumulation by Inducted Coupled Plasma Optical Spectrophotometry (ICP-OES) method, described below.
As +5. removal by HRs from water at short periods of time.
This assay was performed to evaluate HR ability to remove As+5.from water. In order to do this, HR inocula previously grown in MSRT liquid medium with sucrose during 15 d were transferred to modified MSRT with only 30-μM Pi and grown during 7 d. Then, this medium was replaced by water artificially contaminated with As+5.stock solution to reach concentrations of 10, 100, and 200 μM. Control Erlenmeyer without HRs were also analyzed. HRs was harvested after 2 and 6 d of exposition and residual As was determined by a spectrophotometric silver method as described below. This assay was performed in triplicate.
Residual As determination.
After removal assays, residual As concentration was determined with a silver diethylthiocarbamate spectrophotometric method (APHA 2005). Arsenic was reduced with zinc to arsenamine in acid medium and then it was mixed with a chloroformic solution of silver diethylthiocarbamate. Absorbance of the mixture was determined at 520 nm using a Spectronic Genesys 5 (Milton Roy Company) thermostatized spectrophotometer.
For As quantification in naturally contaminated water and its residual As content after removal assays, the commercial kit QUANTOFIX arsenic 10 from Macherey-Nagel® (GmbH & Co. KG, Dueren (DE)) (0.01–0.5 mg/L) was used.
In HR tissue, residual As was determined by ICP-OES by Dr. Eloisa Pajuelo Domínguez collaboration (University of Sevilla, CITIUS). Briefly, As+5 treated HRs were rinsed with 1-mM EDTA to eliminate potential As adsorbed on cell walls and then, repeatedly washed with distilled water. Tissues were oven dried at 60°C until constant weight was reached. Final results, obtained as mean values of ICP-OES determination conducted in triplicate, were expressed as milligrams per kilogram of dry weight (DW).
Statistical analysis.
The results were analyzed with STATISTICA (version 6.0) software. Variance analysis (ANOVA) and post hoc Duncan test were applied to determine significant differences between groups. Results were considered statistically significant when P < 0.05.
Results and Discussion
Behavior of tobacco seedlings exposed to As +5—effects of As +5 on tobacco germination and early root development.
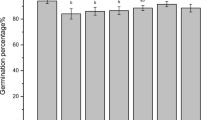
Total seed germination of the variety of tobacco studied (N. tabacum cv. Wisconsin) was not affected by 10- to 200-μM As V (data not shown). This result differs from those reported in previous studies on other species which found that germination decreases significantly with increasing concentrations of As (Abedin and Meharg 2002; Liu et al. 2005; Shri et al. 2009) and from others that have reported an increase in seed germination at low concentrations of the metalloid (Li et al. 2007). The results of our study suggest that this tobacco variety would probably germinate in soils contaminated with these concentrations of As.
Despite the fact that As+5 did not produce negative effects on tobacco germination in any of the assayed concentrations, FW of seedlings was reduced in an As+5 concentration-dependent manner at the range of concentrations analyzed. For instance, biomass production of tobacco seedlings was reduced by 11 and 72% for 10- and 200-μM As+5, respectively (Fig. 1A ). In addition, main root length was significantly reduced by As, which was evident from 100-μM As+5 treatment (Fig. 1A ).
Effect of As+5 on early stages of growth of tobacco seedlings after 10 d. (A) Seedling final FW and main root length. (B) Percentage of seedlings with different number of lateral roots (LR). Error bars represent standard errors from triplicate Petri dishes, each containing 20 seedlings. *Significant differences from the control (Duncan’s test, P < 0.05)
Depending on environmental conditions, the root system develops great plasticity, allowing this organ to change its morphology, distribution topology, and overall architecture (Potters et al. 2009). In order to evaluate the effect of As on tobacco root architecture, root branching was observed and registered as the number of lateral roots for each seedling under As+5 treatment. The major changes in lateral root frequency were observed for 100- and 200-μM As+5 treatments, for which the number of seedlings without lateral roots was higher than that of seedlings treated with 10- and 50-μM As+5. Moreover, for 200-μM As+5 treatment, the number of seedlings with three or more lateral roots was drastically reduced (Fig. 1B ). In many cases, exposure to stress conditions leads to a reorganization of the architecture characterized by an inhibition of primary root growth and the simultaneous stimulation of lateral root formation (López-Bucio et al. 2003). For instance, this response has been observed following exposure to high heavy metal concentrations like cadmium, copper, zinc, and lead (Lequeux et al. 2010). However, as observed in this study, As+5 treatment produced a strong detrimental effect on the tobacco root system since both main root elongation and root branching were reduced by As+5 from 100 μM.
Tolerance of tobacco seedlings to As +5 and effect of phosphate nutrition.
Due to chemical similarity, As+5 and Pi compete for the high-affinity Pi transporters. Thus, in the present study, As+5 tolerance of tobacco seedling was evaluated under different concentrations of potassium phosphate. Tobacco seedlings were treated with solutions containing 10- or 50-μΜ As+5 and 50- or 100-μΜ potassium phosphate during 25 d.
As expected, seedling development was affected by Pi availability, with those seedlings grown with 100-μΜ Pi reaching higher FW, since Pi is an essential plant nutrient (Fig. 2). Seedling growth was not significantly affected by 10-μΜ As+5, compared with control seedlings when 50- or 100-μΜ phosphate were supplied. On the contrary, a significant reduction of tobacco seedling development was observed when the seedlings were exposed to 50-μΜ As+5, since biomass was reduced by 50 and 37.5% with 50- and 100-μΜ Pi treatments, respectively (Fig. 2). Thus, As+5 toxicity in tobacco seedlings was dependent on Pi concentration.
(A) Growth of tobacco seedlings in solutions containing different As+5 concentrations and 50- or 100-μM Pi after 25 d. (B) Photographs of tobacco seedlings growing in hydroponic conditions and exposed to different As+5 and Pi concentrations. Error bars represent standard errors from triplicates. *Significant differences from the control conditions (Duncan’s test, P < 0.05)
Arsenate removal using tobacco seedlings in hydroponic conditions.
In order to evaluate the capability of tobacco seedlings to remove the metalloid, residual As was determined from the remaining solutions after 25 d of growth in medium initially containing 10- and 50-μM As+5 and 100-μM phosphate. Table 1 shows a high removal efficiency of 10-μM As+5, since approximately 46% of As was removed compared with control solutions. For treatments with 50-μM As+5, the removal efficiency was 11.8%. Additionally, tobacco seedlings were also able to remove As from naturally contaminated tap water containing 0.1 mg/L As (1.3 μM). After only 6 d, As concentration was below the detection limit of the highly sensitive QUANTOFIX kit (data not shown).
Behavior of tobacco HRs exposed to As +5—tolerance and effect of Pi nutrition.
In order to evaluate HR tolerance to As+5, HRs were first exposed to a period of Pi starvation so that they consumed their cellular Pi reserves. The presence of 5- or 10-μM As+5 did not significantly affect HR growth, similar to the results obtained for tobacco seedlings. Moreover, HR tissues remained healthy in appearance, whereas treatments with 50- and 100-μM produced a considerable growth reduction (Fig. 3). When Pi concentration was 50- or 100-μM, HR growth in the presence of 50- and 100 -μM As+5 was only around 15% compared with control cultures. However, when Pi concentration was higher, a minor toxic effect on biomass production was observed in presence of 50- and 100-μM As+5. As it could be seen, under low Pi concentrations, the toxicity produced by As+5 in HRs was higher. This is probably due to the fact that under Pi deficiency, high-affinity Pi transporters are induced and thus As+5 is further incorporated in the cell (Vance et al. 2003; Schachtman and Shin 2007). In other words, the mitigation of As toxicity symptoms in tobacco HRs by increasing external Pi concentration could indicate that Pi would be taken up preferentially over As+5, similar to results previously obtained for rice (Wang and Duan 2009). However, if efficient As removal is desired, Pi deficiency would enhance As incorporation from the environment (Fayiga et al. 2008). Thus, the following removal assays were carried out with low Pi (100 μM), in order to improve As removal and accumulation in tobacco HR tissue.
Growth of tobacco HRs under different concentrations of As+5 (0, 5, 10, 50, and 100 μM) and Pi (50, 100, and 1,000 μM) after 25 d. Values are mean of two independent experiments and bars represent standard errors from triplicates. *Significant differences from the corresponding control without As+5 but the same Pi concentration (P < 0.05)
APx and SOD activity in HRs under As +5 treatments.
In order to evaluate the antioxidant response in tobacco HRs treated with As+5, APx and SOD activity were measured. As shown in Table 2, at a low concentration of As+5 (10 μM) APx and SOD activity were higher than control HR enzymatic activities, whereas at high concentration (50 μM) these enzymatic activities were lower than control. However, the changes observed for SOD activity were not statistically significant, whereas APx induction for 10 μM was significantly different compared with control cultures. Since APx enzymes play a vital role in plant defense against oxidative stress, it is evident that As+5 induced these enzymes as an anti-oxidative mechanism to mitigate high levels of H2O2. Although SOD activity did not show statistically significant changes, it should not be discarded that other anti-oxidative enzymes could be induced under As+5 treatment, thus helping with the dissipation of other reactive oxygen species, as described by Gupta et al. (2009) for Brassica juncea L. plants.
As +5 removal and accumulation by tobacco HRs.
After a period of Pi starvation, As removal efficiency of tobacco HRs growing in MSRT medium with 10 μM (=0.75 mg/L) and 50-μM As (=3.5 mg/L) was evaluated (Fig. 4). HRs removed about 90% of As from 20-mL solutions initially containing 10-μM As+5. This high As removal efficiency is interesting since residual As concentration, in post-removal solutions, was only 0.06 mg/L (Table 1), which is quite close to the acceptable limits for human drinking water established by WHO (0.01 mg/L), and removal efficiency could be even improved by changes in tissue/medium volume ratio or Pi availability.
(A) Removal efficiency of HRs from MSRT medium with 10- and 50-μM As+5.and 100-μM Pi after 25 d. (B) As content in tobacco HR tissue from hydroponic cultures initially containing 10- and 50-μM of As+5. The results are expressed as milligrams As per kilograms DW tissue. Error bars represent standard errors from triplicates
Regarding the treatment with 50-μM As+5, a considerable removal of the metalloid (56%) was also found, although HR growth was drastically reduced under this condition. In spite of this, residual As concentration was still high considering the allowed limit for drinking water. However, it could be considered a suitable strategy to reduce the high As levels usually found in industrial effluents.
In order to give complementary evidence of HR removal capability, As content was quantified in HR tissue. To do this, HR tissue was washed with EDTA to discard adsorbed As in cell walls. Thus, the obtained values can be considered as a measure of As accumulation in root cells. HRs treated with 10-μM As+5 incorporated 12 mg As/kg DW, while those exposed to a concentration five times higher (50 μM) accumulated about 32 mg As/kg DW, only around three times more. These results suggest the participation of active detoxification mechanisms, probably induced by the low Pi concentration available (100 μM), which allow high accumulation of the metalloid. Arsenic accumulation was low compared with that reached with As hyperaccumulator plant species; however, it is important to consider that the removal efficiency was enough to reduce the level of residual As to values close to those accepted for human consumption. The removal ability of this tissue was important since 10-μM As (0.75 mg/L) represents a high value compared with the concentrations found for contaminated groundwater in areas such as the Chaco Pampean Plain of Central Argentina (Pérez-Carrera and Fernández Cirelli 2010).
Several authors have studied As removal under hydroponic conditions using different plant species such as the aquatic macrophyte Spirodela polyrhiza L. (Rahman et al. 2007), the plant Cucumis sativus (cucumber) (Hong et al. 2011), a marsh fern Thelypteris palustris (Anderson and Walsh 2007) and others. However, to our knowledge, this is the first report describing HRs as an alternative strategy to efficiently remove As from contaminated solutions. Even though some considerations are still needed, such as the scaling up of HR cultures for removal of contaminants from large volumes of water/effluents, HRs have been considered as a potential system to remove metalloids and other xenobiotics (Guillon et al. 2006; Majumder and Jha 2012).
Arsenic removal from contaminated water by HR cultures at a short period of time.
Even though tobacco HRs efficiently remove As from hydroponic conditions after a long period of treatment (25 d), we were interested in evaluating the ability of HRs to remove As from water and the minimum time required for this. Hence, tobacco HR cultures were exposed to artificially contaminated water containing 10, 100, and 200-μM As+5 and residual As was determined after 2 and 6 d. HRs were able to partially decontaminate water containing 10-μM As+5 in only 2 d (55%), whereas the maximum removal was reached after 6 d (96%; Fig. 5). From a solution initially containing a concentration of 100 μM, tobacco HRs were able to remove 5 and 40% of As after 2 and 6 d of treatment, respectively. However, no considerable removal was found after 2 d for artificially contaminated water containing 200-μM As+5 and only 15% of As was removed after 6 d. As expected, low removal efficiencies were obtained for higher As+5 concentrations.
These results are interesting since they showed that this plant system was able to efficiently remove As from water in a short period of time. Considering the high removal efficiency obtained for a relatively high concentration (0.75 mg/L, equivalent to 10 μM) in comparison to those found in natural As-contaminated water and other environments (Smedley et al. 2009), tobacco HRs could be potentially applied to the treatment of contaminated water. In addition, HRs did not show phytotoxicity symptoms since no necrosis of root apex was observed.
Comparison of the inherent removal ability between tobacco seedlings and HRs to remove As+5 should be made considering tissue biomass. Therefore, As removal expressed as (milligrams per liter)/gram FW was calculated. As shown in Table 2, HRs and seedlings were able to remove 0.24 and 0.31 (mg/L)/g FW, respectively, from solutions initially containing 10 μM As+5. However, the difference of these values was not statistically significant. The major difference between removal efficiencies could be seen for treatments initially containing 50-μΜ As+5. For this condition, HRs showed a higher value of As removed [3.26 (mg/L)/g FW] than that reached for seedlings [0.97 (mg/L)/g FW]. Thus, HR tissue showed a great potential to accumulate As only in root cells, without translocation; although translocation is widely accepted as one of the main factors that contribute to As detoxification in plants (Singh and Ma 2006; Zhao et al. 2010). The higher removal efficiency of HRs could be related to particular phenotypic characteristics of HRs such as the high biomass and high number of root hairs, which increase the surface of uptake.
Conclusions
Several crop species are being researched, not only for determining their usefulness for metal phytoextraction, but also to expand farming to areas containing high concentrations of heavy metals or metalloids. Tobacco has many of the required traits for its use in metal phytoextraction, thus, in this work, tolerance to As+5 was studied. Total tobacco germination was not altered in the presence of As+5. However, biomass and early root growth of tobacco seedlings were negatively affected by As+5 concentrations from 50 μM. This As+5 concentration also affected HR growth except when high Pi was available. As it has been reported for other plant species, As phytotoxicity was mitigated with high Pi availability. As+5 removal capability of tobacco HRs greatly exceeded that of seedlings even when translocation to aerial portions is considered to play an important role in As accumulation in plants. We showed As accumulation in HR tissues, which suggests the presence of mechanisms for metalloid accumulation in tobacco roots. Tobacco has an interesting inherent capacity for As removal and accumulation, and in the future, it could be engineered to achieve additional attributes required to be used in As phytoextraction. However, for achieving this goal, further research in biochemical and molecular basis for As removal and accumulation would be useful to provide scientific tools to outline some new strategies.
References
Abedin M. J.; Meharg A. A. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil 243: 57–66; 2002.
Anderson L.; Walsh M. Arsenic uptake by common marsh fern Thelypteris palustris and its potential for phytoremediation. Sci. Total Environ. 379: 263–265; 2007.
APHA (American Public Health Association) Standard methods for the water and waste water. 21st Edition. Washington DC; 2005.
Beauchamp C. O.; Fridovich I. Isozymes of SOD from wheat germ. Biochim. Biophys. Acta 317: 50–54; 1973.
Boominathan R.; Doran P. M. Cadmium tolerance and antioxidative defenses in hairy roots of the cadmium hyperaccumulator, Thlaspi caerulescens. Biotechnol. Bioeng. 83: 158–167; 2003.
Doran P. M. Application of plant tissue cultures in phytoremediation research: incentives and limitations. Biotechnol. Bioeng. 103: 60–76; 2009.
Eapen S.; Suseelan K. N.; Tivarekar S.; Kotwal S. A.; Mitra R. Potential for rhizofiltration of uranium using hairy root cultures of Brassica juncea and Chenopodium amaranticolor. Environ. Res. 91: 127–133; 2003.
Fayiga A. O.; Ma L. Q.; Rathinasabapathi B. Effects of nutrients on arsenic accumulation by arsenic hyperaccumulator Pteris vittata L. Environ. Exp. Bot. 62: 231–237; 2008.
Flocco C.; Alvarez M.; Giulietti A. Peroxidase production in vitro by Armoracia rusticana (horseradish)-transformed root cultures: effect of elicitation on level and profile of isoenzymes. Biotechnol. Appl. Biochem. 28: 33–38; 1998.
Flores H. E. Use of plant cells and organ culture in the production of biological chemicals. In: LeBaron H. M.; Mumma R. O.; Honeycutt R. C.; Duesing J. H. (eds) Biotechnology in agricultural chemistry, ACS Symp Ser 334. American Chemical Society, Washington DC, pp 66–86; 1987.
Francesconi K.; Visoottiviseth P.; Sridokchan W.; Goessler W. Arsenic species in an arsenic hyperaccumulating fern, Pityrogramma calomelanos, a potential phytoremediator of arsenic-contaminated soils. Sci. Total Environ. 284: 27–35; 2002.
Glebert C.; Ros R.; De Haro A.; Walker D. J.; Pilar Bernal M.; Serrano R.; Navarro-Aviñó J. A plant genetically modified that accumulate Pb is especially promising for phytoremediation. Biochem. Biophys. Res. Commun. 303: 440–445; 2003.
Grill E.; Mishra S.; Srivastava S.; Tripathi R. D. Role of phytochelatins in phytoremediation of heavy metals. In: Singh SN, Tripathi RD (eds) Environmental Bioremediation Technologies Springer, pp 101–146; 2006.
Guillon S.; Tremouillaux-Guiller J.; Pati P. K.; Rideau M.; Gantet P. Hairy root research: recent scenario and exciting prospects. Curr. Opin. Plant Biol. 9: 341–346; 2006.
Gupta M.; Sharma P.; Sarin N. B.; Sinha A. K. Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere 74: 1201–1208; 2009.
Hong S. H.; Choi S. A.; Yoon H.; Cho K. S. Screening of Cucumis sativus as a new arsenic-accumulating plant and its arsenic accumulation in hydroponic culture. Environ. Geochem. Health 33: 143–149; 2011.
Jabeen R.; Altaf A.; Muhammad I. Phytoremediation of heavy metals: physiological and molecular mechanisms. Bot. Rev. 75: 339–364; 2009.
Lequeux H.; Hermans C.; Lutts S.; Verbruggen N. Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Biochem. 48: 673–682; 2010.
Li C. X.; Feng S. L.; Shao Y.; Jiang L. N.; Lu X. Y.; Hou X. L. Effects of arsenic on seed germination and physiological activities of wheat seedlings. J. Environ. Sci. 19: 725–732; 2007.
Liu X.; Zhang S.; Shan X.; Zhu Y. G. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere 61: 293–301; 2005.
López-Bucio J.; Cruz-Ramírez A.; Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 6: 280–287; 2003.
Lugon-Moulin N.; Florian M.; Krauss M. R.; Ramey P. B.; Rossi L. Arsenic concentration in tobacco leaves: a study on three commercially important tobacco (Nicotiana tabacum L.) types. Water Air Soil Pollut. 192: 315–319; 2008.
Majumder A.; Jha S. Hairy roots: a promising tool for phytoremediation. In: Satyanarayana T.; Johri B. N.; Prakash A. (eds) Microorganisms in Environmental Management, Microbes and Environment. Springer, Netherlands, pp 607–629; 2012.
Marano K. M.; Naufal Z. S.; Kathman S. J.; Bodnar J. A.; Borgerding M. F.; Wilson C. L. Arsenic exposure and tobacco consumption: biomarkers and risk assessment. Regul. Toxicol. Pharmacol. 64: 225–232; 2012.
Meharg A. A.; Macnair M. R. An altered phosphate uptake system in arsenate-tolerant Holcus lanatus L. New Phytol. 116: 29–35; 1990.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 15: 473–479; 1962.
Nedelkoska T. V.; Doran P. M. Hyperaccumulation of cadmium by hairy roots of Thlaspi caerulescens. Biotechnol. Bioeng. 67: 607–615; 2000.
Nedelkoska T. V.; Doran P. M. Hyperaccumulation of nickel by hairy roots of Alyssum species: comparison with whole regenerated plants. Biotechnol. Prog. 17: 752–759; 2001.
Pérez-Carrera A.; Fernández Cirelli A. Arsenic and water quality challenges in South America. In: Schneier-Madanes G.; Courel M.-F. (eds) Water and sustainability in arid regions. Springer, Dortrecht Netherlands, pp 275–293; 2010.
Potters G.; Pasternak T. P.; Guisez Y.; Jansen M. A. K. Different stresses, similar morphogenic responses: integrated a plethora of pathways. Plant Cell Environ. 32: 158–169; 2009.
Rahman M. A.; Hasegawa H.; Ueda K.; Maki O. C.; Rahman M. M. Arsenic accumulation in duckweed (Spirodela polyrhiza L.): a good option for phytoremediation. Chemosphere 69: 493–499; 2007.
Robinson B.; Kimb N.; Marchetti M.; Monid C.; Schroeter L.; van den Dijssel C.; Milne G.; Clothier B. Arsenic hyperaccumulation by aquatic macrophytes in the Taupo Volcanic Zone, New Zealand. Environ. Exp. Bot. 58: 206–215; 2006.
Sato K.; Maitani T.; Yoshihira K. Uptake of arsenic by cultured hairy roots of Rubia tinctorum from liquid medium. J. Food Hyg. Soc. Jpn. 32: 414–419; 1991.
Schachtman D. P.; Shin R. Nutrient sensing and signaling: NPKS. Annu. Rev. Plant Biol. 58: 47–69; 2007.
Shalata A.; Mittova V.; Volokita M.; Guy M.; Tal M. Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol. Plant. 112: 487–494; 2001.
Shri M.; Kumar S.; Chakrabarty D.; Trivedi P. K.; Mallick S.; Misra P.; Shukla D.; Mishra S.; Srivastava S.; Tripathi R. D.; Tuli R. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 72: 1102–1110; 2009.
Singh N.; Ma L. Q. Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pteris vittata L. and non-hyperaccumulator Pteris ensiformes L. Environ. Pollut. 141: 238–246; 2006.
Smedley P. L.; Nicolli H. B.; Macdonald D. M. J.; Kinniburgh D. G. Arsenic in groundwater and sediments from La Pampa province, Argentina. In: Bundschuh J.; Armienta M. A.; Birkle P.; Bhattacharya P.; Matschullat J.; Mukherjee A. B. (eds) Natural arsenic in groundwaters of Latin America. CRC Press, London, pp 35–45; 2009.
Sosa Alderete L. G.; Talano M. A.; Ibáñez S. G.; Purro S.; Agostini E.; Milrad S. R.; Medina M. I. Establishment of transgenic tobacco hairy roots expressing basic peroxidases and its application for phenol removal. J. Biotechnol. 139: 273–279; 2009.
Talano M. A.; Wevar Oller A. L.; González P. S.; Agostini E. Hairy roots, their multiple applications and recent patents. Rec Patents Biotechnol. 6: 115–133; 2012.
Vance C. P.; Uhde-Stone C.; Allan D. L. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157: 423–447; 2003.
Wang L.; Duan G. Effect of external and internal phosphate status on arsenic toxicity and accumulation in rice plantlets. J. Environ. Sci. 21: 346–351; 2009.
Wu S.; Zu Y.; Wu M. Cadmium response of the hairy root culture of the endangered species Adenophora lobophylla. Plant Sci. 160: 551–562; 2001.
Zhao F. J.; McGrath P. S.; Meharg A. A. Arsenic as a food chain contaminant: mechanism of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 61: 535–559; 2010.
Zhu Y. G.; Rosen B. P. Perspectives for genetic engineering for the phytoremediation of arsenic-contaminated environments: from imagination to reality? Curr. Opin. Biotechnol. 20: 220–224; 2009.
Acknowledgments
MAT, ALWO, and EA are members of the research career of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET; Argentina). SOG has a fellowship from CONICET. We wish to thanks to PPI (SECyT-UNRC), CONICET, MINCyT Córdoba and PICTO (FONCyT-SECyT-UNRC) for financial support. The authors thank MSc. Iliana A. Martínez, MA, and her research group for language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Talano, M.A., Oller, A.L.W., González, P. et al. Effects of arsenate on tobacco hairy root and seedling growth, and its removal. In Vitro Cell.Dev.Biol.-Plant 50, 217–225 (2014). https://doi.org/10.1007/s11627-013-9557-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-013-9557-1