Abstract
Exclusion of sodium ions from cells is one of the key salinity tolerance mechanisms in plants. The high-affinity cation transporter (HKT1;5) is located in the plasma membrane of the xylem, excluding Na+ from the parenchyma cells to reduce Na+ concentration. The regulatory mechanism and exact functions of HKT genes from different genotypic backgrounds are relatively obscure. In this study, the expression patterns of HKT1;5 in A and D genomes of wheat were investigated in root and leaf tissues of wild and domesticated genotypes using real-time PCR. In parallel, the K+/Na+ ratio was measured in salt-tolerant and salt-sensitive cultivars. Promoter analysis were applied to shed light on underlying regulatory mechanism of the HKT1;5 expression. Gene isolation and qPCR confirmed the expression of HKT1;5 in the A and D genomes of wheat ancestors (Triticum boeoticum, AbAb and Aegilops crassa, MMDD, respectively). Interestingly, earlier expression of HKT1;5 was detected in leaves compared with roots in response to salt stress. In addition, the salt-tolerant genotypes expressed HKT1;5 before salt-sensitive genotypes. Our results suggest that HKT1;5 expression follows a tissue- and genotype-specific pattern. The highest level of HKT1;5 expression was observed in the leaves of Aegilops, 6 h after being subjected to high salt stress (200 mM). Overall, the D genome allele (HKT1;5-D) showed higher expression than the A genome (HKT1;5-A) allele when subjected to a high NaCl level. We suggest that the D genome is more effective regarding Na+ exclusion. Furthermore, in silico promoter analysis showed that TaHKT1;5 genes harbor jasmonic acid response elements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately one third of irrigated lands are affected by salinity (Demidchik and Tester 2002), therefore understanding the mechanisms for salt tolerance plays a key role in improving crop productivity (Huang et al. 2006). One of the vital tolerance mechanisms in cells is maintaining a low concentration of cytosolic Na+ under stress (Kader et al. 2006), with the plasma membrane being the most likely site for selective regulation of ion transport (Kader et al. 2006). Under high levels of Na+, due to similarities in their ion hydration energies and ionic radii, Na+ competes with K+ for uptake through membrane transport systems (Plett and Moller 2010). Because K+ plays an essential role in many enzymatic functions, a high Na+/K+ ratio retards the function of many enzymes (Kader et al. 2006). The uptake of Na+ into cells takes place through multiple Na+-permeable cation channels/transporters, such as outward- and inward-rectifying K+-selective channels, particularly nonselective cation channels in the plasma membrane (Amtmann and Sanders 1999). Loading of xylem vessels with Na+ results in its transportation upward in the plant via the transpiration system (James et al. 2006). This transport triggers ion toxicity when the cytoplasmic concentration of Na+ rises above a threshold level (Kader et al. 2006).
Interestingly, the degree of salt tolerance is not similar among different plant tissues; specifically, shoots are generally more sensitive than roots to Na+ concentrations (James et al. 2006). Salt tolerance, therefore, can be also related to a reduction in the transport of sodium from roots to shoots, and the ability to exclude Na+ from cells to prevent the accumulation of high Na+ levels in leaves (James et al. 2006).
High-affinity potassium transporters (HKTs) are a large superfamily of transporters in plants, bacteria, and fungi. It has been suggested that these transporters play crucial roles in salinity tolerant via removal of Na+ from the xylem during salinity stress (James et al. 2006). In general, little is known about Na+ excluding genes in plants, particularly in wild genotypes, or their degree of genetic diversity. In wheat, group I HKT genes confer salt tolerance through sodium exclusion mechanisms in leaves (Huang et al. 2006). TaHKT1;5-D (Kna1) is a specific Na+ transporter in Triticum aestivum (an allohexaploid, AABBDD genome) that plays the same role as TmHKT1;5-A (Nax2) in Triticum monococcum (diploid, AA genome). While the predicted amino acid identity between TmHKT1;5-A and TaHKT1;5-D is 94% (Byrt et al. 2007).
Wild genotypes represent a potential source for discovering significant novel genes and promoters. Furthermore, erosion of genetic diversity has been suggested to be the main cause of salt sensitivity in modern crops (Dvorak and Akhunov 2005). Iran, as the center of the origin and genetic diversity of wheat, presents rich gene pools for wheat and its wild relatives, such as Triticum and Aegilops species (Tabatabaei and Maassoumi 2001). Recent studies have hypothesized that at least some accessions of diploid wheat varieties with the A genome (i.e., T. boeoticum and T. monococcum) are effective Na+ excluders (Shavrukov et al. 2009). It has been reported that TmHKT1;5-A decreases Na+ concentrations in leaf blades and sheaths to a greater extent than TmHKT1;4-A (Nax1; James et al. 2006). Additionally, high levels of Na+ exclusion and salinity tolerance have been found to be associated with the D genome, such as in Aegilops and bread wheat (Shavrukov et al. 2009). TaHKT1;5-D plays a significant role in salt tolerance via Na+ exclusion from leaves and controlling xylem loading in roots. (Byrt 2008). Identifying new HKT alleles in wild relatives of wheat may provide an opportunity to achieve higher salt tolerance than is associated with the currently known alleles.
In silico promoter analysis can produce valuable information about the function and response of a gene to various cues. Regulatory elements on promoter region of a gene can be more determinant in conferring gene function than its protein structure (Deihimi et al. 2012). We believe that the superiority of an HKT homologue to other homologues can actually be related to its superior promoter structure, rather than its gene structure. Regarding the unknown role of HKT promoters, it appears that promoter analysis of HKT genes may be able to assist in solving many unanswered questions. The regulatory elements in promoters, such as transcription factor binding sites (TFBs; or cis-regulatory elements), are organized into distinct modules that control expression of many genes in systems biology. Thus, the identification of regulatory elements can be a first step in recognition of gene expression patterns (Mariño-Ramírez et al. 2009).
As the HKT gene family encodes one of the most significant Na+ transporters among plants, increasing the expression of key family members has been considered as a viable strategy for improving salinity tolerance (Plett et al. 2010). However, the physiological functions, gene networks, and signaling pathways related to HKT transporters have not been completely elucidated (Hauser and Horie 2010). In addition, the similarities and differences in the expression patterns of HKT genes in different tissues (such as shoots and roots) are relatively unknown. Moreover, while HKT genes such as HKT1;5 can originate from the D, B, or A genomes (Byrt 2008), the effects of the genomic background on the expression and activity of HKTs has not been thoroughly investigated.
In the present study, a comprehensive expression analysis of HKT1;5 under different salinity concentrations using salt-sensitive and salt-tolerant varieties of bread wheat (T. aestivum) and wild wheat ancestors (T. boeoticum and Aegilops crassa) with different genomic backgrounds (A or D genomes, respectively) were carried out. In addition, because the main role of HKT1;5 is to prevent transport of Na+ from shoots to roots, the K+/Na+ ratio was measured in the leaves of salt-sensitive and salt-tolerant wheat cultivars under different salt stress concentrations. The promoter region of HKT1;5-D (T. aestivum) and HKT1;5-A (T. monococcum) were also analyzed in silico.
Materials and Methods
Plant materials.
Seeds of wheat (T. aestivum) cultivars Mahuti and Alamut and wild wheat ancestors (Triticum boeoticum or T. monococcum subsp aegilopoides and A. crassa) were provided by Shiraz and Ilam Universities, Iran. Mahuti is a salt-tolerant cultivar, while Alamut is salt-sensitive. The seeds were soaked in water for 24 h at 4°C on moist filter paper in wrapped Petri dishes. The seedlings were then transferred to hydroponic tanks containing half-strength Hoagland’s solution, pH 6.0 (Genc et al. 2007). The solutions were changed weekly. The plants were grown in a controlled glasshouse with day/night temperatures of 25/21 C under 16 h of light (300 μmol m−2 s−1).
Salinity stress experiment.
For each of the four genotypes (T. aestivum cv. Mahuti and Alamut, T. boeoticum, and A. crassa), a salinity experiment was conducted based on a randomized complete block design (RCBD) with four NaCl concentrations as treatments, six sampling times, and with two biological replications. Seventeen days after germination, the plants were stressed by adding NaCl at concentrations of 0, 50, 100, or 200 mM. Sampling of leaves was carried out at 0 h (before stress), and at 3, 6, 10, 24, and 72 h after initiation of the stress treatments in all genotypes for all treatments. In addition, sampling of root tissue was performed 6, 10, 24, and 72 h after the initiation of salt stress from salt-tolerant (cv. Mahuti) and salt-sensitive (cv. Alamut) wheat cv. Nonstressed control plants of each genotype were grown concurrently and harvested at the same time as a control. Leaf and root tissues were collected during sampling, snap frozen in liquid nitrogen, and immediately stored at −80°C until further analysis was conducted.
Isolation of partial HKT1;5-A and HKT1;5-D sequences.
As HKT1;5 sequence from T. boeoticum (T. monococcum subsp aegilopoides) and Aegilops had not been published, in order to conduct real-time PCR experiments, it was necessary to first isolate HKT1;5 alleles and confirm its expression in the above mentioned genotypes. Thus, primers for the amplification of TbHKT1;5-A (the HKT1;5 isoform in T. boeoticum) were designed based on the protein coding sequence (CDS) of this gene in T. monococcum and for AeHKT1;5-D (HKT1;5 isoform in Aegilops) according to the CDS of this gene in bread wheat (TaHKT1;5-D) available in GenBank, using Vector NTI Suite 9. The following primer sequences were used for amplification of HKT1;5-A and HKT1;5-D: forward, 5′-CTATCACGTGGTGGTGCACC-3′, and reverse, 5′-CGTGCGGCATGACTAGGAGCA-3′. These primers could amplify the partial CDS of HKT1;5 in T. boeoticum and Aegilops. Then, RNA was isolated from the leaves of T. boeoticum (AbAb) and A. crassa (MMDD) for RT-PCR using RNX™ (-Plus) buffer followed by DNase digestion; RNA purification and synthesis of first-strand cDNA from the total RNA were performed according to the manufacturer’s instructions (Fermentas, Ontario, Canada). cDNA was amplified using RT-PCR Master Mix in a Bioer thermocycler. The PCR conditions were as follow: 94°C for 3 min, followed by 30 cycles of 94°C for 1 min, 59°C for 45 s, 2 min at 72°C, and final extension of 10 min at 72°C. PCR product of the partial cDNA sequences from A. crassa and T. boeoticum were sequence verified by Macrogen (http://www.macrogen.com/).
RNA extraction and cDNA synthesis for real-time RT-PCR.
After confirming expression of the HKT1;5 gene in all four genotypes, Mahuti and Alamut, T. boeoticum, and A. crassa, qRT-PCR expression analysis was performed by isolating total RNA from the leaf and root tissues and synthesizing cDNA according to the above mentioned method. For quantitative real-time PCR, 2.5 μl of cDNA was used as a template. The cDNA was amplified using SYBR Green PCR Master Mix (Takara SYBR premix EX-Taq II) in a Bioer thermocycler (Applied Bioer, LineGe-neK, Hangzhou, China). Elongation factor α (elf-α) was selected as a reference gene to normalize the expression data for HKT1;5. The following primer sequences were used for elf-α: 5′-TTTCACTCTTGGAGTGAAGCAGAT-3′ and 5′-GACCTCCTTGACAATTTCTTCATAA-3′. To analyze the expression of HKT1;5-D and HKT1;5-A, the forward and reverse primers sequences were: 5′-CTATCACGTGGTGGTGCACC-3′ and 5′-ACGGAGAAGGTGTGCAGGCTG-3′. The PCR conditions were as follows: 95°C for 2 min, followed by 40 cycles of 95°C for 10 s, 59°C for 15 s, and 72°C for 30 s. Each experiment was repeated independently twice for each genotype as a biological replicate. Standard determination curves were generated using serial dilutions of 10,000, 1,000, 100, and 10 ng cDNA in each well for every experiment. Two independent replicates were performed for each genotype.
Real-time RT-PCR data analysis.
Normalization of the target genes (HKT1;5-A and HKT1;5-D) was carried out based on reference to an endogenous standard (elf-α). The Pfaffl formula (ratio = 2–ΔΔCt) was used to calculate relative expression (Pfaffl 2001), where ΔΔCt = (ΔCt sample − ΔCt control); ΔCt sample = (ΔCt target − ΔCt ref) for all sampling times and NaCl concentrations; and ΔCt control = (ΔCt target − ΔCt ref).
Statistical analysis.
The experiments were conducted based on a RCBD with two biological replications, four NaCl concentrations, and four genotypes per treatment. Six different times were applied as blocks following the initiation of the stress treatment. To compare the differences in expression levels of HKT1;5 between genotypes, a 2 × 2 paired t test at the 0.05 significance level was applied using MINITAB 14 software for the data from leaf and root tissues.
Sodium and potassium analysis.
The K+/Na+ ratio is a reliable index of salt tolerance in plants (Rush and Epstein 1976). This index was determined in leaf tissues from the salt-tolerant (Mahuti) and salt-sensitive (Alamut) bread wheat cv. at four salinity concentrations, 4 wk after the initiation of stress using the Flame-photometry method. This compared the salt-tolerant and salt-sensitive genotypes with respect to the association of HKT genes with the specific ability of the plant to exclude Na+ from leaves and maintain a stable K+/Na+ ratio.
In silico promoter analysis to identify major HKT1;5 elements Triticum genotypes.
As there is currently no available database for promoter identification in wheat or wild wheat relatives, a study published by Byrt (2008) was used in this case. The putative promoter sequences of HKT1;5 genes of T. aestivum and T. monococcum were compared with known cis-regulatory elements in the collection of the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; Lescot et al. 2002). The cis-regulatory elements were noted and counted for each promoter.
Results and Discussion
Isolation of partial HKT1;5 sequences.
Although bread wheat, T. aestivum, is an allohexaploid with an AABBDD genome, the HKT1;5-A gene is not present in this species (Byrt et al. 2007). However, as the sequence of the region where the TaHKT1;5-D allele is located is highly similar to TaHKT1;5-B, it was possible that the B genome isoform could be amplified by PCR (Byrt 2008). Primers designed to amplify HKT1;5 in two cultivars of bread wheat (Mahuti and Alamut) was performed based on the highly conserved coding region, but sequencing the cDNA products showed that only the TaHKT1;5-D allele was amplified. However, we were successfully able to amplify, for the first time, partial CDS of an HKT1;5 gene from T. boeoticum (T. monococcum subsp aegilopoides) and A. crassa. Analysis of the results showed that HKT1;5-A and HKT1;5-D are present in these genotypes, respectively, and are expressed under salt stress in leaf tissues. The sequences amplified for HKT1;5-like genes from T. boeoticum (T. monococcum subsp aegilopoides), A. crassa, and T. Aestivum cv. Mahuti are available from GenBank (accession numbers JQ677810, JQ677811, and JQ677812, respectively).
Expression of TaHKT1;5-D in Mahuti leaf.
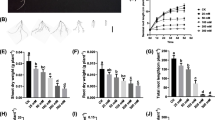
In leaf tissue from Mahuti plants (the salt-tolerant bread wheat cv.), the expression of TaHKT1;5-D varied over time and at different NaCl concentrations (Fig. 1A ). Mean separation values indicated that the highest expression of TaHKT1;5-D (approximately two times higher than control plants not subjected to salt stress) occurred at a concentration of 200 mM Na+ (Fig. 1A ). The strongest upregulation of the TaHKT1;5-D transcripts was observed in this cultivar 3 h after the initiation of stress treatment compared with other concentrations tested. The primary induction of some HKT transporters can result from a number of factors, such as posttranscriptional or protein conformational changes in salt-tolerant cv., including Mahuti (Kader et al. 2006). It appears that due to continuing the application of salt stress, other mechanisms may have been initiated in the leaf; thus, the expression of the TaHKT1;5-D gene was decreased from 10 to 72 h.
Expression analysis of HKT1;5 by real-time RT-PCR; amplification of RNA from leaf and root tissues of wheat cv. Mahuti (salt tolerant) and Alamut (salt sensitive) and leaves of species T. boeoticum and A. crassa (A–F). Sampling was carried out after 3, 6, 10, 24, and 72 h of growing plants under salt stress conditions (50, 100, and 200 mM NaCl). Real-time data were normalized in relation to 0 mM NaCl.
t test analyses confirmed that there was no significant difference in HKT1;5 expression prior to salinity stress (at 0 h before stress) at p = 0.05. Although the level of expression at 0 h was very low so that it was near to control (0 mM treatment), Mahuti leaves exhibited the strongest expression at that time, though it was not significant (data not shown). This result showed that salt stress induced HKT1;5 in all genotypes.
Expression of TaHKT1;5-D in Mahuti root.
The strongest induction of TaHKT1;5-D was nine times higher than in controls of Mahuti root tissue (Fig. 1B ), at 100 mM, occurring 72 h after initiation of the salt stress treatment. The later induction of TaHKT1;5-D in roots likely causes Na+ to increase in upper parts of the plant, such as the leaves. Thus, upregulation of TaHKT1;5-D was induced earlier in leaves. On the other hand, since the induction of HKT1;5 in the Mahuti roots was more severe than leaves, it seems this gene may act to exclude Na+ from absorption by the roots preferentially.
Expression of TaHKT1;5-D in Alamut leaf.
The highest TaHKT1;5-D transcript levels were observed in leaves of the salt-sensitive cv. Alamut at 200 mM, which were 35 times higher than in controls after 10 h of treatment, with the higher salt concentrations showing the greatest response (Fig. 1C ). Studies have indicated that in salt-sensitive rice cultivars, K+-selective channels (in addition to nonselective cation channels) also contribute to Na+ uptake (Kader and Lindberg 2005). It may be that the upregulation of TaHKT1;5-D in the salt-sensitive cv. could also induce strong induction of some Na+ influx transporters that led to an increase of the Na+ concentration in the plant cells, especially the xylem, resulting in greater toxicity to the plant. The reduced expression after 72 h could be related to the toxic effects of the salt treatment on the plant. Alamut exhibited stronger TaHKT1;5-D expression than the salt-tolerant, but this difference was not significant (Table 1).
Expression of TaHKT1;5-D in Alamut root.
The peak induction of TaHKT1;5-D was approximately two and a half times higher than in controls not significantly (Fig. 1D ) In this salt-sensitive cv., Na+ uptake occurs via two pathways (Kader and Lindberg 2005). This process can induce root TaHKT1;5-D activity under low salt conditions, such as at a concentration of 50 mM NaCl. It is probable that the lower TaHKT1;5-D induction at higher NaCl concentrations, including 200 mM, in roots of the salt-sensitive cv. Alamut results in higher Na+ concentrations in leaves. Thus, upregulation occurred under high Na+ conditions in leaves (Table 2).
According to our result, this gene was upregulated late (72 h) in roots, and this late induction provided conditions resulting in earlier and higher TaHKT1;5-D expression in leaves. Previous studies have suggested that the HKT expression pattern is poorly understood and differs among tissues and genotypes (James et al. 2011). Higher expression of TaHKT1;5-D was observed in the salt-tolerant cv. Mahuti than the salt-sensitive cv. Alamut in root tissue, as confirmed by a t test (Table 1). This result confirmed that HKT1;5 exhibited higher expression in the D genome. Although the expression of this gene in T. boeoticum (HKT1;5-A) was higher than in Alamut and Mahuti (including HKT1;5-D), Mahuti is an exceptional cultivar regarding salt tolerance, and Alamut is a salt-sensitive cv.; thus, this result is reasonable.
Comparison of HKT1;5 expression patterns between leaf and root tissues.
HKT1;5 is a remarkable gene for decreasing Na+ concentration in plants. When comparing the expression of TaHKT1;5-D in the different tissues, it was found to be higher in roots than in leaves in the salt-tolerant cv. Mahuti (p = 0.05; Table 1). Therefore, it seems that the main activity for HKT1;5 occurs in the roots of this cultivar which may be related to its exceptional activity with respect to salt tolerance as based on other studies (Ghavami et al. 2004), or is due to varying levels of expression in different tissues (James et al. 2011). Analysis of the K+/Na+ ratio re-confirmed this result where this index was lower in Mahuti leaves than Alamut leaves. Consequently, the Na+ concentration was probably higher in the leaves of Mahuti cultivar than its roots; this may be because of tissue- specificity activity of the HKT1;5.
For Alamut (salt-sensitive wheat), on the other hand, the expression of TaHKT1;5-D in the leaves was not significantly different to the roots (p = 0.05; Table 1), except at the 10 h time-point and high salt treatment where expression in Alamut leaves was very high, far exceeding what was observed for Mahuti leaf tissues. In contrast, in root tissue of Mahuti (tolerant cultivar), the expression of this gene was higher than sensitive cultivar (Alamut) (Fig. 1A–D ) This supports a previous conclusion that HKT gene expression pattern exhibits different activity levels in diverse genotypes (James et al. 2011).
Expression of TbHKT1;5-A in T. boeoticum leaves.
HKT1;5-A in T. boeoticum reduces Na+ concentrations in sheaths and blades of leaves and, thus, leads to increases in K+ concentrations in leaves and induces Na+ removal from the xylem (Byrt 2008). It has been documented that HKT1;5-A is not present in bread wheat (Byrt et al. 2007). In this study, the strongest upregulation of TbHKT1;5-A at 100 mM NaCl was approximately 50 times higher than control levels (Fig. 1E ). Although previous studies have shown that TmHKT1;5-A (in T. monococcum) removes Na+ from the xylem only in the roots (James et al. 2006), the present study shows that TbHKT1;5-A can be highly expressed in leaf tissue of T. boeoticum. The expression of TbHKT1;5-A was low for all time-points of the 200 mM NaCl treatment. Based on our data, there are two likely reasons: TbHKT1;5-A is not effective under high Na+ concentrations, and TbHKT1;5-A expression is different among various tissues (James et al. 2011). For example, upregulation of this gene in roots or shoots may prevent Na+ uptake in leaves, resulting in a decrease in TbHKT1;5-A expression in leaves. However, further verification will require further study; we assume that the first explanation is more probable. Other studies have shown that wheat lines carrying HKT1;5-A exhibit a 3.6 times greater leaf K+/Na+ ratio, and their leaf Na+ levels are approximately 2.5 times lower than those of lines without HKT1;5-A (Huang et al. 2006). Clearly, the Na+ reduction associated with HKT1;5-A is lower compared with HKT1;5-D. Thus, the effect of HKT1;5-D may be stronger than that of HKT1;5-A with respect to the enhancing salt tolerance. HKT1;5-A decreases the Na+ loading and uptake from roots to shoots (Byrt et al. 2007). However, in 2011, James showed that TmHKT1;4-A is more effective than TmHKT1;5-A at reducing Na+ in leaf tissue. The upregulation of HKT1;5-A was higher in T. boeoticum than the salt-tolerant cv. Mahuti, which was confirmed by a t test (Table 1). However, the stronger induction of this gene in T. boeoticum was not significantly different than that in the salt-sensitive cv. Alamut based on a t test.
The relative expression of TbHKT1;5-A was higher 10 h after the induction of stress than at other times. This expression is apparently lower than that of the D genome (HKT1;5-D). It appears that HKT1;5-D carries regulatory elements that allow a rapid response to salinity stress.
Expression of AeHKT1;5-D in A. crassa leaves.
Real-time PCR analysis showed that AeHKT1;5-D expression was maximally upregulated in A. crassa leaf tissue at 200 mM NaCl, to levels 250 times higher than control levels (Fig. 1F ) The three highest expression levels for HKT1;5 was detected in A. crassa at 200, 100, and 50 mM NaCl, respectively. According to real-time PCR, HKT1;5 exhibited higher activity in this wild species compared with cultivated plants under all stress conditions. The D genome is known to contain many genes conferring salt tolerance (Gorham et al. 1990), thus providing useful genetic variation related to Na+ exclusion in each wild species and subspecies (such as Aegilops). There was high expression of AeHKT1;5-D 6 h after stress was initiated. Thus, the earlier and stronger upregulation of AeHKT1;5-D gives raises the question whether the high salinity tolerance of Aegilops led to a high Na+ concentration in its leaves that forces strong AeHKT1;5-D upregulation. We proposed two possibilities: firstly, AeHKT1;5-D is not expressed in lower Aegilops tissues, such as roots and shoots. Second, Na+ accumulates in Aegilops leaf vacuoles via AeHKT1;5-D or other transporters. Although this function of HKT genes has not yet been observed (as the role of these genes is relatively unknown), this function may occur via AeHKT1;5-D. We prefer that the first hypothesis because Byrt showed that the TaHKT1;5-D gene is expressed in Chinese Spring roots, but not shoots, and thus, this gene can show tissue specific expression. The fact that the highest expression of HKT1;5 was observed in A. crassa compared with other wheat species studies (Table 1) presents new options for improving salinity tolerance in wheat. We previously showed that TaSOS1 and TaSOS4, as two important genes in salt tolerance, have higher expression in A. crassa comparing to the other genotypes (Ramezani et al. 2012). Here, t test analyses confirmed the expression of AeHKT1;5-D was higher in Aegilops than the salt-sensitive and salt-tolerant cv. Alamut and Mahuti at p = 0.05 while the increase in the expression of HKT1;5 between Aegilops and T. boeoticum was not significant (Table 1).
K+/Na+ ratio.
Although many researchers agree that K+/Na+ homeostasis is key in plant salinity tolerance (Genc et al. 2007), our results indicated that the Na+ content in the leaves of salt-tolerant cv. (Mahuti) and salt-sensitive cv. (Alamut) increased due to increasing the NaCl concentration in parallel with decreasing the K+ level in both cultivars (Fig. 2). Unexpectedly, tolerant wheat showed a lower K+/Na+ ratio than the salt-sensitive cultivar. t test results showed that the K+/Na+ ratio was significant in Mahuti and Alamut at p = 0.05 (Table 1). Although Mahuti exhibits high tolerance to salinity, this cultivar presents a low K+/Na+ ratio compared with the other cultivars. Thus, it is possible that HKT transporters, or possibly genes involved in the modification of the K+/Na+ ratio are not particularly effective in Mahuti leaves. In agreement with this finding, some reports have noted that Na+ exclusion and tissue tolerance vary independently (Genc et al. 2007). In the present study, because the expression of TaHKT1;5-D was lower in the leaves of the salt-tolerant cv. Mahuti than in the salt-sensitive cv.
It should be noted that TaHKT1;5-D expression and K+/Na+ were also low in the Mahuti leaves than Alamut. In contrast, in Mahuti, the most significant expression for HKT1;5 occurred in the roots. We believe that Mahuti is one of the modern salt-tolerant bread wheat cultivars (Ghavami et al. 2004), it is possible that maybe its mechanisms do not admit to uptake Na+ within its upper parts, so this gene should be active in roots; however the more study can help to understand it more precisely.
Comparative study of promoter regions between HKT1;5-A and HKT1;5-D.
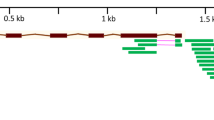
Using an in silico promoter analysis tool (Lescot et al 2002), the results of the PlantCARE analysis showed that the regulatory elements related to the stress and hormone response have some differences between TaHKT1;5-D (T. aestivum) and TmHKT1;5-A (T. monococcum). The cis-elements of the HKT1;5 promoter are shown in Fig 3. TATA box and CAAT elements were more frequent in the TmHKT1;5 promoter than that of TaHKT1;5. The jasmonate response element was two times more frequent in TmHKT1;5 than TaHKT1;5 (Fig. 3). Jasmonate is involved in plant adaptation to biotic and abiotic stresses and is accumulated transiently in response to osmotic/salt stress (Lehmann et al. 1995). The real-time PCR analysis revealed that HKT1;5 expression was low under 200 mM NaCl in T. boeoticum; thus, this genotype is active at lower salinity. However, verifying this hypothesis will require further study. It is likely that the regulatory elements of the TbHKT1;5 promoter are different compared with TmHKT1;5, however, the sequence of TbHKT1;5 is unknown at present.
The sequences and the predicted cis-elements of the putative promoter region of TaHKT1;5-D in T. aestivum (A) and TmHKT1;5-A in T. monococcum (B). The various colored sequences are related to different putative cis-elements in the promoter regions that are identified by PlantCARE database. HSE heat stress responsiveness, LTR low-temperature responsiveness, MYB light responsiveness, MeJA MeJA responsiveness, auxin, gibberellin, TC-rich, and ethylene response elements.
The promoter analysis showed that HSEs (cis-acting elements involved in heat stress responsiveness) elements are more frequent in TaHKT1;5-D than TmHKT1;5; thus, it is likely that these HKTs are also expressed in response to high temperature. Because there was no significant difference between the number of cis-regulatory elements, such as auxin, ethylene, gibberellin and light response elements in the promoters of TaHKT1;5 and TmHKT1;5, we do not expect T. monococcum and T. aestivum to be different when developmental stages and ripening happen.
Conclusions
In conclusion, this study provides a comprehensive expression analysis of HKT1;5 transporters for four wheat genotypes under salt stress by real-time PCR. Because A. crassa showed the highest level of HKT1;5 expression, we predict that Na+ exclusion is the main salinity tolerance mechanism in this species. An important insight from this study was showing that the Mahuti the HKT1;5 gene has a different action compared with other genotypes, which is confirmed with K+/Na+ ratio analysis. However, to fully understand the precise HKT1;5 activity several different plants tissues should be analyzed and compared. According to our results, it is likely that, due to the differences in HKT1;5 function in different tissues, specific promoter elements may be differentially activated in different tissues.
References
Amtmann A, Sanders D (1999) Mechanisms of Na+ uptake by plant cells. Adv Bot Res 29:75–112
Byrt CS (2008) Genes for sodium exclusion in wheat. Ph.D. thesis, University of Adelaide. Available from http://hdl.handle.net/2440/56208. Accessed 24 April 2012
Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol 143:1918–1928
Deihimi T, Niazi A, Ebrahimi M, Kajbaf K, Fanaee S, Bakhtiarizadeh MR (2012) Finding the undiscovered roles of genes: an approach using mutual ranking of coexpressed genes and promoter architecture-case study: dual roles of thaumatin like proteins in biotic and abiotic stresses. SpringerPlus 1:30
Demidchik V, Tester M (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128:379–387
Dvorak J, Akhunov ED (2005) Tempos of gene locus deletions and duplications and their relationship to recombination rate during diploid and polyploid evolution in the Aegilops–Triticum alliance. Genetics 171:323–332
Genc Y, McDonald GK, Tester M (2007) Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant Cell Environ 30:1486–1498
Ghavami F, Malboobi MA, Ghanadha MR, Yazdi-samadi B, Mozaffari J, Jafar-Aghayi M (2004) Evaluation of salt tolerance of Iranian wheat cultivars at germination and seedling stages. Iranian Journal of Agricultural Sciences 35(2):453–464
Gorham J, Win Jones RG, Bristol A (1990) Partial characterization of the trait for enhanced K+−Na+ discrimination in the D genome of wheat. Planta 180:590–597
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ 33:552–565
Huang S, Spielmeyer W, Lagudah ES, James RA, Platten JD, Dennis ES, Munns R (2006) A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol 142:1718–1727
James RA, Blake C, Byrt CS, Munns R (2011) Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J Exp Bot 62:2939–2947
James RA, Davenport RJ, Munns R (2006) Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol 142:1537–1547
Kader MA, Lindberg S (2005) Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice Oryza sativa L. determined by the fluorescent dye SBFI. J Exp Bot 56:3149–3158
Kader MA, Seidel T, Golldack D, Lindberg S (2006) Expressions of OsHKT1, OsHKT2, and OsVHA are differentially regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J Exp Bot 57:4257–4268
Lehmann J, Atzorn R, Brückner C, Reinbothe S, Leopold J, Wasternack C, Parthier B (1995) Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta 197:156–162
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nuc Acids Res 30:325–327
Mariño-Ramírez L, Tharakaraman K, Bodenreider O, Spouge J, Landsman D (2009) Identification of cis-regulatory elements in gene co-expression networks using A-GLAM. Meth Mol Biol 541:3–22
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res 29:e45
Plett DC, Moller IS (2010) Na+ transport in glycophytic plants: what we know and would like to know. Plant Cell Environ 33:612–626
Plett D, Safwat G, Gilliham M, Moller IS, Roy S, Shirley N, Jacobs A, Johnson A, Tester M (2010) Improved salinity tolerance of rice through cell type-specific expression of AtHKT1;1. PLoS One 5:e12571
Ramezani A, Niazi A, Abolimoghadam AA, Zamani Babgohari M, Deihimi T, Ebrahimi M, Akhtardanesh H, Ebrahimie E (2012) Quantitative expression analysis of TaSOS1 and TaSOS4 genes in cultivated and wild wheat plants under salt stress. Mol Biotechnol (in press)
Rush DW, Epstein E (1976) Differences between salt-sensitive and salt-tolerant genotypes of the tomato. Plant Physiol 57:162–166
Shavrukov Y, Langridge P, Tester M (2009) Salinity tolerance and sodium exclusion in genus Triticum. Breed Sci 59:671–678
Tabatabaei SMF, Maassoumi TR (2001) Triticum boeoticum ssp thaoudar exist in Iran. Cereal Res Commun 29:121–126
Acknowledgments
The authors would like to thank the Institute of Biotechnology for supporting this research and the Bioinformatics Research Group in the College of Agriculture (Shiraz University). We thank Dr. Mehrabi (Ilam University) for kindly supplying seeds of wild genotypes for this study. We thank Mr. Amin Ramezani for help in performing the real-time PCR experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Zamani Babgohari, M., Niazi, A., Moghadam, A.A. et al. Genome-wide analysis of key salinity-tolerance transporter (HKT1;5) in wheat and wild wheat relatives (A and D genomes). In Vitro Cell.Dev.Biol.-Plant 49, 97–106 (2013). https://doi.org/10.1007/s11627-012-9478-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9478-4