Abstract
A genetic transformation protocol was developed using the transfer of a synthetic CP4 EPSPS transgene, as a conditional positive selectable marker, into commercially relevant zonal pelargoniums using an Agrobacterium tumefaciens strain in combination with a novel step-down glyphosate selection system. The transformation efficiency based on independent T-DNA integration events averaged 1.9 % over 10 experiments. Some 273 independent transformants were produced within an average time of 6 mo from explant inoculation with Agrobacterium to plantlet recovery. For plantlet recovery, three aromatic amino acids were incorporated into the rooting medium to ameliorate the accumulative effects of glyphosate selection. The T-DNA also contained a mutant ethylene receptor (etr1-1) cDNA from Arabidopsis thaliana, under control of the petunia flower-specific, floral-binding protein promoter, to confer ethylene insensitivity. However, delayed flower senescence was not obtained. The transformation protocol provides a reliable method to add herbicide resistance and other traits to zonal pelargonium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelargonium × hortorum L. H. Bailey (pro sp.), commonly called zonal pelargonium or zonal geranium in the USA, is an internationally popular and economically significant bedding plant (Clark 1992). Molecular breeding technologies provide opportunities to genetically engineer zonal pelargonium to improve cultivar characteristics, for example, to produce a true yellow-flowered cultivar (Mitchell et al. 1998). However, practical transformation systems are a prerequisite for such ventures.
Two genetic transformation protocols have been reported previously for P. × hortorum. Robichon et al. (1995) described a hygromycin selection-based protocol for the tetraploid cv. “Alain” using the disarmed Agrobacterium tumefaciens strain EHA101 harboring a binary vector containing CaMV35S:gusA, CaMV35S:hpt, and nos:nptII transgenes. Among the 57 plants selected for hygromycin resistance, only one plant presented beta-glucuronidase (GUS) activity, and this was restricted to the margins of the leaves. In addition, the T-DNA transferred was truncated in 17 out of 18 lines, and independent transformation was demonstrated by Southern analysis for only two lines. Hassanein et al. (2005) used two disarmed strains of A. tumefaciens, strain LBA4404 and strain EHA101, both harboring CaMV35S:gusAintron and nos:nptII with a kanamycin-based selection system for cv “Panaché sud,” and verified the transgenic nature of regenerated plants by PCR. However, because shoot regeneration occurred via a callus intermediate, the isolation of more than one putative physically independent cluster of shoots and buds from one leaf disk (Hassanein et al. 2005) could have represented duplicate transformation events. Also, the presence of Agrobacterium in transformed tissues, even after the use of bacteriostatic antibiotics, can give false-positive PCR results. Unfortunately, Southern analysis, which provides the most reliable molecular evidence of transgene integration and the independence of transformed lines, was not reported in this study.
Transformation of zonal pelargoniums using glyphosate selection has not been reported previously. Glyphosate selection has a number of advantages over other commonly used selectable markers. For example, in maize, glyphosate selection can yield a very low frequency of non-transgenic escapes compared to kanamycin, where selection is quite inefficient. Unlike kanamycin, glyphosate is not detoxified, and consequently, there is no cross-protection afforded to adjacent cells. Also, glyphosate is a highly mobile selection agent and translocates throughout the plant and so is less dependent on direct contact of the target tissue than some other selection agents (Howe et al. 2002).
Using the CP4 EPSPS transgene as a conditional positive selectable marker (Miki and McHugh 2004), transgenic zonal pelargoniums that were resistant to glyphosate-containing herbicides were obtained. We report a reliable transformation method to produce glyphosate-resistant zonal pelargoniums using glyphosate as the selection agent and a mutant ethylene receptor (etr1-1) complementary DNA (cDNA) from Arabidopsis thaliana, under control of the petunia flower-specific, floral-binding protein promoter.
Materials and Methods
Plant material, seed disinfection, and germination.
Seeds of a parental line of F1 hybrid zonal pelargonium, P. × hortorum cv. “Deep Scarlet” F50 (Syngenta Seeds Inc, Downers Grove, IL) were surface sterilized in 30 % Janola® (Toops' Wholesale Ltd., Palmerston North, New Zealand) for 25 min and rinsed five times with sterile distilled water. Disinfected seeds, usually 250 seeds per experiment, were germinated in Petri dishes (90 mm) containing 7.5 g/L Davis bacteriological agar (Danisco NZ Ltd, Auckland, New Zealand). Dishes were sealed with one layer of Glad Wrap® polyethylene film and then wrapped in aluminum foil. Petri dishes were maintained at 28°C in the dark in an ECHOTherm chilled incubator (Torrey Pines Scientific, Carlsbad, CA) for 7 d to obtain etiolated hypocotyls.
Explant preparation, preculture, and culture conditions.
Explants 8–10 mm long were cut from the etiolated hypocotyls with a scalpel and used for shoot regeneration, glyphosate shoot regeneration inhibition curves, and transformation experiments. For transformation, up to 1,240 explants were used per experiment. Explants were placed, 20 per Petri dish, on 70-mm-diameter qualitative filter paper (LabServ, ThermoFisher Scientific, Auckland, New Zealand) on a preculture medium (PFR#879, Table 1). Explants were precultured in the dark at 25°C for 5 d prior to inoculation with Agrobacterium.
Development of shoot regeneration and shoot elongation media.
Shoots were regenerated from etiolated hypocotyls on filter paper placed on thiadiazuron (TDZ)-containing medium PFR#1602. Shoots were harvested and moved to one of two shoot elongation media, either with 0.3 mg/L TDZ (PFR#1619) or with TDZ replaced by 6-benzylaminopurine (BAP), gibberellic acid (GA3), and indole-3-acetic acid (IAA) (PFR#1670). Eight replicate medium tubs were used per treatment, and the quality of shoots (color, size, development, rooting) were compared after a 9-wk culture.
Determination of glyphosate levels that inhibit all shoot regeneration from explants.
The effects of five glyphosate concentrations in shoot regeneration medium PFR #1602 were tested on shoot regeneration frequencies from etiolated hypocotyl explants over 31 d to establish a glyphosate concentration that inhibited all shoot regeneration. The glyphosate concentrations tested were: 0, 60, 90, 120, and 150 μM. The explants used in these treatments were first precultured for 4 d on preculture medium (PFR#879) in the dark, then cultured for 2 d in the dark on co-cultivation medium (PFR#1260) to simulate the transformation protocol, before transfer to the selection media containing five different concentrations of glyphosate. Subsequently, each transformation experiment listed in Table 2 had three replicate plates of glyphosate kill controls and two replicate plates of shoot regeneration controls embedded in it.
Agrobacterium strain and binary vector.
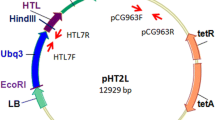
For the 35 transformation experiments reported in this study, A. tumefaciens strain ABI, which contained the disarmed pTiC58 plasmid pMP90RK (Koncz and Schell 1986), and the binary vector pSFPD06 (Fig. 1) were used. This binary vector contained on its T-DNA a synthetic CP4 EPSPS gene (5-enolpyruvylshikimate-3-phosphate synthase), derived from Agrobacterium spp. strain CP4 and optimized for expression in plants (Pagette et al. 1995). This gene encoded for the chloroplast-localized enzyme EPSPS in the shikimic acid pathway. A modified chloroplast transit peptide sequence, CTP2, derived from the EPSPS coding region of A. thaliana (Klee et al. 1987) was fused to the CP4 EPSPS gene sequence to target the CP4 EPSPS protein to chloroplasts. The CTP2 CP4 EPSPS fusion construct, under control of a Figwort Mosaic Virus 34S constitutive promoter, confers resistance to glyphosate (N-phosphonomethylglycine) (Barry et al. 1992). This construct also allowed glyphosate to be used as a conditional positive selectable marker (Miki and McHugh 2004) in transformation experiments. The T-DNA also contained an etr1-1 cDNA from A. thaliana that encoded a mutant ethylene receptor, which was unable to bind to ethylene and which should confer ethylene insensitivity in the presence of wildtype ethylene receptors (Wilkinson et al. 1997; Zhao et al. 2002). The receptor gene was under regulatory control of a flower-specific, floral-binding protein (FBP1) promoter from petunia (Angenent et al. 1993).
T-DNA of pSFPD06: left to right: RB right border; FBP1 floral-binding protein promoter from petunia; etr1-1 cDNA from A. thaliana encodes an ethylene receptor that is unable to bind to ethylene; E9-poly A terminator from Pisum sativum; FMV 34S promoter figwort mosaic virus 34S promoter; ctp2 a modified chloroplast transit peptide sequence derived from the epsps coding region of A. thaliana; cp4-epsps selectable marker gene–synthetic cp4 epsps gene, derived from Agrobacterium spp. strain CP4 and optimized for expression in plants; E9-poly A terminator from P. sativum; LB left border. The 3.2-Kb HindIII etr1-1 fragment probe used for Southern border fragment analysis is shown. The 724-bp internal BamHI fragment found in all transgenic lines and the plasmid control is also shown.
Agrobacterium culture and vir gene induction.
A. tumefaciens strain ABI was streaked from microbead stocks stored at −80°C on to LB agar plates (Luria and Burrows 1957) containing 25 mg/L chloramphenicol (ABI chromosomal selective marker), 40 mg/L kanamycin (pMP90RK selective marker), and 40 mg/L spectinomycin (pSFPD06 selective marker). Agrobacterium suspensions were grown overnight in 10 ml LB broth in 100-ml Erlenmeyer flasks (Schott, Wertheim, Germany) with the same antibiotics detailed above, at 28°C and 250 rpm in an orbital incubator (Sanyo-Gallenkamp, Loughborough, UK). Cultures were then centrifuged at 12,000×g for 10 min, the supernatant containing the antibiotics was discarded, and the pellet was resuspended in 80 ml of AB vir gene pre-induction medium (Gelvin and Liu 1994) containing 200 μM acetosyringone. Agrobacterium suspensions were then cultured for 4 h at 28°C and 250 rpm in an orbital incubator (Sanyo-Gallenkamp) to induce vir genes and maximize T-stand production and T-complex formation (Culianez-Marcia and Hepburn 1988).
Inoculation explants and their co-cultivation with Agrobacterium.
Explants, 8–10 mm long, which were cut from the etiolated hypocotyls with a scalpel, were transferred in batches of 100 after 5 d on preculture medium (PFR#879) to 50-ml beakers and inoculated with a 30-ml suspension of A. tumefaciens strain ABI that was grown in AB medium (Gelvin and Liu 1994). Inoculation of explants was performed by vacuum infiltration to −95 kPa, until the suspension bubbled. Explants were then transferred to 70-mm-diameter qualitative filter paper (LabServ, ThermoFisher Scientific), which was placed on co-cultivation medium (PFR#1260 Table 1), 20 explants per Petri dish for 2 d of co-cultivation in the dark at 25°C. After co-cultivation, explants were transferred into media (PFR#1612) containing 250 mg/L ticarcillin disodium (Timentin®, ProPharma, Auckland, New Zealand) to stop Agrobacterium growth.
Selection of transgenic cells, shoot regeneration, shoot elongation, and rooting.
Transgenic tissues were selected from etiolated hypocotyl explants where tissues were transferred to media containing lower concentrations of glyphosate over time. Initially, tissues were placed on medium PFR#1612 with 150 μM glyphosate for 21 d and then transferred to medium PFR#1754 containing 150 μM glyphosate with increased concentration of ticarcillin disodium salt (500 mg/L) to control Agrobacterium overgrowth and 40 μM CuSO4 to assist shoot regeneration. After 42 d, tissues were transferred to a medium containing 100 μM glyphosate (PFR#1814), and at 63 d, tissues were transferred to a medium containing 50 μM glyphosate (PFR#1778). The shoot regeneration controls were cultured onto equivalent media without glyphosate in parallel with the inoculated tissues, e.g., PFR#1755 at 21, 42, and 63 d. Only one putative transgenic shoot was harvested from each explant so that physical and molecular independence could be guaranteed. Putative transformants were harvested from explants on selection media from 63 to 105 d after the Agrobacterium inoculation, and explants were discarded after shoot harvest. Small glyphosate-resistant shoots (<1 cm long) were transferred to shoot regeneration medium PFR#1602 without glyphosate for 3 wk. Then, 1.5- to 2-cm-long, glyphosate-resistant shoots were transferred to shoot elongation medium without glyphosate, PFR#1771 for 3 wk. Glyphosate-resistant shoots (>3 cm long) were transferred to rooting medium (PFR #2010) supplemented with 0.1 μM of three aromatic amino acids (0.0165 mg/L l-phenylalanine, 0.018 mg/L l-tyrosine, and 0.0242 mg/L l-tryptophan), to ameliorate the effects of accumulated glyphosate. Transgenic plants were transferred to a containment house about 6 mo after explants had been inoculated with Agrobacterium.
Transgenic plant verification—lateral flow tests for CP4 EPSPS protein.
All shoots that arose via adventitious organogenesis were tested for the presence or absence of the CP4 EPSPS protein by using the trait RoundUp Ready® lateral flow tests (Strategic Diagnostics Inc., Newark, DE), which use a double antibody, sandwich immunoassay. Shoots positive for CP4 EPSPS protein were harvested off the hypocotyl explants to the recovery medium (PFR#1602), elongated in medium PFR#1771 without glyphosate present, and rooted in medium PFR#2010 supplemented with three aromatic amino acids (phenylalanine, tyrosine, and tryptophan).
DNA extraction and Southern border fragment analysis to determine independent lines.
Genomic DNA was extracted from young leaves of one untransformed control and nine independent transgenic zonal pelargonium lines, using a modified cetyltrimethylammonium bromide (CTAB) method (Boase et al. 1996) and digested with Bam-HI restriction enzyme. The digested DNA, 20 μg/lane, was loaded and separated electrophoretically on a 1 % (w/v) Tris-acetate-EDTA (TAE) agarose gel and transferred to an Amersham Hybond-N+ blotting membrane under alkaline conditions, according to the manufacturer's instructions (Amersham Pharmacia Biotech Inc., Piscataway, NJ). The membrane was hybridized overnight in Church and Gilbert buffer solution (Church and Gilbert 1984) at 65°C with a α[32P]-dCTP radioactively labeled probe consisting of a 3.2-Kb Hind-III etr1-1 fragment from pSFPD06 (Fig. 1). The membrane was washed with 0.1× SCC, 0.1 % SDS at 65°C, and placed on Kodak® Biomax® Maximum Resolution (MR) film (Radiographic Supplies, Christchurch, New Zealand) for a 2-wk exposure before development.
Northern hybridization analysis of CP4 EPSPS transcripts.
RNA was extracted from leaves of three control and nine independent transgenic lines using a modified hot borate method (Hunter et al. 2010). RNA was separated by electrophoresis on a 1 % (w/v) RNA formaldehyde agarose gel and transferred to a Hybond XL membrane (Amersham). This was hybridized overnight in Church and Gilbert solution (Church and Gilbert 1984) at 65°C with a α[32P]-dCTP radioactively labeled to a CP4 EPSPS probe (Fig. 1), amplified from pSFPD06 using PCR. The membrane was washed with 0.1× SCC, 0.1 % SDS at 65°C. The sequence of the CP4 EPSPS forward primer for PCR of the probe was 5′-GATTTCGACAGCACCTTCATC-3′ and for the reverse primer, 5′-GCCATCAGGTCCATGAACTC-3′. The Kodak® BioMax® MR film (Radiographic Supplies) was exposed for 1 h, 2 h, and overnight (ON), and the autoradiographs were overlain electronically to show the low abundance of transcript for lines 518 and 778 relative to the other transgenic lines. The membrane was also hybridized to a cDNA probe corresponding to a 25/26S rRNA [pTIP6] (King and Davies 1992), to show evenness of RNA loading.
RT-PCR analysis of etr1-1 mRNA transcripts.
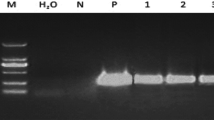
To investigate if the FBP1:etr1-1 recombinant gene, which was also on the T-DNA along with the CP4 EPSPS transgene, was expressing in the transgenic plants, RT-PCR analysis was performed on RNA extracted from petals using a modified hot borate method (Hunter et al. 2010). Nineteen transgenic lines and five untransformed control lines were tested. Four control lines, which were all CP4 EPSPS negative, were also tested by RT-PCR for expression of the introduced FBP1:etr1-1 recombinant gene. The QIAGEN® One step RT PCR kit (QIAGEN, Germantown, MA) was used as well as the following A. thaliana etr1-1 primers: forward 5′-CAATCTTTAGCGACGAGAAGC-3′ and reverse, 5′-CGTTGGTGGCGTTGTTTTGTG-3′. A reverse transcriptase control with no RNA template was included. RNA from each of the zonal pelargonium lines was diluted to 100 ng/μl for reverse transcription, which was performed with oligo dT primer, and then, 1 μl of the resulting cDNA per line was used for the PCR. For PCR, initial denaturation was at 94°C for 2 min followed by 40 cycles of melting (94°C/30 s), annealing (55°C/30 s), and extension (72°C/1 min). A volume of 10 μl PCR products per line was loaded onto the gel for electrophoresis. PCR products were separated electrophoretically on a 1 % (w/v) TAE agarose gel containing ethidium bromide. The fluorescent image of this stained gel was captured using an FLA-5100 Fluorescent Image Analyser (Fujiphotofilm Co. Ltd, Tokyo, Japan).
Glyphosate spray test.
A spray test was carried out with Roundup®, a broad-spectrum, foliar-applied, non-selective, post-emergence herbicide, which contained 3.6 g/L of glyphosate, on three representative untransformed control lines and all nine transgenic lines for which Northern analysis was conducted. Plants were photographed before the application of a single application of spray and again 22 and 50 d after spray application.
Results
Production of transgenic plants and transgenic plant verification—lateral flow tests for CP4 EPSPS protein.
A total of 24,082 hypocotyl explants of cv. “Deep Scarlet” were inoculated with Agrobacterium in 35 experiments conducted over a 9-mo period (Table 1). These experiments produced 273 shoots that were positive for CP4 protein using lateral flow strip tests (Fig. 2). The average time from explant inoculation with Agrobacterium to transfer of the plantlets to a containment house was about 6 mo. The transformation efficiency (TE) based on the number of physically independent CP4-positive shoots, as a percentage of inoculated explants, for the 35 experiments ranged from 0 to 3.17 % (Table 2). For the first 25 experiments when the transformation protocol was under development, TE ranges from 0 to 1.46 % and averaged 0.38 % ± 0.36 SE. For the last 10 experiments when the optimized transformation protocol (Fig. 3) was used, the TE ranged from 0.85 to 3.17 % and averaged 1.91 % ± 0.64 SE. Plantlets of 51 independent transgenic lines and their shoot regeneration controls were transplanted to soil and grown to flowering in a containment house (Figs. 4 and 5).
Trait RoundUp Ready® lateral flow test results of a control and some glyphosate-resistant transgenic shoots. The upper line on the membrane indicates the strip is functional, and the lower line indicates a sample that is positive for the CP4 EPSPS protein. Strips 1–3 untransformed control lines 33013, 32670, and 32671; strips 4–12 nine transgenic lines 518, 540, 586, 717, 724, 746, 778, 789, and 792.
Regeneration control (RC), glyphosate kill (GK), and transgenic (TR) explant cultures at different subculture stages of the optimized transformation protocol, days 21, 42, 63, 84, and 105. Plants in the containment house (CH) are shown in the bottom row: P plantlets immediately after removal from in vitro culture; controls lines, front row; transgenic lines, middle and back rows. Q–R plants 22 d after spraying with herbicide containing 3.6 g/L of glyphosate; Q representative control line, R representative transgenic line.
Shoot elongation medium.
In medium PFR#1602 (Table 2), 93 % of non-inoculated etiolated hypocotyl explants on filter paper regenerated at least one shoot (Fig. 4). However, when transferred to elongation media PFR #1619 or PFR#1670 (Table 2) and cultured for 9 wk, untransformed shoots were greener, larger, and better formed, and more were rooting in PFR#1670 (in the absence of TDZ) than in PFR #1619 with TDZ. These observations were considered in the formulation and deployment of PFR #1771 medium for transgenic shoot elongation in the absence of TDZ and PFR #2010 for rooting of transgenic shoots.
Glyphosate concentrations that inhibit all shoot regeneration from explants.
The number of explants regenerating shoots decreased in frequency from 100 % observed at 0 μM glyphosate to lesser frequencies at 60 and 90 μM glyphosate, to 0 % of explants regenerating shoots at 120- and 150-μM glyphosate concentrations. Therefore, the 150-μM glyphosate concentration was used in selection medium 1 for the first 42 d to provide a buffer against shoots escaping selection. The effects of stepping down the glyphosate concentration were monitored photographically at each subculture stage of the optimized transformation protocol (days 21, 42, 63, 84, and 105 after explant inoculation) in each experiment by embedded glyphosate kill controls (Fig. 4).
Southern analysis.
Genomic DNA extracted from the leaves of nine lines and digested with Bam-H1 was probed with a radioactively labeled 3.2-Kb Hind III etr1-1 fragment designed to hybridize to a 724-bp Bam-H1 internal fragment and the right plant-DNA–T-DNA junction region (Fig. 1). The 724-bp internal fragment was clearly visible in all transgenic lines and the plasmid control (Fig. 6) but not in the untransformed control (6). Two bands were common to the untransformed control line and the transgenic lines (Fig. 6) which were due to the Arabidopsis etr1-1 probe having sequence homology to the endogenous ethylene receptor sequences in the zonal pelargonium genome (Dervinis et al. 2000). These two endogenous bands, together with the internal 724-bp fragment, show that the DNA was cut by the Bam-H1 restriction enzyme. Several border fragments were visible greater than 3,354 bp (minimum size of right-border junction fragment) in all transgenic lines, and no evidence existed for right-border deletion mutants. The varying sizes resulted from the different sites of Bam-H1 in the native plant DNA. Transgene copy number varied from two to five among the nine lines (Fig. 6). The different banding pattern for each transgenic line showed that all nine lines resulted from independent T-DNA integration events (Fig. 6).
Southern border fragment analysis of zonal pelargonium lines. Lane 1 pSFPD06 plasmid DNA; lane 2 line 33013 untransformed control; lanes 3–11 nine transgenic lines 518, 540, 586, 717, 724, 746, 778, 789, and 792. DNA was extracted from leaves using a modified CTAB method and digested with BamHI. The membrane was hybridized to a radioactive 3.2-Kb HindIII etr1-1 fragment probe made from pSFPD06. White arrows mark the positions of transgene bands. The two endogenous bands found in the untransformed control line and all transgenic lines are indicated by black arrows.
Northern analysis of CP4 EPSPS transcripts.
Northern analysis of RNA extracted from leaves of three control and nine transgenic lines showed CP4 EPSPS transcripts only for the transgenic lines (Fig. 7) with some lines, e.g., #778, expressing weakly compared to other lines.
Northern analysis of three control lines (left) and nine transgenic lines (right). Lanes 1–3 untransformed control lines 33013, 32670, and 32671; lanes 4–13 nine transgenic lines 518G, 518, 540, 586, 717, 724, 746, 778, 789, and 792. Line 518G was sprayed with glyphosate before collection of leaves and RNA extraction. The film was exposed for 1 h, 2 h, and overnight (ON) (autoradiographs overlain) to show the low abundance of transcript for lines 518 and 778 relative to the other transgenic lines. The bottom panel is an overlay of the autoradiograph of the membrane hybridized to a cDNA probe corresponding to a 25/26S rRNA (pTIP6) to show evenness of RNA loading.
RT-PCR of etr1-1 mRNA transcripts.
To investigate the expression of the introduced FBP1:etr1-1 recombinant gene, RT-PCR analysis was performed on RNA extracted from petals of 19 transgenic lines and five untransformed control lines (data not shown). All transgenic lines showed a positive band for the etr1-1 cDNA product, and all control lines were negative. Data for a subset of nine transgenic lines and three control lines (Fig. 8) show that PCR-amplified, 450-bp etr1-1 cDNA products were detected only in the nine transgenic lines and not in the untransformed control lines (Fig. 8).
RT-PCR analyses of etr1-1 expression. Lanes 1–3 untransformed control lines 33013, 32670, and 32671; lanes 4–13 nine transgenic lines, 518G, 518, 540, 586, 717, 724, 746, 778, 789, and 792. Line 518G was sprayed with glyphosate before collection of leaves and RNA extraction. Lane 14 RT control, lane 15 negative PCR control.
Glyphosate spray test.
A spray test with herbicide containing 3.6 g/L of glyphosate showed that three representative untransformed control lines died within 22 d after a single application of spray but that eight of nine representative transgenic lines were largely tolerant to glyphosate (Fig. 4). One transgenic line, #778, suffered substantial spray damage as evidenced by leaf dieback and survival of only shoot tips. This was the weakest CP4 expressor as shown by the Northern analysis (Fig. 7).
Discussion
We have developed a transformation system for diploid inbred zonal pelargonium cultivars based on shoots arising by adventitious organogenesis from etiolated hypocotyl segments under glyphosate selection. This transformation system was used to produce a large number of independent glyphosate-resistant shoots. Shoots were shown to be positive in a CP4 protein lateral flow immunoassay. A selection of these shoots was rooted, transplanted to a containment house, and grown to flowering. Southern analysis confirmed that the lines produced were from independent transformation events. Nine lines were shown by Northern analysis to be expressing the CP4 EPSPS transcripts. A spray test with herbicide containing 3.6 g/L of glyphosate showed that eight of nine independent transgenic lines were largely tolerant to glyphosate while three representative untransformed control lines died within 22 d after spray application.
The TE based on the number of CP4-positive shoots as a percentage of inoculated explants was initially in the range 0.0–1.46 % with an average of 0.38 % for the first 25 experiments. During the development of the protocol, three technical issues occurred that were solved via qualitative observations followed by adjustments to the tissue culture media deployed. To control Agrobacterium overgrowth after co-cultivation, ticarcillin levels were increased from 250 to 500 mg/L at 21 d, and the explants were cultured with Agrobacterium on filter paper (instead of direct culture on the medium) for the first 42 d after inoculation, including during the critical co-cultivation period. The benefits of co-cultivation on filter paper rather than directly on agar-solidified medium when employing Agrobacterium-mediated transformation have been reported for other crops (Nontaswatsri et al. 2004; Zhijian et al. 2008). This, in concert with supplementation with CuSO4 (which is beneficial to shoot regeneration in some crops (Purnhauser and Gyulai 1993)), allowed more transgenic shoots to regenerate via organogenesis.
The alleviate a problem with CP4-positive shoots which failed to survive and elongate, the protocol was changed from use of media containing a constant 150-μM glyphosate concentration to a step-down glyphosate selection protocol: 150 μM glyphosate to 42 d after inoculation with Agrobacterium, then to 100 μM glyphosate to 63 d, and finally 50 μM after 63 d until independent shoots were harvested. The survival rate of CP4-positive shoots improved dramatically from an average of 24 %, when using a constant level of 150 μM glyphosate throughout selection, to 85 % after deploying the step-down glyphosate selection protocol. A glyphosate selection protocol which also deployed a reducing level of glyphosate after set time periods has been reported previously for the Agrobacterium-mediated transformation of wheat (Hu et al. 2003). However, in that report, 2 mM for 1 wk was used followed by 100 μM for an additional 2 wk and then 20 μM for regeneration from embryogenic calli (Hu et al. 2003).
An initial delay in root initiation on elongated transgenic shoots was overcome by supplementing the rooting medium with three aromatic amino acids known to be inhibited by glyphosate (l-phenylalanine, l-tyrosine, and l-tryptophan). The enzyme encoded by the CP4-EPSP transgene may not have been produced initially at a high enough level, or it was not active enough in small shoots to counter the growth-inhibiting effect of accumulated glyphosate on meristems, particularly root meristems. The same three aromatic amino acids at the same 0.1 μM concentration were added to the regeneration medium after glyphosate selection of transgenic wheat from embryogenic calli (Hu et al. 2003). After solutions to the three technical issues were incorporated into the final improved protocol, the TE ranged from 0.85 to 3.25 % and averaged 1.9 %, a fivefold increase on the average obtained for the first 25 experiments.
In this study, several petal retention assays were conducted in the presence and absence of exogenous ethylene. When ethylene was supplied in the 2- to 4-ppm range for 1, 2, or 3 h, no statistically significant response was found between control and transgenic lines. When petal retention was assayed for five control lines and 22 transgenic lines in a constant temperature room without the supply of exogenous ethylene at five different times of the year, no statistically significant difference was found between the control and transgenic lines. This zonal pelargonium genotype transformed with Arabidopsis etr1-1 under the control of the petunia FBP1 promoter did not yield distinct, uniform, and stable ethylene-insensitive phenotypes, even though RT-PCR analysis of etr1-1 showed cDNA products of the expected size. Clearly, the construct was functional, but the fbp1:etr1-1 expression levels may not have been high enough or in the appropriate tissue locations to give stable phenotypes with delayed flower senescence.
Conclusions
We have developed a transformation system mediated by A. tumefaciens for commercial zonal pelargonium cultivars, based on shoots arising by adventitious organogenesis from etiolated hypocotyl segments under glyphosate selection. This protocol was used to produce a large number of independent glyphosate-resistant shoots, which were shown to be positive in a CP4 protein lateral flow test. Plantlet recovery involved incorporation of three aromatic amino acids in the medium to ameliorate the accumulative effects of glyphosate selection. A selection of these plantlets was transplanted to a containment house and grown to flowering. Southern analysis confirmed that the lines produced were from independent transformation events. Nine lines were shown by Northern analysis to be expressing the CP4 EPSPS transcripts. A spray test with herbicide containing 3.6 g/L of glyphosate showed that eight of nine independent transgenic lines were largely tolerant to glyphosate while three representative untransformed control lines died within 22 d after spray application. This transformation protocol provides a reliable method to add glyphosate-based herbicide resistance and other traits to the zonal pelargonium genome.
References
Angenent G. C.; Franken J.; Busscher M.; Colombo L.; van Tuten A. J. Petal and stamen formation in petunia is regulated by the homeotic gene fbp1. The Plant J. 2(1):101–112; 1993.
Barry G.; Kishore G.; Padgette S.; Taylor M.; Kolacz K.; Weldon M.; Re D.; Eichholtz D.; Fincher K.; Hallas L. Inhibitors of amino acid biosynthesis: strategies for imparting glyphosate tolerance to crop plants. In: Singh B. K.; Flores H. E.; Shannon J. C. (eds) Biosynthesis and molecular regulation of amino acids in plants. American Society of Plant Physiologists, Rockville, MD USA, pp139-145; 1992.
Boase M. R.; Deroles S. C.; Winefield C. S.; Butcher S. M.; Borst N. K.; Butler R. C. Genetic transformation of regal pelargonium (Pelargonium Xdomesticum ‘Dubonnet’) by Agrobacterium tumefaciens. Plant Sci. 121:47–61; 1996.
Church G. M.; Gilbert W. Genomic sequencing. Proc. Natl. Acad. Sci. U.S.A. 81:1991–1995; 1984.
Clark D. Pelargoniums. Timber Press., Portland, OR USA; 1992.
Culianez-Marcia F. A.; Hepburn A. G. The kinetics of T-strand production in a nopaline-type helper strain of Agrobacterium tumefaciens. Plant Microbe Interact 1950:207–214; 1988.
Dervinis C.; Clark D. G.; Barrett J. E.; Nell T. A. Effect of pollination on accumulation of ETR1 homologue transcripts during flower petal abscission in Geranium (Pelargonium ✕ hortorum) L.H. Bailey). Plant Mol. Biol. 26:847–856; 2000.
Gamborg O. L.; Miller R. A.; Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research 50:148–151; 1968.
Gelvin S. B.; Liu C. N. Genetic manipulation of Agrobacterium tumefaciens strains to improve transformation of recalcitrant plant species. Plant Mol Biol Manual B4:1–13; 1994.
Hassanein A.; Chevreau E.; Dorion N. Highly efficient transformation of zonal (Pelargonium ✕ hortorum) and scented (P. capitatum) geraniums via Agrobacterium tumefaciens using leaf discs. Plant Sci. 169:532–541; 2005.
Howe A. R.; Gasser C. S.; Brown S. M.; Padgette S. R.; Hart J.; Parker G. B.; Fromm M. E.; Armstrong C. L. Glyphosate as a selective agent for the production of fertile transgenic maize (Zea mays L.) plants. Molecular Breed 10:153–164; 2002.
Hu T.; Metz S.; Chay C.; Zhou H. P.; Biest N.; Chen G.; Cheng M.; Feng X.; Radionenko M.; Lu F.; Fry J. Agrobacterium-mediated large-scale transformation of wheat (Triticum aestivum L.) using glyphosate selection. Plant Cell Re. 21:1010–1019; 2003.
Hunter D. A.; Pinkney T. T.; Watson L. M.; Trivellini A.; Janssen B. J.; Brummel D. A.; Heyes J. A. Effect of postharvest water deficit stress on gene expression in heads of broccoli (Brassica oleracea var. italica ). PostharvestBiol Technol. 59:113–123; 2010.
King G. A.; Davies K. M. Identification, cDNA cloning and analysis of mRNAs having altered expression in tips of harvested asparagus spears. Plant Physiol 100:1161–1169; 1992.
Klee H. J.; Muskopf Y. M.; Gasser C. S. Cloning of an Arabidopsis thaliana gene encoding 5-enolpyruvylshikimate-3-phosphate synthase:sequence analysis and manipulation to obtain glyphosate-tolerant plants. Mol Gen Genetics 210:437–442; 1987.
Koncz C.; Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genetics 204: 383–396; 1986.
Linsmaier E. M.; Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiologia Plantarum 18:100–127; 1965.
Luria S. E.; Burrows J. W. Hybridisation between Escherichia coli and Shigella. J. Bact. 74: 461–476; 1957.
Miki B.; McHugh S. Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J Biotech 107:193–232; 2004.
Mitchell K. A.; Markham K. R.; Boase M. R. Pigment chemistry and colour of pelargonium flowers. Phytochem 47(3):355–361; 1998.
Murashige T.; Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15:473–497.
Nontaswatsri C, Fukai S, Goi M (2004) Revised co-cultivation conditions produce effective Agrobacterium-mediated genetic transformation of carnation (Dianthus caryophyllus L.) Plant Sci 166:59–68
Pagette S. R.; Kolacz K. H.; Delannay X.; Re D. B.; LaValee B. J.; Tinius C. N.; Rhodes W. K.; Otero Y. I.; Barry G. F.; Eichholtz D. A. Development, identification, and characterization of a glyphosate-tolerant soybean line. Crop Sci 35:1451–1461; 1995.
Purnhauser L.; Gyulai G. Effect of copper on shoot regeneration in wheat, triticale, rape and tobacco tissue cultures. Plant Cell Tiss Org Cult. 35:131–139; 1993.
Robichon M. P.; Renou J. P.; Jalouzot R. Genetic transformation of Pelargonium ✕ hortorum. Plant Cell Rep. 15:63–67; 1995.
Wilkinson J. Q.; Lanahan M. B.; Clark D. G.; Bleecker A. B.; Chang C.; Meyerowitz E. M.; Kee H. J. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nature Biotech. 15: 444–447; 1997.
Zhao X. C.; Qu X.; Mathews D. E.; Schaller G. E. Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1-1 from Arabidopsis. Plant Physiol. 130:1983–1991; 2002.
Zhijian T.; Li Z. T.; Dhekney S. A.; Dutt M.; Gray D. J. An improved protocol for Agrobacterium-mediated transformation of grapevine (Vitis vinifera L.). Plant Cell Tiss Org Cult. 93:311–321; 2008.
Acknowledgments
Lisa Harvey, Deepa Patel, David Horgan, and John Javellana are thanked for technical assistance. Ian King is thanked for transplanting plants to the soil in the containment house and growing them to flowering. Syngenta Seeds, IL, USA, is thanked for the supply of seeds. The Scotts Company and the New Zealand Institute for Plant & Food Research are thanked for co-funding this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Finer
Rights and permissions
About this article
Cite this article
Boase, M.R., Harriman, R.W., Smith, F.D. et al. Herbicide-resistant, transgenic zonal pelargoniums produced by step-down glyphosate selection and plantlet recovery in the presence of aromatic amino acids. In Vitro Cell.Dev.Biol.-Plant 48, 313–323 (2012). https://doi.org/10.1007/s11627-012-9437-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9437-0