Abstract
A protocol for in vitro propagation of the wild germander (Teucrium polium L.) was developed. In vitro plants were developed from ex vitro axillary buds. Then, shoot tips were excised and established on Murashige and Skoog medium. Proliferation of shoots was tested with different levels of 6-furfurylaminopurin, 6-benzyladenine, or thiadiazuron. The highest proliferation of T. polium was obtained when 6-benzyladenine and 6-furfurylaminopurin were used at 2.0 and 1.6 mg l−1, respectively. Thiadiazuron gave the lowest response for shoot proliferation. Rooting was experimented at different levels of Indol-3-butric acid, Indol-3-acetic acid, or 1-naphthaleneacetic acid. 1-Naphthaleneacetic was the only growth regulator which promoted root induction. Rooted plants were acclimatized successfully with 75% survival and grown in the greenhouse. In vitro- and in vivo-grown plants were analyzed for essential oil production. In vitro-grown T. polium on MS medium supplemented with 6-benzyladenine and 1-naphthaleneacetic gave higher oil yield than that grown on hormone-free Murashige and Skoog medium. In vivo (wild)-grown T. polium produced different oil yield when collected in different months (April and October). β-caryophyllene, used as a marker compound in the essential oil, was identified and quantified by gas chromatography (GC) analysis. Gas chromatography/mass (GC-MS) spectrometry analysis was also used to identify other components of in vitro cultures and to compare with in vivo-grown plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Germander (Teucrium polium L.) or Jaa’deh, as it is locally known in Jordan, belongs to the Labiatae family (Oran and Al-Eisawi 1998). It flourishes mainly in the wild in the Mediterranean region and can be found in Iraq, Saudi Arabia, and Egypt (Feinbrun-Dothan 1986). It is found flowering from March to April, usually in rocky mountains and marginal areas, and is characterized as a dwarf, pubescent aromatic herb possessing oval leaves with enrolled margins 1–3 cm long, sessile, oblong, or linear; the stems end in a shortly paniculate or corymbose inflorescences (Feinbrun-Dothan 1986) and dense heads of white flowers (Al-Eisawi 1982). T. polium is an edible medicinal plant which possesses diverse biological activities. It is widely used in folk medicine; an infusion of the aerial parts is used for abdominal colic and headache, as vermifuge, depurative, antispasmodic, and antidiabetic, and to treat kidney stones (Al-Khalil 1995; Oran and Al-Eisawi 1998; Abu-Irmaileh and Afifi 2000). Different chemical compounds have been isolated and identified from T. polium worldwide, principally: tridoids and flavonoids (Rizk et al. 1986), neo-clerodanediterpenoids (Alcazar et al. 1992; Bedir et al. 1999), abietane diterpenoids (Cuadrado et al. 1992), and furanoidditerpenes (Malakov and Papanov 1983). Utilizing gas chromatography (GC) and gas chromatography/mass spectrometry (GC-MS) analysis, several research groups have evaluated the essential oil of T. polium grown in different geographic areas and revealed the presence of several compounds varying in their major constituent(s) and their concentration depending on the geographic origin (Hassan et al. 1979; Rizk et al. 1986; Cakir et al. 1998; Eikani et al. 1999; Aburjai et al. 2006; Kabouche et al. 2007).
T. polium biodiversity has declined dramatically in recent years, and some have become extinct totally in the wild, which could be attributed to several reasons: habitat encroachment by urban and agricultural development, deforestation, deterioration of rangelands by overgrazing and soil erosion, illegal collection, and depletion of major water resources (Wardam 2006). Therefore, there is a need to propagate T. polium for its medicinal use and to conserve it from being an endangered medicinal plant. Plant tissue culture techniques are considered as powerful tools for propagating high numbers of plant species and production of the essential oil (Conger 1981). Acclimatization is the final, but necessary, step in all micropropagation schemes (Van Huylenbroeck and Debergh 1996). Most species grown in vitro require an acclimatization process to support plant survival and growth when transferred to soil (Ebrahim et al. 2007). Several medicinal plant species of the Labiatae family were studied in vitro: Mentha spp. (Čellárová 1992), Origanium (Borovec 1988; Kumari and Saradhi 1992; Shetty et al. 1995; Svoboda et al. 1995; Arafeh et al. 2003), and Salvia (Arikat et al. 2004). There are some in vitro studies that have been carried out with Teucrium species, such as an in vitro plant regeneration system for Teucrium stocksianum Boiss (Bouhouche and Ksiksi 2007) and T. stocksianum (Bouhouche and Ksiksi 2003).
Essential oil studies on T. polium collected from different geographical regions revealed that β-caryophyllene was found in multiple analyses (Hassan et al. 1979; Rizk et al. 1986; Cakir et al. 1998; Eikani et al. 1999; Aburjai et al. 2006; Kabouche et al. 2007). The sesquiterpene β-caryophyllene is a major plant volatile found in large amounts in the essential oils of many different spice and food plants, such as oregano (Origanum vulgare L.), cinnamon (Cinnamomum spp.), and black pepper (Piper nigrum L.); (Mockute et al. 2001; Jayaprakasha et al. 2003; Orav et al. 2004). In T. polium, the composition of the essential oil is varied according to region. In previous studies and a preliminary work conducted in our lab, β-caryophyllene was found as a component of the essential oil of T. polium in most regions studied (Hassan et al. 1979; Rizk et al. 1986; Cakir et al. 1998; Eikani et al. 1999; Aburjai et al. 2006; Kabouche et al. 2007). Therefore, it was used as a standard to study the essential oil in the current study.
To our knowledge, there is no reported literature on the in vitro propagation or secondary metabolites (i.e., essential oils production) of in vitro-grown T. polium. Therefore, this study was initiated to develop a protocol for in vitro establishment, multiplication, rooting, and acclimatization of T. polium L. as well as to study the essential oil content and its constituents of volatile components in vivo and to compare them with in vitro-grown plants.
Materials and Methods
Plant material.
Axillary buds of T. polium L. were collected from the wild in November and December 2007 in the Northern part of Jordan from Ain Janna in Ajloun (long. 35°45′36″ E, lat. 32°19′48″ N). Axillary buds of T. polium L. were surface-sterilized by washing thoroughly under running tap water for 15 min with a few drops of mild detergent, antibacterial soap (devomycin 5 mL L−1 water), and fungicide powder (Benomyl 10 g l−1 water). Then, they were dipped in antiseptic solution of 3.5% sodium hypochlorite for 5 min, followed by washing under running tap water for 15 min. Axillary buds were transferred into ethanol 70% (v/v) for 30 s and then rinsed with sterile distilled water three times (15 min each) under laminar air flow cabinet.
In vitro establishment.
Axillary bud growth and development. Sterilized buds were inoculated on the surface of half-strength Murashige and Skoog (1962) (MS) media supplemented with 2.0 mg l−1 gibberellic acid (GA3). Afterwards, aseptically grown cultures were directly sub-cultured to MS media without growth regulators for further growth in the growth room (16/8-h light/dark), photoperiod (photosynthetic photon flux density = 40–45 μmol m−2 s−1), at 24 ± 1°C). Then, microshoots (10–12 mm) were sub-cultured on the surface of MS media supplemented with 0.5 mg l−1 6-furfurylaminopurin (kinetin) and 0.1 mg l−1 naphthalene acetic acid (NAA) for mother stock multiplication.
In vitro multiplication and rooting.
Shoot multiplication. Nodal segments (10–12 mm) were sub-cultured in Erlenmeyer flasks (250 mL) containing 50 mL of solid MS medium. The medium was supplemented with plant growth regulators 6-benzyladenine (BA), kinetin, or thiadiazuron (TDZ) at different concentrations (0.0, 0.4, 0.8, 1.2, 1.6, or 2.0 mg l−1) and 0.1 mg l−1 NAA. Data were reported after 5 wk for the number of proliferated shoots, shoot height, and number of leaves/explants. Treatments were arranged in a completely randomized design with five replications (with two explants in each replicate).
Rooting. Microshoots (10–12 mm long) were grown on a plant growth regulator-free medium for 2 wk to eliminate any carryover effect of cytokinin that might inhibit or reduce rooting. Individual 10 to 12-mm-long microshoots were transferred to 1.5 × 150-mm culture test tubes containing 12 mL of solid MS rooting medium supplemented with different plant growth regulators Indol-3 butyric acid (IBA), NAA, or Indol-3 acetic acid (IAA) at 0.0, 0.1, 0.4, 0.8, 1.2, 1.6, or 2.0 mg l−1. Each level was represented in ten replicates. Data were reported after 5 wk for the number of roots, root length, and shoot height for each treatment.
Ex vitro acclimatization.
Three days before acclimatization, the cotton plugs were removed from culture tubes containing in vitro-rooted microshoots. In vitro-rooted plants were then removed from test tubes and the agar was removed by gentle washing with tap water. Then, plants were transferred to peat/perlite 1:1 mixture in plastic pots mixed with 10 mL of benomyl fungicide solution (10 g l−1) and covered with a beaker; water was sprayed continuously to avoid wilting. Plants were acclimatized under 16 h of supplementary light of 40 μmol m−2 s−1, 8-h dark for 3 wk at 24 ± 2°C. Relative humidity was gradually reduced from 95% at the beginning of acclimatization to 70% at the end of acclimatization period. Survival percentage was recorded at the end of 3 wk. Acclimatized plants were transferred to a greenhouse (air temperature 25 ± 2°C day/19 ± 2°C night) and planted in a mixture of soil (clay-loam)/perlite 1:1 in 17.5 × 17.5-cm plastic pots and overhead irrigated.
Determination of essential oil content in T. polium L.
Plant material. Fresh samples of T. polium L. were collected from the wild in April and October 2008 in the Northern part of Jordan from Ain Janna in Ajloun (the same place from which the axillary buds of T. polium L. have been collected as outlined in “Plant material” under Materials and Methods”). The collected materials were preserved in paper bags for in vivo essential oil analysis. Two in vitro plant samples were also processed for essential oil analysis. One was grown in plant growth regulator-free MS medium and the other one on solid MS medium supplemented with 0.5 mg l−1 BA and 0.1 mg l−1 NAA.
Essential oil extraction.
Essential oils were extracted from fresh finely chopped plant material by steam distillation using essential oil distillation apparatus. Distillation was performed using 100 g of fresh plant material in 250 mL distilled water for 3 h. The temperature was set at 70°C and then adjusted after boiling to 50°C. The distillate was collected in a flask surrounded by ice to aid cooling. The essential oil was recovered from the distillate by shaking with a mixture of hexane/dichloromethane (1:1) using a separatory funnel. The organic layer was then evaporated using a rotary evaporator, leaving the essential oil. The essential oils obtained were dried over anhydrous sodium sulfate and stored in dark glass vials at 4°C until required for analysis. The percentage yield of the obtained oil for the in vivo and in vitro samples was calculated as weight (g) of essential oil per 100 g of plant fresh material.
Essential oil analysis.
Gas chromatographic method was used for the identification and quantification of the marker compound β-caryophyllene in T. polium essential oil using external reference standard of β-caryophyllene (Sigma-Aldrich, St. Louis, MO). β-caryophyllene was identified by matching its recorded mass spectrum with the NIST library database (National Institute of Standards and Technology, Gaithersburg, MD) provided by the instrument software and by comparing its retention time value with the reference standard analyzed under the same experimental conditions. Essential oil of the in vitro and in vivo samples, 3.5 and 9.17 mg, respectively, were accurately weighed and diluted separately with n-hexane (96% for pesticide residue analysis) in 10-mL volumetric flasks. Aliquots (1 μl) were injected into the GC. The concentration of β-caryophyllene was calculated by interpolation using a constructed six-point external standard calibration curve (5, 10, 20, 40, 80, and 95 ppm).
The composition of the essential oils of the in vivo and in vitro T. polium was determined using GC-MS (Varian 3800 GC equipped with Varian Saturn 2200 MS, Santa Clara, CA). VF-ms-5 column was used (30 m × 0.25 mm × 0.25 μm). The carrier gas was He (99.999 pure) with a flow rate of 0.9 mL L−1. The injector temperature was held at 250°C, while the column oven temperature was held at 100°C for 3 min and then increased at a rate of 10°C to 250°C. The total run time was 19 min. The mass detector was set to scan ions between 40 and 500 m/z. The volatile oil compounds were identified by matching their recorded spectra with the data bank mass spectra (NIST) provided by the instrument software and by comparing their retention index values with those in the literature. Only matching spectra of a large degree of certainty using reverse-fit modes were accepted.
Statistical analysis.
Each experiment was set up as a completely randomized design. The collected data were statistically analyzed using Statistical Analysis System (SAS, Cary, NC, 2001). Means were separated according to the least significant difference (LSD) test at the 0.05 level probability.
Results
In vitro establishment.
Successful in vitro establishment of T. polium L. was achieved in this study from axillary buds development. Growth from buds was shown after 2 wk of inoculation. Also, some plants were regenerated from the callus at the base of the explants. The highest percentage of developed shoots was achieved on MS or half-strength MS medium supplemented with 2.0 mg l−1 GA3.
In vitro shoot multiplication and rooting.
In this study, no significant differences were noticed between control treatments—C1 (without 0.1 mg l−1 NAA) and C2 (with 0.1 mg l−1 NAA)—during the 5 wk of incubation, and no callus was observed on the basis of these microshoots. Table 1 shows the effect of different concentrations of BA, kinetin (Kin), and TDZ in combination with 0.1 mg l−1 NAA on shoot multiplication of T. polium L. It is clear that after 5 wk of culture, multiplication parameters and growth performance of T. polium L. responded significantly to increased BA concentrations up to 2.0 mg l−1, as shown in Fig. 1a . Shoot length and number of leaves produced from microshoots cultured on MS medium supplemented with 0.4, 0.8, 1.2, and 1.6 mg l−1 BA were not significantly different from each other. Callusing occurred at the base at all BA concentrations, the largest at 1.6 mg l−1 BA.
It is obvious that the continuous increase in Kin concentrations up to 1.6 mg l−1 increased significantly the number of shoots up to 15.3 (Table 1 and Fig. 1b ), but when the concentration of Kin increased to 2.0 mg l−1, the proliferation was inhibited as with control treatments C1 and C2. The highest shoot length of 2.9 cm was obtained at the low level, 0.4 mg l−1 of Kin. Highest leaf numbers were obtained at 1.2 mg l−1 Kin (11.6 average of leaves/explants).
No significant difference was shown between both controls as compared with TDZ concentrations, except for 0.8 mg l−1 which gave 8.9 shoot/explants (Table 1 and Fig. 1c ) and 2.6-cm shoot height. Also, there were no significant differences between all treatments with TDZ on shoot length and number of proliferated shoots. The highest average leaf number (11.6 of leaves/explants) was obtained at 1.2 mg l−1. A large callus was obtained at the base of microshoots in all concentrations of TDZ used, except the control treatments.
There was no rooting induction among rooting experiments conducted with IBA or IAA at the given concentrations. Table 2 shows the effect of different concentrations of NAA on the rooting parameters of T. polium L. Roots were obtained only at levels of 0.8, 1.2, and 1.6 mg l−1 of NAA. Rooting percentages for these levels were 100%. The best rooting was obtained at 0.8 mg l−1 NAA (Fig. 2a ). It gave the highest number of roots, root length, and shoot height, but it gave a large callus on the base of the plant.
Ex vitro acclimatization.
In vitro-rooted plants of T. polium L. showed survival percentage of 75%. Acclimatized plants appeared normal and did not exhibit any morphological abnormalities, as shown in Fig. 2b .
Determination of essential oil content in T. polium L.
The percentage yield of the obtained oil for the in vivo and in vitro samples was calculated as weight (g) of essential oil per 100 g of plant fresh material, and the results obtained are given in Table 3. In vivo (wild) T. polium samples showed higher percentage oil yield than in vitro samples. Wild T. polium sample, which was collected in April 2008, showed the highest percentage yield (0.55%, w/w) of oil, while the oil’s percentage yield of the wild sample which was collected in October 2008 was found to be 0.47% (w/w). For the in vitro samples, the sample which was grown on solid MS media supplemented with 0.5 mg l−1 BA and 0.1 mg l−1 NAA showed the highest percentage of (0.40%, w/w) oil yield. The other in vitro sample, which was grown on hormone-free MS medium, showed the lowest percentage yield of only 0.18% (w/w). In the current study, the difference in the amounts of essential oils extracted from in vitro and in vivo plant materials could be attributed to the fate of the glandular trichomes to which the synthesis of chemotype is restricted.
Essential oil analysis.
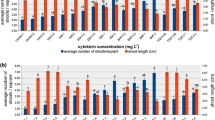
β-caryophyllene was detected in the in vivo sample which was collected in April 2008 (Table 3 and Fig. 3) and in the in vitro sample which was grown on solid MS media supplemented with 0.5 mg l−1 BA and 0.1 mg l−1 NAA (Table 3 and Fig. 4). No β-caryophyllene was detected in either the in vivo sample which was collected in October 2008 or in the in vitro sample which was grown on hormone-free MS media. T. polium sample which was grown on solid MS medium supplemented with 0.5 mg l−1 BA and 0.1 mg l−1 NAA was found to have the highest (3.0%, w/w) total β-caryophyllene content, while T. polium sample which was collected from the wild in April 2008 showed total β-caryophyllene content of only (0.4%, w/w), (Table 3).
The composition of the essential oils of the in vivo T. polium, which was collected in October 2008, and the in vitro T. polium, which was grown on hormone-free MS medium, was determined using GC-MS. The results are presented in Table 4. The volatile components that were commonly identified in the two samples (in vitro and in vivo) were linalool, verbenol, endobornyl acetate, and (+)-Spathulenol.
Discussions
In vitro establishment.
Solid MS medium supplemented with 0.1 mg l−1 NAA and 0.5 mg l−1 Kin promoted satisfactory plant size, growth, and development. Axillary buds can be considered as a good starting material in T. polium L., especially if they were cultured on half-strength MS medium supplemented with 2.0 mg l−1 GA3. The lower osmotic pressure of half-strength MS media with the effect of GA3 concentrations might affect the high axillary bud development. Mahanta and Paswan (2006) propagated Anthurium andreanum in vitro from axillary buds on MS medium containing 0.8 mg l−1 BA and 0.1 mg l−1 IAA. Also, in vitro propagation of Talinum portulacifolium L. through axillary bud culture was done using MS medium supplemented with 1.35 mg l−1 BA and 0.35 mg l−1 IAA (Thangavel et al. 2008).
In vitro shoot multiplication and rooting.
In our study, high concentrations of BA (2.0 mg/L) and kinetin (1.6 mg/L) gave higher proliferation. Results of Owies et al. (2004) showed successful in vitro proliferation of Gundelia tournefortii on MS media containing 2.0 or 3.0 mg l−1 BA. Sudha and Seeni (1996) demonstrated that using 3.0 mg l−1 BA induced a high frequency of shoot number in Rauwfolfia micrantha (a rare medicinal plant). Rollinia mucosa, an important medicinal plant, was reported to display the highest regeneration frequency on a medium containing 2.0 mg l−1 BA and 0.1 mg l−1 NAA (Figueiredo et al. 2001). In our study, all levels of Kin except the control treatments showed callus formation on the basal parts of proliferated shoots. It was reported that highest in vitro proliferation rate of T. stocksianum Boiss. was achieved on the medium containing 3 mg l−1 Kin and 0.5 mg l−1 IAA (Bouhouche and Ksiksi 2007). For Salvia fructosia Mill, Arikat et al. (2004) found that low level of Kin 0.4 mg l−1 resulted in best proliferation shoots.
In our study, TDZ failed to give proliferation at most levels, but gave good results at 0.8 mg/L. For in vitro-grown Origanium vulgare and Origanium syriacum, it was reported that TDZ failed to promote proliferation at used levels and that it was a callus-inducing factor (Arafeh et al. 2003, 2006). In all studied concentrations of TDZ, in vitro explants of T. polium L. showed large dark green leaves and some brown sections at the edges of leaves. On the other hand, Jiang et al. (2005) found that the maximum number of shoots was obtained when 1.0 mg l−1 TDZ was used for in vitro proliferation of Arnebia euchroma (an important Chinese traditional medicinal plant). They found that other cytokinins (Kin and 6-benzyladenine) and auxin (α-naphthaleneacetic acid) were not efficient in inducing regeneration on cotyledon explants (Jiang et al. 2005).
Rooting was achieved only with NAA; best rooting was achieved at 0.8 mg/L in the current study. Also, in Drimia robusta, optimal root initiation was achieved on media supplemented with 1 mg l−1 NAA (Ngugi et al. 1998). Similarly for Schizobasis intricate (an important medicinal plant of the Hyacinthaceae family), rooting medium of half-strength MS with 1 mg l−1 NAA gave the best results (Drewes et al. 1993).
Ex vitro acclimatization.
T. polium microshoots were successfully acclimatized. In Cussonia, rooted plants were acclimatized for planting ex vitro, with 63% survival after acclimatization (Tetyana and Van Staden 2001). In A. andreanum, a survival rate of 60% was obtained when in vitro-raised plants were transferred to in vivo condition in plastic pots containing soil/perlite (10:1) mixture (Mahanta and Paswan 2006).
Determination of essential oil content in T. polium L.
In our study, in vitro T. polium, which was grown on solid MS medium supplemented with 0.5 mg l−1 BA and 0.1 mg l−1 NAA, showed a high percentage oil yield of 0.40% (w/w), which is comparable to that of the in vivo-grown T. polium (0.55%). Sudriá et al. (1999) found that incorporation of 0.1 mg l−1 BA in the culture medium had a marked positive effect on the capacity of Lavandula dentate plants to produce and accumulate essential oils; it showed an increase of 150% over that produced in the absence of plant growth regulators (Sudriá et al. 1999). Arikat et al. (2004) found that the percentage yield of the oil of Salvia fructicosa Mill, which was grown on MS medium containing 0.02 mg l−1 NAA, 0.5 mg l−1 BA, and 0.03 mg l−1 GA3, increased by two fold (0.7%) over the in vivo (Arikat et al. 2004). Hiarat et al. (1990) reported that the total amount (0.9–1.3 mg g−1 plant) of the essential oil produced in Mentha spicata shoot tip cultures on B5 medium was almost equal to that (1–1.5 mg g−1 plant) produced by mother plants (Hiarat et al. 1990).
Arafeh et al. (2006) determined the oil content of the in vitro- and in vivo-grown plant samples of O. syriacum. Our findings were in agreement with their results in which the oil percentage for in vitro-grown O. syriacum was less than greenhouse-grown plants. The oil percentage of T. polium grown wild in Salt (Jordan) and Algeria was found to be 0.8% and 1.7%, respectively (Aburjai et al. 2006; Kabouche et al. 2007), while Teucrium yemens yielded 0.45% (w/w) essential oil (Nasser et al. 2008). Furthermore, the oil percentage for T. yemens which was collected in April was more than that of the same plant collected in the flowering stage, which was found to be 0.1–0.3% (w/w; Nasser et al. 2008). These findings clearly show that the geographical origin of T. polium greatly influences the essential oil quality and quantity, and a new chemotype may be found in plants from different countries (Aburjai et al. 2006).
Essential oil analysis.
There are many medicinal plants containing β-caryophyllene as a major component of their essential oil. For example, the genus Piper has β-caryophyllene contents in fruits and leaves with different percentages. The essential oil of Piper guineense contains (20.8%) of β-caryophyllene in the fruit, P. nigrum contains (12.8%) of β caryophyllene in the fruit essential oil, and Piper capense contains (12.4%) β-caryophyllene in the leaf essential oil (François et al. 2009). Furthermore, the composition of the essential oils of the leaves and flowers of Tithonia diversifolia (Hemsl) was reported to have 20.1% β-caryophyllene (Moronkola et al. 2007).
Also, there are many studies on Teucrium spp., especially in T. polium, which revealed the presence of many compounds such as β-caryophyllene. In the literature, there was great variability in β-caryophyllene content in the oil of wild T. polium samples collected from different geographical regions. For example, the percentage content of β-caryophyllene in T. polium collected from Jordan (Aburjai et al. 2006), Turkey (Cakir et al. 1998), and Iran (Eikani et al. 1999) was found to be 8.7%, 17.8%, and 18%, respectively, while the oils of T. polium grown in Saudi Arabia (Hassan et al. 1979) and Algeria (Kabouche et al. 2007) revealed no presence of β-caryophyllene at all. These findings clearly demonstrate that the geographical origin of T. polium greatly influences the essential oil quality. The findings of our study clearly show that the time of harvest affects the oil percentage and the composition of the oil. Oil of O. syriacum was reported by Daouk et al. (1995) to contain 4% thymol in August and 14% in October (Daouk et al. 1995). Werker et al. (1985) also found striking differences in the essential oil content and constituents in four chemotypes of O. vulgare grown under the same management (Werker et al. 1985). Putievsky et al. (1996) reported that June harvest showed the highest thymol percentage (57%), while the lowest percentage was obtained in October harvest (37%, Putievsky et al. 1996). In a study carried out by Hiarat et al. (1990), the contents of monoterpenoids, especially carvon and limonene, in Menthaspicata plants grown on B5 media were higher than those in the mother plant (Hiarat et al. 1990).
Essential oil and its constituents are strongly affected by several factors such as nutrient media, light, and temperature (Arikat et al. 2004; Arafeh et al. 2006; Hachicha et al. 2006; Nasser et al. 2008). Thus, a change in β-caryophyllene can be achieved if any multiplication in the in vitro conditions happens (pH, nutrient, light, etc.). In our study, the in vitro-grown plants showed more β-caryophyllene than the in vivo-grown plants, while in vivo-grown plants yielded more oil than in vitro-grown plants. This could be because of the production of β-caryophyllene during a specific stage of the plant growth. Under in vitro conditions, harvested shoots are young; thus, chemical compounds that are secreted by trichomes accumulate in the shoot and their amount is concentrated. Furthermore, auxin and cytokinin incorporated into the culture medium have a marked influence on the production of secondary metabolites (Sudriá et al. 1999). In the current study, the major constituents of the essential oil which was extracted from in vitro cultures were different from in vivo-grown plants, while essential oil composition in clonally propagated Minthostachys mollis, an important medicinal plant belonging to the Labiatae, was similar to that of plants growing in the wild (del Chebel et al. 1998). These conflicting results confirm that the variability of oil composition in different populations of the same plant species might be attributed mainly to genetic diversity (Skoula et al. 2000).
In our study, microshoots showed a greater amount of β-caryophyllene than in vivo plants, which may be related to the significant effect of BA in the culture medium on the biosynthesis and the level of β-caryophyllene. Tawfik et al. (1992) and Arikat et al. (2004) found BA to have a significant effect on the production of camphor in the in vitro-grown shoots of Salvia officinalis and S. fructosia Mill, respectively. From the above results and findings, it can be concluded that plant growth regulators such as BA not only affected growth and development of proliferated shoots but also affected the proportion of chemotypes such as β-caryophyllene. Consequently, plant growth regulators may be used in tissue culture medium for regulating or increasing the production of specific important secondary metabolites.
Conclusions
The current study shows that axillary buds of T. polium L. are a good starting material for in vitro establishment due to the high plant development. High concentrations of BA (2.0 mg/L) and kinetin (1.6 mg/L) showed higher proliferation, while TDZ showed good results at 0.8 mg/L. Best rooting was achieved only with NAA at 0.8 mg/L. In vitro-grown T. polium L. can be considered for oil production if cultured on MS medium supplemented with suitable growth regulators. Wild-grown T. polium L. yielded a similar oil percentage in spring and in autumn, but with different compositions. β-caryophyllene was identified in microshoots of T. polium L. grown on a media supplemented with growth regulators and in those in vivo-collected samples in spring where the former showed a higher percentage yield of β-caryophyllene.
References
Abu-Irmaileh B.; Afifi F. U. Treatment with medicinal plants in Jordan. Dirasat Med Bio Sci 27: 53–74; 2000.
Aburjai T.; Hudaib M.; Cavrini V. Composition of the essential oil from Jordanian germander (Teucrium polium L.). J Essent Oil Res 18: 97–99; 2006.
Al-Eisawi D. M. List of Jordan vascular plants. Mitt Bot Munchen 18: 79–182; 1982.
Al-Khalil S. A survey of plants used in Jordanian traditional medicine. Pharm Biol 33: 317–323; 1995.
Alcazar R.; DeLaTorre M. C.; Rodriguez B.; Bruno M.; Piozzi F.; Savona G. Neo-clerodanediterpenoids from three species of Teucrium. Phytochemistry 31: 3957–3960; 1992.
Arafeh R. M.; Shibli R. A.; Mahmoud M. In vitro seed propagation of wild Syrian marjoram (Origanum syriacum L.). AdvHortSci 17: 241–244; 2003.
Arafeh R. M.; Shibli R. A.; Mahmoud M.; Shatnawi M. A. Callusing, cell suspension culture and secondary metabolites production in Origanum vulgare L. and Origanum syriacum L. Jordan J AgricSci 2: 274–282; 2006.
Arikat N. A.; Jawad F. M.; Karam N. S.; Shibli R. A. Micropropagation and accumulation of essential oils in wild sage (Salvia fruticosa Mill). ScientiaHort 100: 193–202; 2004. doi:10.1016/j.scienta.2003.07.006.
Bedir E.; Tasdemir D.; Çalis I.; Zerbe O.; Sticher O. Neo-clerodanediterpenoids from Teucrium polium. Phytochemistry 51: 921–925; 1999. doi:10.1016/S0031-9422(99)00052-7.
Borovec V. Micropropagation of clones of marjoram (Origanum vulgare L.) under in vitro conditions. Bull VyzSlech Us ZelOlom 32: 49–54; 1988.
Bouhouche N; Ksiksi T. In vitro propagation of two important native medicinal plants in the United Arab Emirates (UAE). Proceeding of the Seventh Annual U.A.E. University Research Conference, UAE, 2003, 63–68 pp.
Bouhouche N.; Ksiksi T. An efficient in vitro plant regeneration system for the medicinal plant Teucrium stocksianum Boiss. Plant Biotech Rep 1: 179–184; 2007. doi:10.1007/s11816-007-0033-4.
Cakir A.; Duru M. E.; Harmandar M.; Ciriminna R.; Passannanti S. Volatile constituents of Teucrium polium L. from Turkey. J Essent Oil Res 10: 113–115; 1998.
Čellárová E. Micropropagation of Mentha L. In: Bajaj Y. P. S. (ed) Biotechnology in agriculture and forestry, high-tech and micropropagation. III. Springer, Berlin, pp 262–276; 1992.
del Chebel A. V.; Koroch A. R.; Juliani H. R.; Juliani H. R.; Trippi V. S. Micropropagation of Minthostachys mollis (H.B.K.) Grieseb. and essential oil composition of clonally propagated plants. In Vitro Cell DevBiol Plant 34: 294–251; 1998. doi:10.1007/BF02822716.
Conger B. V. Cloning agricultural plants via in vitro techniques. CRC, Boca Raton; 1981.
Cuadrado M. J. S.; Bruno M.; De La Torre M. C.; Piozzi F.; Savona G.; Rodríguez B. Rearranged abietanediterpenoids from the root of two Teucrium species. Phytochemistry 31: 1697–1701; 1992.
Daouk R. K.; Dagher S. M.; Sattout E. J. Antifungal activity of the essential oil of Origanum syriacum L. J Food Prot 58: 1147–1149; 1995.
Drewes F. E.; Bayley A. D.; Van-Staden J. Tissue culture of Schizobasis intricate, a medicinal plant. South Afr J Bot 59: 105–106; 1993.
Ebrahim N.; Shibli R.; Makhadmeh I.; Shatnawi M.; Abu-Ein A. In vitro propagation and in vivo acclimatization of three coffee cultivars (Coffea arabica L.) from Yemen. World ApplSci J 2: 142–150; 2007.
Eikani M. H.; Goodarznia L.; Mirza M. Comparison between the essential oil of supercritical carbon dioxide extract of Teucrium polium L. J Essent Oil Res 11: 470–472; 1999.
Feinbrun-Dothan N. Flora Palestina. The Israel Academy of Sciences and Humanities, Jeruselum, Israel; 1986.
Figueiredo S. F. L.; Albarello N.; Viana V. R. C. Micropropagation of Rollinia mucosa (JACQ.) Baill. In Vitro Cell Dev B. Plant 37: 471–475; 2001. doi:10.1007/s11627-001-0083-1.
François T.; Michel J.; Lambert S.; Ndifor F.; Vyry W.; Henri A.; Chantal M. Comparative essential oils composition and insecticidal effect of different tissues of Piper capense L., Piper guineense Schum. Et Thonn., Piper nigrum L. and Piper umbellatum L. grown in Cameroon. African Journal of Biotechnology 8: 424–431; 2009.
Hachicha S. F.; Skanji T.; Barrek S.; Ghrabi Z. G.; Zarrouk H. Composition of the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flav Frag J 22: 101–104; 2006. doi:10.1002/ffj.1764.
Hassan M. M. A.; Muhtadi F. J.; Badr A. A. A. GLC–mass spectrometry of Teucrium polium oil. J Pharm Sci 68: 800–801; 1979. doi:10.1002/jps.2600680639.
Hiarat T.; Murakami S.; Ogihara K.; Suga T. Volatile monoterpenoid constituents of the plantlets of Mentha spicata produced by shoot tip culture. Phytochemistry 29: 493–495; 1990.
Jayaprakasha G. K.; Jagan Mohan Rao L.; Sakariah K. K. Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J Agric Food Chem 51: 4344–4348; 2003.
Jiang B.; Yang Y. G.; Guo Y. M.; Guo Z. C.; Chen Y. Z. Thidiazuron-induced in vitro shoot organogenesis of the medicinal plant Arnebia euchroma (Royal) Johnst. In Vitro Cell Dev B. Plant 41: 677–681; 2005. doi:10.1079/IVP2005650.
Kabouche A.; Kabouche Z.; Ghannadi A.; Sajjadi S. E. Analysis of the essential oil of Teucrium polium ssp. aurasiacum from Algeria. J Essent Oil Res 19: 44–46; 2007.
Kumari N.; Saradhi P. P. Regeneration of plants from callus cultures of Origanum vulgare L. Plant Cell Rep 11: 476–479; 1992.
Mahanta S.; Paswan L. In vitro propagation of Anthurium andreanum from axillary buds. J OrnamHort 68: 544–550; 2006.
Malakov P. Y.; Papanov G. Y. Furanoidditerpenes from Teucrium polium. Phytochemistry 22: 2791–2793; 1983. doi:10.1016/S0031-9422(00)97698-2.
Mockute D.; Bernotiene G.; Judzentiene A. The essential oil of Origanum vulgare L. ssp. growing wild in Vilnius district (Lithuania). Phytochemistry 57: 65–69; 2001.
Moronkola D.; Ogunwande I.; Walker T.; Setzer W.; Oyewole I. Identification of the main volatile compounds in the leaf and flower of Tithonia diversifolia (Hemsl) Gray. J Nat Med 61: 63–66; 2007.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–497; 1962.
Nasser A.; Ali A.; Wurster M.; Arnold N.; Lindequist U.; Wessjohan L. Chemical composition of the essential oil of Teucrium yemense Deflers. Rec Nat Prod 2: 25–32; 2008.
Ngugi G. W.; Jager A. K.; van Staden J. In vitro propagation of Drimia robusta Bak. South Afr J Bot 64: 266–268; 1998.
Oran S. A.; Al-Eisawi D. M. Chick-list of medicinal plants in Jordan. Dirasat, Med Bio Sci 25: 84–112; 1998.
Orav A.; Stulova I.; Kailas T.; Müürisepp M. Effect of storage on the essential oil composition of Piper nigrum L. fruits of different ripening states. J Agric Food Chem 52: 2582–2586; 2004.
Owies D.; Shibli R.; Ereifej K. In vitro propagation of Gundelia tournefortil. Adv. Hort. Sci. 18: 127–131; 2004.
Putievsky E; Dudai N; Ravid U. Cultivation, selection and conservation of Oregano species in Israel. Padulosi S (ed) Oregano, CIHEAM, Proceedings of the International Plant Genetic Resources Institute (IPGRI)Valenzano (Bari), Italy, 1996.
Rizk A. M.; Hammouda F. M.; Rimpler H.; Kamel A. Iridoids and flavonoids of Teucrium polium herb. Planta Med 2: 87–88; 1986.
Shetty K.; Curtis O. F.; Levin R. E.; Witkowsky R.; Ang W. Prevention of vitrification associated with in vitro shoot culture of oregano (Origanum vulgare) by Pseudomonas spp. J Plant Physiol 147: 447–451; 1995.
Skoula M.; Abbes J. E.; Johnson C. B. Genetic variation of volatiles and rosmarinic acid in populations of Salvia fruticosa mill growing in Crete. Biochem Sys Eco 28: 551–561; 2000. doi:10.1016/S0305-1978(99)00095-2.
Sudha G. G.; Seeni S. In vitro propagation of Rauwolfia micrantha, a rare medicinal plant. Plant Cell, Tiss Org Cult 44: 243–248; 1996. doi:10.1007/BF00048530.
Sudriá C.; Piñol M. T.; Palazón J.; Cusidó R. M.; Vila R.; Morales C.; Bonfill M.; Cañigueral S. Influence of plant growth regulators on the growth and essential oil content of cultured Lavandula dentata plantlets. Plant Cell, Tiss Org Cult 58: 177–184; 1999. doi:10.1023/A:1006377003962.
Svoboda K. P.; Finch R. P.; Cariou E.; Deans S. G. Production of volatile oils in tissue culture of Origanum vulgare and Tanacetum vulgare. Acta Hort 390: 147–152; 1995.
Tawfik A. A.; Read P. E.; Cuppet S. L. Stimulation of growth and monoterpene production of sage (Salvia officinalis) by benzyladenine in vitro. Plant Growth RegulSoc Am 20: 200–206; 1992.
Tetyana P.; Van Staden J. Micropropagation of Cussonia paniculata—a medicinal plant with horticultural potentials. South Afr J Bot 67: 367–370; 2001.
Thangavel K.; Maridass M.; Sasikala M.; Ganesan V. In vitro micropropagation of Talinum portulacifolium L. through axillary bud culture. Ethnobot Leaflets 12: 413–418; 2008.
Van Huylenbroeck J. M.; Debergh P. C. Physiological aspects in acclimatization of micropropagated plantlets. Plant Tissue Culture and Biotechnology. 2: 136–141; 1996.
Wardam B. Plant diversity in Jordan and the threats it faces. Biodiversity and Ecosystems, Jordan, Amman. Available from: http://www.arabenvironment.net/archive/2006/7/73595.html, cited July 24 2009, 2006.
Werker E.; Ravid U.; Putievsky E. Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae. Isr J Bot 34: 31–45; 1985.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: D. T. Tomes
Rights and permissions
About this article
Cite this article
Al-Qudah, T.S., Shibli, R.A. & Alali, F.Q. In vitro propagation and secondary metabolites production in wild germander (Teucrium polium L.). In Vitro Cell.Dev.Biol.-Plant 47, 496–505 (2011). https://doi.org/10.1007/s11627-011-9352-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-011-9352-9