Abstract
Echinacea, better known as purple coneflower, has received a global attention because of its increasing medicinal value. There is enormous potential for the discovery of new medicinal compounds in this species and an immediate need for techniques to facilitate the production of high quality, chemically consistent plant material for drug development and clinical trials. In vitro tissue culture of Echinacea can play a vital role in the development of novel germplasm, rapid multiplication, and genetic modifications for an enhanced phytochemical production. Recent establishment of liquid culture techniques, large-scale bioreactors, and Agrobacterium-mediated transformation are changing the production parameters of the Echinacea species. This review provides an overview of the recent developments in in vitro technologies and challenges that remain in the Echinacea biotechnology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Echinacea: An Important Medicinal Plant
Echinacea is widely used in Europe and North America for the treatment of common cold. Echinacea, also referred to as purple coneflower, is geographically confined to America (Macchia et al. 2001; Binns et al. 2004) and is distributed in dry prairies from Texas to Saskatchewan and from west of the Rocky mountains to Minnesota (McGregor 1968; Kindscher 1989; Bauer and Foster 1991; Foster 1991 ). Major producers of Echinacea in Europe are in Germany, Switzerland, The Netherlands, Italy, and Spain (Galambosi 2004). In the last few years, the areas of cultivation of Echinacea extended beyond North America and Europe into South America, Australia, and other areas of the world (Yu and Kaarlas 2004). The global cultivation area of Echinacea was roughly estimated at several thousand hectares (Commonwealth Secretariat 2001). Consumption of herbal products rose from 15 to 35% in the last few years, and garlic and Echinacea were the most popular self-care herbs (Saskatchewan Nutraceutical Network 2001). In 1998, Echinacea was the tenth most important medicinal plant sold in Europe with annual sales of about $120 million. In North America, Echinacea is listed as the first among 11 top-selling herbal extracts (Yu and Kaarlas 2004). Retail sales of Echinacea products are more than $158 million annually in the USA and have been estimated at $1,300 million annually worldwide (Blumenthal 2003).
Echinacea species are members of the Asteraceae family (Perry et al. 2001) and include E. angustifolia, E. pallida, E. simulata, E. paradoxa, E. tennesseensis, E. laevigata, E. sanguinea, E. atrorubens, E. gloriosa, along with E. purpurea (McGregor 1968). The word Echinacea is derived from the Greek Echinos for sea urchin or hedge hog, a reference to the spiny appearance of the plant (Speroni et al. 2002). Echinacea products are made from roots, flower heads, seeds, or juice of the whole plant (Hu and Kitts 2000). Three species of Echinacea are generally used medicinally: E. purpurea Moench (roots and tops), E. angustifolia DC (roots), E. pallida Nutt (roots; Perry et al. 2001). Echinacea species may produce in access of 50 tons of fresh and 10 tons of dry mass/ha, depending on the species, with E. purpurea often producing the highest yield. The drying ratio between the fresh and dry herb yield of Echinacea species ranges from approximately 2.5–5:1 with a generally lower drying ratio for root tissues because of the higher dry matter content (Galambosi 2004).
A recent NAPRALERT search revealed the presence of 216 different medicinally active compounds in E. purpurea (Murch et al. 2006). Phenolic acids, alkamides, polyacetylenes, glycoproteins, and polysaccharides have been detected as biologically active components in different Echinacea species (Bauer and Wagner 1991). Known phenolic compounds in Echinacea species include caffeic acid derivatives such as cichoric acid in E. purpurea and E. pallida, and echinacoside in E. angustifolia (Harborne and Williams 2004). More recent studies have questioned the bioavailability and absorption of phytochemical components of Echinacea species and suggest alkamides as possible candidates for medicinal efficacy (Matthias et al. 2005; Woelkart et al. 2005).
Medicinally, Echinacea is thought to create activity in the immune system by stimulating T-cell production, phagocytosis, lymphocytic activity, cellular respiration (anti-oxidation) activity against tumor cells (Bauer and Foster 1991; Barrett 2003), and inhibiting hyaluronidase enzyme secretion (Bergeron et al. 2002). Echinacoside, found in E. angustifolia, is a broad spectrum antibiotic, inhibiting a broad range of viruses, protozoa, bacteria, and fungi. However, there may not be enough echinacoside in most tissues for the effects to be significant (Cervellati et al. 2002). Another unique compound, echinacein, has been shown to counteract the activity of hyaluronidase, an enzyme that microbes produce to penetrate tissues and cause infection (Pons 1992). Echinacea products differ considerably in their composition mainly because of the use of variable plant material, extraction methods, and addition of other components. The effects of Echinacea preparations tested in clinical trials also differ greatly. Overall, the clinical evidence of the efficacy of Echinacea is inconsistent, and reported benefits have not been confirmed with replicated rigorous trials (Linde et al. 2006; Thygesen et al. 2007).
Conventional Propagation Practices
There is a little available information on optimized growing methods for any Echinacea species, and protocols for optimum growth, yield, and chemical composition are somewhat limited (Dafault et al. 2003; Zheng et al. 2006). Conventional propagation of Echinacea employs seeds, crown divisions, and root cuttings. Seeds are generally directly seeded in the field, or germinated in the greenhouse and the established seedlings transplanted in the field (Miller 2000). However, the efficiency of Echinacea seed germination and transplant production is rather low and inconsistent, ranging from no germination to variable frequency, depending on the physiology of the seed and the growth environment, soil pH, and moisture (Hobbs 1998; Macchia et al. 2001). Echinacea seed dormancy also varies with species (Li 1998). E. pallida and E. angustifolia exhibit higher levels of dormancy than E. purpurea (Hobbs 1998). Seed germination is dependent on temperature (Kochankov et al. 1998; Hassel et al. 2004), light (Smith-Jochum and Albrecht 1987), and harvest time (Wartidiningsih et al. 1994). E. purpurea seeds harvested at physiological maturity, but before drying, have higher germination rates than seeds harvested after desiccation (Wartidiningsih and Geneve 1994). However, even in favorable conditions, seeds still fail to germinate because of physical or physiological dormancy. The proven methods of improving Echinacea seed germination include stratification (Baskin et al. 1992; Wartidiningsih et al. 1994; Feghahati and Reese 1994; Parmenter et al. 1996; Van Gaal et al. 1998) and osmotic and matric priming (Bradford 1986; Samfield et al. 1990; Pill et al. 1994; Wartidiningsih et al. 1994, 1991; Pill and Haynes 1996). The efficiency and duration of stratification treatments can be further improved with plant growth regulators (PGRs) such as ethylene (Jones 1968; Sari et al. 2001; Macchia et al. 2001) and gibberellic acid (GA3; Pill and Haynes 1996). It is obvious that Echinacea seeds collected from different locations require a different set of treatments and show unique germination responses, and the optimization of seed treatments and cultivation techniques is essential for normal crop growth with higher concentration of active ingredients (Li 1998).
Although propagation by seed in Echinacea is a predominant technique, it does not ensure pathogen-free plants, is seasonally dependant, time-consuming, and prone to poor yield because of seed dormancy and diseases. A high level of fungal and microbial contamination of in vitro germinated Echinacea seeds has been reported previously (Choffe et al. 2000b). To fulfill the increasing demand for this important medicinal plant, different methods and strategies have been developed, which include rapid multiplication of axenic, healthy plants, and faster introduction of new cultivars with desired traits. In this regard, in vitro tissue culture techniques are proved to be very valuable. Research on in vitro regeneration of Echinacea has resulted in the development of several protocols and strategies for controlled environment production, which are discussed in this review.
In Vitro Technologies for Mass Propagation of Echinacea
In vitro culture and regeneration of plants offer improvements over traditional vegetative propagation because of the faster rate of plant multiplication (Lineberger, 1983) and may also be effective in propagating species that are less responsive to cloning by conventional means (Bridgen 1986; Harbage 2001). Echinacea species have been regenerated from a range of tissues from in vitro seedlings to mature, field-grown plants.
In Vitro seed germination.
Being largely an organically grown crop (Li 1998), Echinacea plants, including seeds, may be heavily infected with microorganisms (Mechanda et al. 2003; Perry et al. 2004). Seed is an important explant in establishing Echinacea cultures in vitro (Lakshmanan et al. 2002). Different methods have been adopted for seed sterilization including surface sterilization with ethanol and sodium hypochloride (Lakshmanan et al. 2002; Mechanda et al. 2003; Koroch et al. 2003; Zobayed and Saxena 2003) along with the detergent Tween 20 (Choffe et al. 2000a; Koroch et al. 2002a). However, the use of surface sterilants alone may not eliminate microbial contamination completely (Choffe et al. 2000a). Hence, plant preservation mixture (PPM; Phytotechnology Laboratories Lexena, KS), the broad-spectrum antimicrobial agent, was used to control the systemic fungal contamination of Echinacea seeds to obtain sterile seedlings (Choffe et al. 2000a; Mechanda et al. 2003). Alternately, Harbage (2001) proposed removing the seed coat layers to prevent contamination of seeds. In our experience with E. purpurea seeds, a sequential process of surface sterilization by immersion in 10% PPM followed by a 30-s immersion in 70% ethanol and 18-min immersion in 5.4% sodium hypochloride containing traces of Tween 20 proved highly efficient in ensuring contamination-free seed germination (Murch et al. 2006). Basal media components are sufficient to support the in vitro germination of Echinacea seeds (Li 1998). Seed explants develop shoots when exposed to a cytokinin (Gockel et al. 1992; Harbage 2001). However, recent studies have shown that endophytic, antibiotic-resistant bacteria can survive in Echinacea cultures without detrimental effects on the growing plants in the culture environment (Lata et al. 2006).
Explants.
Choice of explant varies with species and plays an important role in determining the efficiency of propagation. Several regeneration methods have been reported for commercially relevant Echinacea species (Jones et al. 2007; Zhao et al. 2006; Pan et al. 2004; Sauve et al. 2004; Zobayed and Saxena 2003; Koroch et al. 2003; Lakshmanan et al. 2002; Park et al. 2002; In Sup So et al. 2002a; Harbage 2001; Coker and Camper 2000, 2004; Choffe et al. 2000a, b), and nearly all protocols have utilized embryonic or in vitro grown seedling explants. In early experiments, anther, mesophyll protoplast, petiole, stem, seed, flower stalks, leaf sections, hypocotyls, cotyledons, and roots have been used as explant for induction of callus that subsequently differentiated into shoots and roots (Table 1). These choices of explant material reflect a preference for juvenile tissues that generally have high organogenic competence and in vitro seed germination is relatively less difficult (Koroch et al. 2003). The development of in vitro methods for regeneration of Echinacea species using leaf tissue is a non-destructive approach in contrast to the methods using embryonic tissues as explant source (Koroch et al. 2003; Jones et al. 2007). Furthermore, the out-crossing reproductive nature of the genus presents an added uncertainty in the use of seed or seedling tissues, which could lead to clones with substantial genetic variation from the parent plant. Therefore, leaf is more suitable for regeneration of Echinacea. It is noteworthy that the same explant may show different morphogenic responses under specific culture conditions (Choffe et al. 2000a; Murch et al. 2006).
Regeneration.
In vitro regeneration of Echinacea can occur both by organogenesis and by somatic embryogenesis. Murashige and Skoog (MS; Murashige and Skoog 1962) culture medium has been the medium of choice in most studies on Echinacea micropropagation. However, the use of other media such as woody plant medium (WPM) has also been reported (Gockel et al. 1992; Harbage 2001; Mechanda et al. 2003).
Shoot organogenesis.
Several biochemical processes are required for differentiation during shoot morphogenesis in plants (Chawla 2000). In general, the explant type, its orientation in the culture medium, and PGRs play a key role in regulating the differentiation process (Kumar et al. 2005; Jones et al. 2007). Koroch et al. (2002a) induced callus and indirect shoot organogenesis from the leaf explant of E. purpurea with different auxin/cytokinin combinations (α-naphthaleneacetic acid/6-benzylaminopurine (NAA/BAP)). The use of BAP alone at lower concentrations (0.44–8.88 μmol/l) stimulated adventitious shoot formation and increased callus production compared to a low-shoot initiation response with increasing NAA concentrations. In another report on E. purpurea, Mechanda et al. (2003) induced direct shoot regeneration from fully developed leaves of potted mature plants with various levels of BAP, whereas callus was induced with a combination of NAA (1 μmol/l) and BAP (6 μmol/l).
The BAP and NAA combination was found to be useful for regeneration of whole plantlets via indirect shoot organogenesis from the leaf explant of E. pallida (Koroch et al. 2003). Sauve et al. (2004) found NAA in combination with Thidiazuron (TDZ) or BAP to be effective for shoot organogenesis from the leaf explant of E. tennesseensis, an endangered species (Walck et al. 2002). Lower concentrations of BAP (0.45–4.5 μmol/l) were also effective for shoot organogenesis of seed explants (Gockel et al. 1992; Harbage 2001). Bhatti et al. (2002) found that factorial combinations of BAP with NAA were effective in inducing shoot organogenesis from hypocotyl explants for E. angustifolia, E. purpurea, and E. pallida. However, Coker and Camper (2000) found the combination of NAA and kinetin to be more effective than 2, 4-dichlorophenoxyacetic acid (2,4-D) and kinetin. Hypocotyl and cotyledon tissues have also shown similar responses to BAP (Bhatti et al. 2002; Sauve et al. 2004). In our studies, shoot organogenesis was observed in petiole explant cultures of E. purpurea (Choffe et al. 2000b). The sub-epidermal cell layers of petiole explant formed callus and shoots on media supplemented with BAP or TDZ in combination with indoleacetic acid (IAA). Histological examinations of petiole sections revealed BAP-induced periclinal division in the subepidermal cell layers within 3 to 7 d, leading to the formation of numerous meristematic centers. Cells in these meristematic centers were small with dense cytoplasm and prominent nuclei (Choffe et al. 2000b).
Somatic embryogenesis.
Propagation by somatic embryogenesis is likely to generate much higher number of plantlets (Ammirato 1983; Khibas 1995; Mithila et al. 2001). Somatic embryos of E. purpurea were first observed in petiole explants cultured in the presence of BAP, TDZ, or TDZ and IAA (Choffe et al. 2000b). Histology of these cultures showed a well-defined protoderm comprised of distinct rectangular cells, and there was no evidence of vascular connections to maternal vasculature. In another study, Lakshmanan et al. (2002) used hypocotyls from four species of Echinacea (E. purpurea, E. pallida, E. paradoxa, E. angustifolia) for inducing somatic embryogenesis on MS media supplemented with 9 μM 3,6-dichloro-o-anisic acid (dicamba, DC) or 2,4-D. Whereas well-defined embryos were observed in all cultures, E. angustifolia and E. pallida were more embryogenic than E. paradoxa and E. purpurea. These results were in contrast to those for petiole culture of E. purpurea (Choffe et al. 2000b) in which 2,4-D was found to be inhibitory to embryogenesis. Zobayed and Saxena (2003) reported enhanced somatic embryogenesis in E. purpurea with the use of indolebutyric acid (IBA) as an auxin and 14-d dark pre-incubation of cultures. In a recent report on E. purpurea, Murch et al. (2006) observed significant difference in the development of shoots or somatic embryos on petiole sections of clonally propagated lines derived from different individual seeds and cultured onto a medium supplemented with only the cytokinin (BAP); there was up to 30-fold variation in the capacity of individual seedling-derived germplasm to regenerate shoots and greater than 50-fold variation in the competence of cells to undergo somatic embryogenesis. As well, the culture of leaf disks of each seedling-derived line onto auxin- and cytokinin-supplemented medium and incubation in the dark also induced both de novo shoots and somatic embryos. There was a significant variation in relative ratios of the different modes of regeneration, depending on the origin of the seedling-derived lines.
It is apparent that the source of explant significantly affects the regenerative response of Echinacea. The inconsistencies observed may also be a result of differences in the culture methods, the genetic background of the parent plants, and the physiological status of the explant tissue used. Differences in the nature and frequencies of regenerative responses are known to vary considerably with different types of explants (Annadana et al. 2000), with hypocotyls being more responsive than other explants in many species including Echinacea (Lakshmanan et al. 2002; Chae et al. 2004). The genotype has also been linked to the differences in embryogenic capacity of different species and cultivars (Lakshmanan and Taji 2000). Despite extensive research, the key regulatory factors that determine the morphogenic competence of plant cells are unknown. It has become increasingly apparent that different types of explants from the same plant and different cells within the same explant exist in different states of morphogenic competence and, thus, require different cues to enter into a particular morphogenic pathway (Ammirato 1983; Hicks 1994). It is, thus, logical to conclude that variable efficiencies of explants in response to auxin and cytokinin combinations reflect different states of morphogenic competence of cells in the petiole, hypocotyl, and other tissues requiring different inductive signals to elicit a specific regenerative response (Choffe et al. 2000b; Lakshmanan et al. 2002).
Auxin, cytokinin, and TDZ in Echinacea regeneration.
In most studies, the presence of BAP in the culture medium was essential for shoot organogenesis/multiplication in all of Echinacea species tested (Choffe et al. 2000a; Harbage 2001; Mechanda et al. 2003; Koroch et al. 2003). Also, in our studies with a range of explants and a diverse plant population, the medium supplemented with BAP alone or in combination with other PGRs induced high rates of shoot proliferation in E. purpurea (Choffe et al. 2000a, b; Zobayed and Saxena 2003; Murch et al. 2006; Jones et al. 2007). Kinetin with NAA also showed potential in inducing shoot organogenesis in E. purpurea (Coker and Camper 2000; Zhao et al. 2006). The efficiency of cytokinin-induced regeneration was further increased with the addition of coconut milk (Mechanda et al. 2003) and paclobutrazol (Lakshmanan et al. 2002). However, the role of TDZ in the regeneration of Echinacea is rather interesting. TDZ is structurally quite different from a cytokinin or an auxin and has been shown to effectively substitute for both auxin and cytokinin requirements of organogenesis and somatic embryogenesis of Echinacea. In earlier studies, Choffe et al. (2000b) observed limited shoot organogenesis by TDZ, which was enhanced by the addition of IAA for E. purpurea. In the cultures of E. tennesseensis, the combination of TDZ and NAA stimulated a three-fold increase in the number of shoots than with BAP alone (Sauve et al. 2004).
Recently, a high efficiency system has been developed for TDZ-induced regeneration in both liquid and solid media. Additionally, the higher concentrations of TDZ were found to stimulate somatic embryogenesis compared to lower concentrations, which favored shoot organogenesis (Jones et al. 2007). This concentration-dependant mode of TDZ-induced morphogenesis may facilitate scale-up production of somatic embryos and artificial seeds. Because TDZ alone was able to induce callus proliferation, organogenesis, and somatic embryogenesis of Echinacea, the culture system offer a unique opportunity for investigating basic scientific questions related to the regulation and expression of morphogenesis in higher plants. The mode of action of TDZ is unknown, but it may act via modulation of endogenous auxins, cytokinins, and possibly other growth substances (Hutchinson et al. 1996; Singh et al. 1996; Visser et al. 1992). The Echinacea plants produced by TDZ-mediated regeneration, regardless of the mode of regeneration, matured and flowered within 4 mo. of being transplanted, a significantly shorter period than traditional practices.
Regeneration of protoplasts.
Cell manipulation techniques to effect somaclonal variation and somatic hybridization using protoplasts are useful to produce new and improved cultivars. Protoplast fusion between closely and distantly related species may allow bulk DNA transfer compared to one or two genes by current methods of genetic transformation. Although this technique is expected to have a large impact in the development of new varieties, its application has been limited by the difficulty in regenerating plants from fused protoplast. Practical applications have resulted from somatic hybridization between closely related species. Somatic hybrids among various Echinacea species are likely to generate novel germplasm, which may potentially exhibit synergistic effects of a range of medicinal compounds. Protoplasts have been isolated from various Echinacea tissues such as leaf and callus using enzymatic digestion with cell-wall-degrading enzymes such as cellulase, pectinase, hemicellulase (Al-Atabee and Power 1990; Zhu et al. 2005). Successful plant regeneration system for mesophyll-isolated protoplasts of E. purpurea was first developed using an alginate-embedding culture system (Pan et al. 2004). Cell colony formation from protoplasts, callus proliferation, and shoot organogenesis occurred in response to commonly used combinations of auxins and cytokinins. Further optimization of protoplast regeneration and fusion from several genotypes within a species and among different species holds potential for fundamental studies, as well as novel commercial products.
Root organogenesis.
Considerable work has been done to enhance rooting efficiency in different species of Echinacea. In most reports, varying concentrations of different auxins were used for root induction, although a basal medium without an auxin supplement has also been used (Choffe et al. 2000a; Harbage 2001; Koroch et al. 2002a; Lakshmanan et al. 2002; Pan et al. 2004; Sup So et al. 2002; Mechanda et al. 2003; Koroch et al. 2003; Zobayed and Saxena 2003; Sauve et al. 2004). Generally, rooting of Echinacea shoots was best induced on MS medium supplemented with IBA or IAA, and IBA seems to be more effective for induction from a variety of explants (Choffe et al. 2000b). Very few reports are available on the induction of root organogenesis with NAA (Table 1). However, Lakshmanan et al. (2002) concluded from their detailed work on the micropropagation of four Echinacea species that the presence of auxin, even at a very low concentration, was inhibitory to Echinacea rooting. Inhibition of rooting in the presence of an auxin is indicative of potentially high endogenous auxin content.
Light, temperature and pH affect regeneration
Light plays an important role in the shoot proliferation of Echinacea. A 16-h photoperiod of 40 to 80 μmol m−2 s−1 usually from Gro-lux type white fluorescent lamps or cool white fluorescent light at commonly used temperature of 25 ± 2°C appears to be sufficient for shoot regeneration (Choffe et al. 2000a, b; Bhatti et al. 2002; Lakshmanan et al. 2002; Koroch et al. 2003). In some cases, an initial incubation in the dark increased the efficiency of regeneration in Echinacea (Zobayed and Saxena 2003). However, Harbage (2001) incubated plant material of four commercially important Echinacea species in the dark or 40 μmol m-2 s-1 continuous cool white fluorescent light at 21 or 30°C and concluded that rooting performance was significantly affected by species but not by light or temperature. Specific effects of pH variations of the culture medium on regeneration of Echinacea species have not been reported, and most protocols utilize the standard pH of 5.6 ± 0.3. Echinacea species in the wild grow in soils with a pH ranging from 5.9 to 8.0. The best field production of Echinacea was recorded for crops grown in soil with a neutral pH (6.8–7.0; Galambosi 2004), although the species such as Echinacea pallida are typically found on lime-rich soils (Zheng et al. 2006).
Transplant and survival ex vitro.
The successful acclimatization of micropropagated plants and their subsequent transfer to field is a crucial step for commercial exploitation of in vitro technology and can be accomplished in the greenhouse as well as in the open field (Preece and Sutter 1991; Sutter et al. 1992). Relative humidity plays a vital role in acclimatization and survival of tissue-culture-raised plants. Initially, the rooted plantlets are transferred to pots after about 2–4 d in a mist chamber and kept covered with polyethylene sheets. The sheets are later removed for acclimatization of the plants in a growth chamber or greenhouse and no misting is required. Echinacea plants derived from micropropagation appeared normal, grew vigorously, and flowered within 3 mo. of transplantation (Mechanda et al. 2003; Zobayed and Saxena 2003; Jones et al. 2007), whereas conventionally grown Echinacea plants do not flower until the second season in the field (Miller 2000). Most plantlets transplanted into pots grew to maturity and appeared morphologically normal, resembling the source material with no obvious difference in their growth and development (Lakshmanan et al. 2002; Sauve et al. 2004). Thus, the combination of in vitro propagation and greenhouse production could enable a substantial reduction of time required to produce a mature plant (Jones et al. 2007).
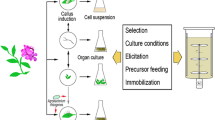
In general, different explants and procedures require different sets of PGRs and culture conditions such as growth medium and light. This is a reflection of the interaction of plant growth substances applied exogenously and consequent changes in absolute and available concentrations of endogenous hormones and nutrients. It is, therefore, difficult to develop a protocol that would produce optimum results with most genotypes. However, based on the efficiency and time required for regeneration, the BAP–IBA and the TDZ-induced regeneration systems may provide a solid basis for the development of efficient production systems for specific genotypes, even with a limited range of explants in some cases. A general regeneration/transformation protocol is proposed in Figs. 1.
Genetic Engineering of Echinacea
Genetic engineering techniques offer many advantages for the development of value-added crops (Wang and To 2004), as well as the ability to transfer foreign genes into plants has provided new ways to study regulation of developmental and biosynthetic processes (Guillon et al. 2006). However, there has been limited research on genetic engineering of Echinacea with only a few reports of transgenic hairy roots and plants. In general, the type of explant and the conditions of co-cultivation, selection, and regeneration of transformed tissues affected overall transformation efficiency of Echinacea species.
Trypsteen et al. (1991) first reported the Agrobacterium rhizogenes-mediated transformation of E. purpurea roots, but the transgenic roots showed limited growth, the typical morphological characteristics were not observed, and some of the roots produced callus and shoots. Recently, we have developed an efficient procedure to initiate and propagate hairy roots for the isolation of medicinally active compounds (Liu et al. 2006). Leaf explants of E. purpurea transformed with A. rhizogenes (ATCC 43057) formed hairy roots, which attained maximum dry biomass in MS basal medium within a 40-d culture period. The high-performance liquid chromatography (HPLC) analyses of methanolic extracts from these hairy roots revealed the presence of important biologically active caffeic acid derivatives (CADs): cichoric acid (19.21 mg g−1 dry biomass), caftaric acid (3.56 mg g−1 dry biomass), and chlorogenic acid (0.93 mg g−1 dry biomass). The most striking aspect of this work was to discover major CADs production in hairy-root cultures at levels comparative to those in the source mother plant. In further studies, we found that light exposure enhanced CAD biosynthesis (Abbasi et al. 2007). The E. purpurea hairy roots offer an excellent biological model to study the biosynthetic pathway of medicinally important CADs and a potentially efficient system for the production of medicinally important CADs.
Recently, Wang and To (2004) developed transgenic Echinacea plants using a unique Agrobacterium-mediated transformation method in which the β-glucuronidase (GUS) reporter gene of the pBI119-based expression vector was substituted by the Petunia chalcone synthase (CHS) gene. These transgenic plants overexpressing Petunia chalcone synthase can be used as a model system for studying the accumulation of plant secondary metabolites in Echinacea. A. tumefaciens strains EHA 105 and GV 26801 with binary vector pBISN1 (Roesler et al. 1991) have also been used for developing protocols for stable integration of transferred DNA into E. purpurea genome (Koroch et al. 2002b).
Phytochemicals Produced in In Vitro Cultures
Efficient and consistent production of secondary metabolites and other bioactive natural products can be achieved more easily with in vitro cultured cells and organs than with field-grown plants (Wang et al. 2006). Echinacea cell cultures have been successfully used for small and large-scale production of polysaccharides (Misawa 1994; Li and Barz 2005) for the assessment of their potential in immunological functions with some success (Wagner et al. 1986; Wagner et al. 1988; Luettig et al. 1989; Melchart et al. 2002; Roesler et al. 1991; Bauer and Foster 1991; Steinmuller et al. 1993). For example, arabinogalactan isolated from Echinacea cell cultures stimulated macrophages to excrete tumor necrosis factor (TNF; Roesler et al. 1991). Hydrophilic pharmacological components such as cichoric acid and echinacoside were also detected from cell cultures of E. angustifolia (Smith et al. 2002), whereas cinnamic acid and caffeic acid were isolated from callus cultures of Echinacea (Sicha et al. 1991). Anthocyanins were also extracted from E. pallida and E. purpurea, and identified as cyanidin 3-malonylglucoside (Cheminat et al. 1989). Li and Barz (2005) reported that the elicitation of cell culture of E. purpurea with yeast produced two new 8,4′-oxynorneoligans that were not detected in the intact plants and have cytotoxicity to human tumor cells. Therefore, cell cultures and their elicitation may be a promising way of finding novel compounds offering an opportunity of creating molecular diversity in nature.
Differentiated organs and whole plants in culture offer another efficient system for Echinacea phytochemical production. Recently, it has been shown that genetic diversity in seed populations of E. purpurea controls the capacity for regeneration and the production of cichoric acid, caftaric acid, chlorogenic acid, cynarin, and echinacoside in regenerated plantlets (Murch et al. 2006; Fig. 2). This chemodiversity offers an opportunity to select individuals with specific levels of preferred phytochemicals. The hairy root cultures also provide a promising source for Echinacea secondary metabolites due to their genetic stability, hormone-independent growth, and ability to produce medicinal metabolites at a rate comparable to the parent plants.
Together, the in vitro grown cell, callus, and hairy root cultures can be exploited to study biosynthetic pathways of important phytochemicals. As Echinacea is a good source of caffeic acid derivatives, alkamides, polyacetylenes, glycoproteins, and polysaccharides, it can also be used as a model system to study factors that influence the production of these compounds (Hu et al. 2004).
Conclusions and Future Prospects
The efficiency of medicinal plant regeneration systems must be defined in terms of the production of specific medicinally active metabolites. In this context, substantial progress has been made in the development of in vitro regeneration systems of Echinacea in the past 5 yr. As a result, several protocols are currently available for the establishment of axenic cultures and regeneration of tissues, organs, and whole plants. The regenerated plants can be grown to maturity in controlled environments. These in vitro production systems of Echinacea generate large numbers of plants and are coupled with rapid maturity of regenerants in the greenhouse, and they can provide physiologically consistent plant material for year-round extraction of pharmaceuticals (Murch et al. 2006; Zheng et al. 2006; Jones et al. 2007). However, further research in a number of fundamental and applied areas is warranted to fully exploit the potential of this species. The future research priorities of Echinacea research include the selection of elite germplasm lines, biochemical, and molecular characterization of biosynthetic pathways of the compounds of interest, and enhanced phytochemical production by undifferentiated cells, as well as organized tissues and whole plants. Research on the development of large-scale bioreactors has tremendous potential in the discovery of new compounds that are synthesized in low quantities. Additionally, the large-scale bioreactors for somatic embryo and artificial seed production are likely to reduce chemical variability of Echinacea and improve the prospects of germplasm preservation. In this context, the occurrence of both organogenesis and somatic embryogenesis in Echinacea cultures is important, as it offers an opportunity for the selection of germplasm that are suitable for bioreactor-based production systems. More research is also needed to identify suitable elicitors to enhance the bioactive constituents in cell suspensions or regenerating cultures and elucidation of the key factors responsible for their biosynthesis.
Echinacea secondary metabolites could be a source of new drugs for pharmaceutical industry. In addition, natural and induced genetic variations of medicinal plants have significant potential to produce unusual or novel compounds. Similarly, cloning the genes controlling the production of medicinal compounds and more efficient and robust transformation systems will yield commercially useful transgenic roots and plants capable of producing important secondary metabolites. The selection of elite germplasm and their complete metabolic profiling may be very rewarding for new drug discovery.
The overall progress made in Echinacea biotechnology is likely to accelerate the industry trend toward controlled cultivation of plants rather than collection from the wild. There is an increased recognition in the natural product industry of the requirement of new standards of the quality of the raw and processed plant material. The emerging herbal marketplace is expecting and demanding improved and consistent quality control procedures including standardization protocols. It is here that the in vitro technologies has immediate and a very significant role to play in providing large quantities of high quality, chemically consistent, raw material to the Echinacea industry.
References
Abbasi, B. H., Tian, C. L., Murch, S. J., Saxena, P. K. and Liu, C. Z. Light enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. (in press).DOI 10.1007/s00299-007-0344-5. 2007
Al-Atabee, J. S. and Power, J. B. Protoplast isolation and plant regeneration in ornamental Compositae. Acta Hort.280:255–258.1990
Ammirato, P. V. Embryogenesis. In: DA Evans, WR Sharp, PV Ammirato and Y Yamada (eds) Handbook of plant cell culture I. MacMillan, New York, USA, pp 82–123.1983
Annadana, S., Radamaker, W., Rammana, M., Udayakumar, M. and de Jong, J. Response of stem explants to screening and explant source as a basis for methodical advancing regeneration protocols for chrysanthemum. Plant Cell Tissue Organ Cult.62:47–55.2000
Barrett, B. Medicinal properties of Echinacea: a critical review. Phytomedicine.10:66–86.2003
Baskin, C. C., Baskin, J. M. and Hoffman, G. R. Seed dormancy in the prairie for Echinacea angustifolia (Asteraceae): after-ripening pattern during cold stratification. Int J Plant Sci.153:239–243.1992
Bauer, R and Foster, S. Analysis of alkamides and caffeic acids derivatives from Echinacea simulata and E. paradoxa roots. Planta Med.57:447–449.1991a
Bauer, R and Wagner, H. Echinacea species as potential immunostimulatory drugs. In: H Wagner and NR Farnsworth (eds) Economic and medicinal plant research. Academic, New York, pp 253–321.1991b
Bergeron, C., Gafner, S., Batcha, L. L. and Angerhofer, K. Stabilization of caffeic acid derivatives in Echinacea purpurea L. glycerin extract. J Agric Food Chem.5:3967–3970.2002
Bhatti, S. M., Myles, E. L., Long, D. E. and Sauve, R. In vitro regeneration of St. Johns wort and coneflowers. SNA research conference, Vol. 47. pp 340–342.2002
Binns, S. E., Arnason, J. T. and Baum, B. R. Taxonomic history and revision of the genus Echinacea. In: S Miller (ed) Echinacea. The genus Echinacea. CRC Press, Boca Raton, FL, pp 3–11.2004
Blumenthal, M. The ABC clinical guide to herbs. American Botanical Council. Thieme, New York.2003
Bradford, K. J. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. HortScience.21:1105–1113.1986
Bridgen, M. Do-it-yourself cloning. Greenh Grow.4:46–47.1986
Cervellati, R., Renzulli, C., Clelia, M., Guerra, M. C. and Speroni, E. Evaluation of antioxidant activity of some natural polyphenolic compounds using Briggs–Rauscher reaction method. J Agric Food Chem.50:7504–7509.2002
Chae, WB., Choi, GW. and Chung, IS. Plant regeneration depending on explant type in Chrysanthmum coronarium L. J Plant Biotechnol.6:253–258.2004
Chawla, H. S. Introduction to plant biotechnology. International Book Distributing, Lucknow, India, pp 39–56.2000
Cheminat, A., Brouillard, R., Guerne, P., Bergmann, P. and Rether, B. Cyanidin 3-malonylglucoside in two Echinacea species. Phytochemistry.28:3246–3247.1989
Choffe, K.L., Murch S.J. and Saxena, P. K. Regeneration of Echinacea purpurea: induction of root organogenesis from hypocotyls and cotyledon explants. Plant Cell Tissue Organ Cult.62:227–234.2000a
Choffe, K. L., Victor, R. M. J., Murch, S. J. and Saxena, P. K. In vitro regeneration of Echinacea purpurea L.: direct somatic embryogenesis and indirect shoot organogenesis in petiole culture. In Vitro Cell Dev Biol Plant.36:30–36.2000b
Coker, P. S. and Camper, N. D. In vitro culture of Echinacea purpurea L. J. Herbs Spices Med Plants.7(4):1–7.2000
Coker, P. S. and Camper, N. D. In vitro culture of Echinacea species. In: S Miller (ed) Echinacea. The genus Echinacea. CRC Press, Boca Raton, FL, pp 23–28.2004
Commonwealth Secretariat. A guide to the European market for medicinal plants and extracts. Commonwealth Secretariat.2001
Dafault, R. J., Rushing, J., Hassell, R., Shepard, B. M., McCutcheon, G. and Ward, B. Influence of fertilizer on growth and marker compound of field-grown Echinacea species and feverfew. Sci Hortic.98:61–69.2003
Feghahati, S. M. J. and Reese, R. N. Ethylene, light and pre-chill enhanced germination of Echinacea angustifolia seeds. J Am Soc Hortic Sci.119:853–858.1994
Foster, S. Echinacea: nature’s immune enhancer. Healing Arts, Rochester, VT, pp 35–48.1991
Gockel, C., Wawrosch, C. H., Leonhardt, W. and Kopp, B. Micropropagation of Echinacea angustiofolia. Planta Med.58:626–629.1992
Galambosi, B. Cultivation in Europe. In: S Miller (ed)Echinacea. The genus Echinacea. CRC Press, Boca Raton, FL, pp 29–52.2004
Guillon, S., Tremouillaux-Guiller, J., Pati, P. K., Rideau, M. and Gantet, P. Hairy root research: recent scenario and exciting prospects. Curr Opin Plant Biol.9:341–346.2006
Hassel, R. L., Dafault, R. and Phillips, T. Relationship among seed size, source and temperature on germination of Echinacea angustifolia, pallida, and purpurea. Acta Horti.629:239–244.2004
Harbage, J. F. Micropropagation of Echinacea angustifolia, E. pallida, and E. purpurea from stem and seed explants. Hortic Sci.36:360–364.2001
Harborne, J. B. and Williams, C. A. Phytochemistry of the genus Echinacea. In: S Miller (ed) Echinacea. The genus Echinacea. CRC Press, Boca Raton, FL, pp 55–71.2004
Hicks, G. S. Shoot induction and organogenesis in vitro: a developmental perspective. In vitro Cell Dev Biol Plant.30:10–15.1994
Hobbs, C. R. The Echinacea handbook. Botanica, Capitola, CA.1998
Hu, C. and Kitts, D. Studies on the antioxidant activity of Echinacea root extract. J Agric Food Chem.48:1466–1472.2000
Hu, C., Kitts, D. D. and Zawistowski, J. The chemistry of antioxidant constituents of Echinacea. In: S Miller (ed) Echinacea. The genus Echinacea. CRC Press, Boca Raton, FL, pp 23–28.2004
Hutchinson, M. J., Krishna Raj, S. and Saxena, P. K. Morphological and physiological changes in TDZ induced somatic embryogenesis in geranium hypocotyls culture. Int J Plant Sci.1:440–446.1996
Jones, M. P. A., Yi, Z., Murch, S. J. and Saxena, P. K. Thidiazuron-induced regeneration of Echinacea purpurea L. micropropagation in solid and liquid culture systems. Plant Cell Rep.26:13–19.2007
Jones, R. L. Ethylene enhanced release of ∝-amylase from barley aleurone cells. Plant Physiol.43:442–444.1968
Khibas, J. S. Somaclonal variation in Rudbekia. Dirasat B Pure Appl Sci.22:171–181.1995
Kindscher, K. Ethnobotany of purple coneflower (Echinacea angustifolia, Asteraceae) and other Echinacea species. Econ Bot.43(4):498–507.1989
Kochankov, V. G., Grzesik, M., Chojnowski, M. and Nowak, J. Effect of temperature, growth regulators and other chemicals on Echinacea purpurea (L.) Moench seed germination and seedling survival. Seed Sci. Tech.26:547–554.1998
Koroch, A, Juliani, H. R., Kapteyn, J. and Simon, J. E. In Vitro regeneration of Echinacea purpurea from leaf explants. Plant Cell Tissue Organ Cult.69:79–83.2002a
Koroch, A., Kapteyn, J., Juliana, H. R. and Simon, J. E. In vitro regeneration and Agrobacterium transformation of Echinacea purpurea leaf explants. Trends in new crops and new uses. ASHS Press, Alexandria, VA, pp 522–526.2002b
Koroch, A., Kapetyn, J., Juliana, H. R., Simon, J. E. In vitro regeneration of Echinacea pallida from leaf explants. In Vitro Cell. Dev Biol Plant.39:415–418.2003
Kumar, V., Gururaj, H. B., Prasad, N., Giridhar, P. and Ravishankar, G. A. Direct shoot organogenesis on shoot apex from seedling explants of Capsicum annuum L. Sci Hortic.106:237–246.2005
Lakshmanan, P. and Taji, A. Somatic embryogenesis in leguminous plants. Plant Biol.2:136–148.2000
Lakshmanan, P., Danesh, M. and Taji, A. Production of four commercially cultivated Echinacea species by different methods of in vitro regeneration. J. Hortic. Sci. Biotechnol.77:158–163.2002
Lata, H., Li, X. C., Silva, B., Moraes, R. M. and Halda-Alija, L. Identification of IAA producing entophytic bacteria from micropropagated Echinacea plants using 16S rRNA sequencing. Plant Cell Tissue Organ Cult.85:353–359.2006
Li, T. S. C. Echinacea: cultivation and medicinal value. Hortic. Tech.8:122–129.1998
Li, W. W. and Barz, W. Biotechnological production of two new 8,4′-oxynorneolignans by elicitation of Echinacea purpurea cell cultures. Tetrahedron Lett.46:2973–2972.2005
Linde, K., Barrett, B., Wolkart, K., Bauer, R. and Melchart, D. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev.1.2006
Lineberger, D. Micropropagation for the perennial industry. In: EM Smith and SM Still (eds) Proceedings of the Herbaceous Perennial Symposium, Columbus, Ohio. Ohio Coop. Ext. Ser. Bull. 717:7–9.1983
Liu, C. Z., Abbasi, B. H., Min, G., Murch, S.J. and Saxena, P.K. Caffeic acid derivatives production by hairy root cultures of Echinacea purpurea. J Agric Food Chem.54:8456–8460.2006
Luettig, B., Steinmuller, C., Gifford, G. E, Wagner, H. and Lohmann-Matthes, M. L. Macrophage activation by the polysaccharide Arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst.81(9):669–675.1989
Macchia, M., Angelini, L. G. and Ceccarini, L. Methods to overcome seed dormancy in Echinacea angustifolia DC. Sci Hortic.89:317–324.2001
Matthias, A., Addison, R.S., Penman, K.G., Dickinson, R.G., Bone, K. M. and Lehmann, R.P. Echinacea alkamide disposition and pharmacokinetics in human after tablet ingestion. Life Sci.77:s2018–s2019.2005
McGregor, R. L. The taxonomy of the genus Echinacea (Compositae). Univ Kans Sci Bull.48(4):113–142.1968
Mechanda, S. M., Baum, B. R., Johnson, D. A. and Aranson, J. T. Direct shoot regeneration from leaf segments of mature plants of Echinacea purpurea L. In Vitro Cell Dev Biol Plant.39:505–509.2003
Melchart, D., Clemm, C., Weber, B., Draczynski, T., Worku, F., Linde, K., Weidenhammer, W., Wagner, H. and Saller, R. Polysaccharides isolated from Echinacea purpurea herba cell cultures to counteract undesired effects of chemotherapy—a pilot study. Phytother Res.16:138–142.2002
Miller, A. Echinacea 2000: technical crop report. Richters: The herb specialists. Goodwood, ON, Canada, pp 1–17.2000
Misawa, M. Equipment and facilities. In: Plant tissue culture: an alternative for production of useful metabolites. Bio International, Toronto.1994
Mithila, J., Murch, S.J., KrishnaRaj S. and Saxena, P. K. Recent advances in Pelargonium in vitro regeneration systems. Plant Cell Tissue Organ Cult.67:1–9.2001
Murashige, T. and Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant.15:473–497.1962
Murch, S. J., Peiris, S. E., Shi, W. L., Zobayed, S. M. A. and Saxena, P. K. Genetic diversity in seed populations of Echinacea purpurea controls the capacity for regeneration, route of morphogenesis and phytochemical composition. Plant Cell Rep.25:522–532.2006
Pan, Z. G., Liu, C. Z., Zobayed, S. M. A. and Saxena P. K. Plant regeneration from mesophyll protoplasts of Echinacea purpurea. Plant Cell Tissue Organ Cult.77:251–255.2004
Park, C., Koroch, A., Kapteyn, J., Juliana, H. and Simon, J. In vitro shoot multiplication of Echinacea purpurea. XXXVI Horticulture conference S06-P-81.2002
Parmenter, G.A., Burton, L.C. and Littlejohn, R.P. Chilling requirement of commercial Echinacea seed. N Z J Crop Sci.24:109–120.1996
Perry, B., Burges, E. and Glennie, V. Echinacea standardization: analytical methods for phenolic compounds and typical levels in medicinal species. J Agric Food Chem.49:1702–1706.2001
Perry, N. B., Wills, R. B. H. and Stuart, D. L. In: S Miller (ed) Echinacea. The genus Echinacea. CRC Press, Boca Raton, FL, pp 111–126.2004
Pill, W. G., Crossan, C. K., Frett, J. J. and Smith, W. G. Matric and osmotic priming of Echinacea purpurea Moench seeds. Sci Hortic.59:37–44.1994
Pill, W. G. and Haynes, J. G. Gibberellic acid during priming of Echinacea purpurea in the Poltava region. Byulleten Glavnogo Botanicheskogo Sada.160:7–10.1996
Pons, T. L. Seed responses to light. In: M Fenner (ed) Seeds: the ecology of regeneration in plants. CAB International, Wallingford, UK, pp 259–284.1992
Preece JE and Sutter EG. Acclimatization of micropropagated plants to the green house and field. In: PC Debergh, RH Zimmerman (ed) Micropropagation. Kluwer, The Netherlands, p 71–93.1991
Roesler, J., Emmendorffer, A., Steinmuller, C., Luettig, B., Wagner, H., Lohmann-Matthes, ML. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to test subject mediates activation of phagocyte system. Int J Immunopharmacol.13:931–941.1991
Samfield, D. M., Zajicek, J. M. and Cobb, B. G. Germination of Coreopsis lanceolata and Echinacea purpurea seeds following priming and storage. Hortic Sci.25:1605–1606.1990
Sari, A. O., Morales, M. R. and Simon, J. E. Ethephon can overcome seed dormancy and improve seed germination in purple coneflower species Echinacea angustifolia and E. pallida. HortTechnology.11:202–205.2001
Saskatchewan Nutraceutical Network. Nutraceutical market and industry information. Available at http://www.nutranet.org/subpages/market.htm. 2001
Sauve, R. J., Mmbaga, M.T. and Zhou, S. In Vitro regeneration of the Tennessee coneflower (Echinacea tennesseensis). In Vitro Cell Dev Biol Plant.40:325–328.2004
Sicha, J. H., Becker, H., Dusek, J., Hubik, J., Siaka, T. and Hrones, I. Callus cultures of the genus Echinacea II. Effect of phenylalanine on the growth of cultures and production of cinnamic acids. Pharmazie.46:363–364.1991
Singh, R. P., Murthy, B. N. S. and Saxena, P. K. In vitro morphogenetic competence of diploid zonal geranium cotyledonary tissue induced with phenylurea compounds. Physiol Mol Biol Plants.2:53–58.1996
Smith, M. A. L., Kobayashi, H., Gawienowski, M. and Briskin, D. P. An in vitro approach to investigate medicinal chemical synthesis by three herbal plants. Plant Cell Tissue Organ Cult.70:105–111.2002
Smith-Jochum, C. and Albrecht, M. L. Field establishment of three Echinacea species for commercial production. Acta Horti.208:115–120.1987
So, I. S., Cho, K. H. and Lee, C. W. De novo regeneration system for seedling leaf tissue of E. purpurea. XXXVI Horticulture conference. S06-P-47.2002
Speroni, E., Covoni, P., Guizzardi, S., Renzulli, C. and Guerra, M. C. Anti-inflammatory and cicatrizing activity of E. pallida. J Ethnopharmacol.79:265–272.2002
Steinmuller, C., Roesler, J., Grottrup, E., Franke, G., Wagner, H. and Lohmann-Matthes, M. L. Polysaccharides isolated from plant cell culture of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria monocytogenes. Int J Immunopharmacol.15:605–614.1993
Sutter E. G., Shackel K. and Diaz J. C. Acclimatization of tissue cultured plants. Acta Horti.314:115–118.1992
Thygesen, L., Thukin, J., Mortensen, A., Skibsted, L. H. and Molgaard, P. Antioxidant activity of cichoric acid and alkamides from Echinacea purpurea, alone and in combination. Food Sci.101:74–81.2007
Trypsteen, M., Lijsebettens, M. V., Severen, R. V. and Montagu, M. V. Agrobacterium rhizogenes-mediated transformation of Echinacea purpurea. Plant Cell Rep. 10:85–89.1991
Van Gaal, T. M., Galatowitsch, S.M. and Strefeler, M. S. Ecological consequences of hybridization between a wild species (Echinacea purpurea) and related cultivar (Echinacea purpurea, White swan). Sci Hortic.76:73–88.1998
Visser, C., Qureshi, J. A., Gill, R. and Saxena, P. K. Morphoregulatory role of TDZ: substitution of auxin-cytokinin requirement of somatic embryogenesis in hypocotyls culture of geranium. Plant Physiol. 99:1704–1707.1992
Wagner, H., Stuppner, H., Puhlmann, J., Jurcic, K., Zenk, M. H. and Lohmann-Matthes, M. L. Immunologically active polysaccharides from tissue cultures of Echinacea purpurea. Planta Med.5:428.1986
Wagner, H., Stuppner, H., Schafer, W. and Zenk, M. Immunologically active polysaccharides of Echinacea purpurea cell culture. Phytochemistry 27:119–126.1988
Walck, J. L., Hemmerly, T. E. and Hidayati, S. N. The endangered Tennesse purple coneflower, Echinacea tennesseensis (Asteraceae): its ecology and conservation. Nativ Plants J.3:54–64.2002
Wang, B., Zhang, G., Zhu, L., Chen, L. and Zhang, Y. The genetic transformation of Echinacea purpurea with Agrobacterium rhizogenes and bioactive ingredients analysis in transformed cultures. Colloids Surf B Biointerfaces.53:101–104.2006
Wang, H. M. and To, K. Y. Agrobacterium-mediated transformation in the high value medicinal plant Echinacea purpurea. Plant Sci.166:1087–1096.2004
Wartidiningsih, N. and Geneve, R. L. Seed source and quality influence germination in purple coneflower (Echinacea purpurea). Hortic Sci.29:1443–1444.1994
Wartidiningsih, N., Geneve, R. L. and Kester, S. T. Osmotic priming or chilling stratification improves seed germination of purple coneflower. HortScience.29:1445–1448.1994
Woelkart, K., Koidl, C., Grisold, A., Gangemi, J. D., Turner, R. B., Marth, E. and Bauer, R. Bioavailability and pharmacokinetics of alkamides from the roots of E. angustifolia in human. J. Clin. Pharmacol.45:683–689.2005
Yu, H. C. and Kaarlas, M. Popularity, diversity, and quality of Echinacea, In: S Miller (ed) Echinacea. The genus Echinacea. CRC Press, Boca Raton, FL. pp 29–52.2004
Zhao, F.-C., Nilanthi, D., Yang, Y-S. and Wu, H. Anther culture and haploid plant regeneration in purple coneflower (Echinacea purpurea). Plant Cell Tissue Organ Cult.86:55–62.2006
Zheng, Y., Dixon, M. A. and Saxena, P. K. Growing environment and nutrient availability affect the content of some phenolic compounds in Echinacea purpurea and Echinacea angustifolia. Planta Med.72:1407–1414.2006
Zhu, L., Wang, B., Zhou, J., Chen, L., Dai, C. and Duan, C. Protoplast isolation of callus in E. angustifolia. Colloids Surf B Biointerface.44:1–5.2005
Zobayed, S. M. A. and Saxena, P. K. In Vitro regeneration of Echinacea purpurea L.: enhancement of somatic embryogenesis by indolebutyric acid and dark pre-incubation. In Vitro Cell Dev Biol Plant.39:605–612.2003
Acknowledgment
The authors acknowledge the financial support from the Chinese Academy of Sciences and the Natural Sciences and Engineering Research Council of Canada. Financial support of a Ph.D. scholarship to Bilal H. Abbasi by the Higher Education Commission of Pakistan is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: H. Wagner
Rights and permissions
About this article
Cite this article
Abbasi, B.H., Saxena, P.K., Murch, S.J. et al. Echinacea biotechnology: Challenges and opportunities. In Vitro Cell.Dev.Biol.-Plant 43, 481–492 (2007). https://doi.org/10.1007/s11627-007-9057-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-007-9057-2