Abstract
Nowadays, adoptive T cell immunotherapy is emerging as a novel and potent treatment for cancer. To prepare enough effective T cells for treatment use, their rapid expansion is favorable. Our study compared 6 commonly used cultural media for human T cells, including serum-containing media and serum-free media, namely RPMI 1640, IMDM, Gibco OpTmizer CTS T Cell Expansion SFM, Gibco AIM-V Medium CTS, LONZA X-VIVO 15, and StemSpan SFEM with or without Dynabeads Human T-Activator CD3/CD28, on in vitro T cell expansion, apoptosis, and immune phenotype. Our study results suggest that serum-free media provide better proliferation environment for T cells. Among the 3 serum-free media, we identify OpTmizer and AIM-V as better T cell culture environments compared with X-VIVO as T cells are proved to have higher viability in the first two media. Besides, we found that in vitro human T cells keep relatively resting status among non-CD3/CD28 groups, since they have weak proliferation and apoptosis abilities. The phenotypes of T cells in different cultural environments over time indicate T cells maturation during culture duration. These results provide a firm foundation of adoptive T cell immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human T cell, widely known as a major category of human immune cell, plays an important role in immunoregulation and cell cytotoxicity. With the understanding of human T cell function improves, adoptive T cell immunotherapy is emerging as a novel and potent treatment for cancer. T cells modified to express chimeric antigen receptors (CARs), for example, is an advanced cell immunotherapies with high expectation. During the treatment process of this immunotherapy, T cells are sorted from patients’ peripheral blood, transfected with CARs, and then cultured in vitro under the stimulation of certain cytokine. T cells generally needs to be expanded to 106~108 for transfusion, depending on specific clinical situation (Dai et al. 2016).
Therefore, in vitro human T cell culture technique is widely taken into application. To meet the enlarging demands of in vitro T cells, here we focus on various medium environment that were used for in vitro human T cell culture, namely RPMI 1640, IMDM, Gibco OpTmizer CTS T Cell Expansion SFM, Gibco AIM-V Medium CTS, LONZA X-VIVO 15, and StemSpan SFEM with or without Dynabeads Human T-Activator CD3/CD28 (Bruserud and Tronstad 2005; Chang and Silver 2015; Janetzki et al. 2010; Kochenderfer et al. 2009; Mailer et al. 2017; Schiller et al. 2016), on in vitro T cell expansion, apoptosis, and immune phenotype. We aim at optimizing ex vivo T cell cultural condition based on the findings of our experiments.
Materials and Methods
Isolation of human T cells
Three healthy donors voluntarily joined this study with informed consents. Fifty milliliters of peripheral blood was collected from each healthy donor with hepar tubes, separated with Ficoll-Paque PLUS (Solarbio, Beijing, China) for peripheral blood mononuclear cells (PBMCs) isolation. PBMCs were firstly harvested and then CD3+ T cells were sorted with CD3 magnetic microbeads (Miltenyi, Bergisch Gladbach, Germany). T cells were suspended with PBS at a final concentration of 5.0 × 105 per mL.
CSFE labeling
Half of the CD3+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) from the CellTrace CFSE Cell Proliferation Kit (Thermo Fisher Scientific, Waltham, MA) to measure their proliferating function in different culture media. Briefly, CFSE was firstly dissolved in dimethylsulfoxide (DMSO), then diluted with PBS to a final concentration of 5 uM as the working solution. T cells were incubated with CFSE at 37°C for 20 min. On days 3, 7, and 10, T cells were collected, washed with PBS for three times, and analyzed on a BD FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ). Proliferation index was calculated with FCS express version 4.07 software (De Novo software, Los Angeles, CA).
Culture media
T cells (labeled with CFSE or not) were cultured in 6 commonly used T cell culture media as follows: RPMI-1640 (Solarbio) plus 10% fetal bovine serum (Thermo Fisher Scientific), IMDM (Solarbio) plus 10% fetal bovine serum, Gibco OpTmizer CTS T Cell Expansion SFM, Gibco AIM-V Medium plus 10% GemCell human AB serum (Gemini Bioproducts, West Sacramento, CA), LONZA X-VIVO 15, and StemSpan SFEM (STEMCELL Technologies, Vancouver, BC). All culture media groups were added with 1000 IU/mL recombinant human IL-2 (PeproTech, Rocky Hill, NJ) and were further separated into with or without Dynabeads Human T-Activator ANTI (Thermo Fisher Scientific) groups. Cells were cultured in 24-well plates with the density of 5.0 × 105 per mL. Cells were then maintained at 37°C and 5% CO2 for over 10 d.
Cell morphology and cell counting
Human T cells with CD3/CD28 stimulation were collected on days 3, 7, and 10, respectively. Cell morphology was observed using an inverted microscope IX71, under magnification × 200. A small proportion of cell suspension was stained with trypan blue for the measurement of cell density, numbers, and viability.
Apoptosis assays
Cells were collected on day 3 and day 7, respectively. Apoptosis assays were performed using the Dead Cell Apoptosis Kit with Annexin V Alex Fluor 488 and Propidium Iodide (Thermo Fisher Scientific) as per the manufacturer’s protocol on a BD FACSCalibur flow cytometer (BD Biosciences).
Subgroups analysis
T cell cultures were analyzed for various lymphocyte subgroups, a.o. CD4+ and CD8+ T cells and T cell maturation subgroups using following markers: CD45RA, CD45RO, CD62L, and CCR7 (BD Biosciences). Specifically, T cells were identified into different subgroups according to the following phenotype combinations. Tn (naïve T cells): CD3 + CD45RO-CD45RA + CCR7+, Tcm (central memory T cells): CD3+ CD45RO + CD45RA-CCR7+, Tem (effective memory T cells): CD3+ CD45RO + CD45RA-CCR7-, Tef (effective T cells): CD3+ CD45RO-CD45RA + CCR7-. Samples were measured on the BD FACSCalibur flow cytometer, and then analyzed with FCS express version 4.07 software (De Novo software).
Statistical analysis
All statistical analyzes were performed with SPSS software (SPSS Inc., Chicago, IL). Results are shown as mean ± SD. Comparison of results was carried out using the ANOVA test. P < 0.05 was considered to indicate a statistically significant difference.
Results
In vitro human T cells demonstrate weak expansion ability without CD3/CD28 stimulation
According to the relevant studies about ex vivo human T cells culture (Bruserud and Tronstad 2005; Chang and Silver 2015; Janetzki et al. 2010; Kochenderfer et al. 2009; Mailer et al. 2017; Schiller et al. 2016), we identified 6 commonly used cultural medium systems for T cell expansion as follows: 1640, IMDM, OpTmizer, AIM-V, X-VIVO, and SFEM, with their additional ingredients illustrated in the “Materials and methods” section. 1640 and IMDM require serum as being the medium of T cell expansion, while the other media are designed serum-free regarding their specifications. However, AIM-V did not show satisfactory effect of T cell proliferation in non-serum environment (Sato et al. 2009); therefore, 10% human AB serum was added to AIM-V medium during our experiments.
We firstly examined human T cell expansion status in the 6 medium systems without CD3/CD28 stimulation. T cells were cultured in 24-well plates at the density of 5.0 × 105 per mL and cell number was counted at specific time points (Fig. 1a). On day 3, cells barely proliferated. The total number of T cells was largest in OpTmizer and smallest in SFEM, which were 12.0 ± 8.0 × 105 and 7.6 ± 2.1 × 105, respectively. On day 7 and day 10, we found that T cells grew much faster in OpTmizer, AIM-V, and X-VIVO as compared to those in 1640, IMDM, and SFEM, with the cell number of 62.1 ± 26.8 × 105, 48.7 ± 9.0 × 105, 43.5 ± 17.8 × 105, 20.7 ± 6.4 × 105, 28.0 ± 4.0 × 105, and 17.3 ± 4.6 × 105, respectively. Even for OpTmizer, in which T cells expanded by approximately 10 times during a 10-d culture duration, the proliferation rate was still far below expectation. According to these results, we concluded that in vitro human T cells do not have the capability of strong expansion without CD3/CD28 stimulation, regardless of the cultural medium.
Expansion of in vitro human T cells in 1640, IMDM, OpTmizer, AIM-V, X-VIVO and SFEM over time. The numbers of human T cells cells in the 6 media are described from day 0 to day 10 with (b) and without (a) CD3/CD28 stimulation. The numbers of cells obtained in the 6 media with CD3/CD28 stimulation are compared on day 3 (c) day 7 (d) and day 10 (e). ANOVA showed a statistically significant difference between the mean values of all the groups (P value equals 0.0027 in Fig. c, 0.000 in Fig. d and Fig. e).
In vitro human T cells demonstrate various expansion abilities in different cultural media
As the next step, we cultured human T cells in all of the 6 medium systems under CD3/CD28 stimulation to see whether cells could grow faster, and the results turned out to be significantly different from the non-CD3/CD28 group (Fig. 1b). On day 3, the number of T cells in AIM-V was 55.3 ± 11.0 × 105, which already increased nearly tenfold, while the other groups remained at relatively slower proliferation rates. T cells grew up to 12.8 ± 1.4 × 105 in OpTmizer, which was the slowest group, significantly less than the AIM-V group (P = 0.0027). (Fig. 1c). On day 7, a rapid increase to 278.0 ± 22.0 × 105 was observed in the OpTmizer group, much higher than that in AIM-V group (88.0 ± 32.0 × 105, P = 0.0011) and other groups (Fig. 1d). When day 10, the OpTmizer group was still in the leading position (549.0 ± 82.7 × 105), which had increased more than a hundredfold. The AIM-V, X-VIVO, IMDM, and 1640 groups also progressed into a rapidly increasing phase, with the cell numbers of 426.0 ± 46.6 × 105, 374.0 ± 98.0 × 105, 344.0 ± 87.0 × 105, and 272.0 ± 42.3 × 105, respectively. The SFEM group, on the other hand, kept a relatively slow proliferation rate during the 10-d culture duration, and finally reached the number of 110.6 ± 23.4 × 105 (Fig. 1e).
To better quantify the expansion rate, CFSE labeling assay was performed. T cells were labeled with CFSE, collected on days 3, 7, and 10, respectively, and then analyzed on the flow cytometer (Fig. 2b). During the same cultural time duration, T cells passaged a larger number of generations in OpTmizer and AIM-V. Proliferation index was also calculated with software (Fig. 2d). It was clear that AIM-V group grew fastest in the first 7 d, and then slowed down. The proliferation index of OpTmizer group exceeded that of AIM-V group and became the fastest one by day 7. The SFEM group maintained a low index throughout the cultural duration. In addition, we performed CFSE labeling assay on non-CD3/CD28 groups (Fig. 2a, c), and all the proliferation index were no surprisingly at low levels (T cells were too few to analyzed on day 7). The CFSE results were consistent with that of the cell counting.
Proliferation of in vitro human T cells assessed by CFSE assay in 1640, IMDM, OpTmizer, AIM-V, X-VIVO, and SFEM over time. Results show CFSE proliferation in the 6 media with CD3/CD28 stimulation on day 3, day 7, and day 10 (b) and without the stimulation on day 3 and day 10 (a) for failing to collect enough cells for examination on day 7 without CD3/CD28 stimulation. Correspondingly, the proliferation rates are described in the 6 media with (d) and without (c) CD3/CD28 stimulation over time.
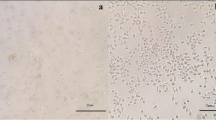
The morphology of in vitro human T cells was observed on days 3, 7, and 10, respectively (Fig. 3). On day 3, a proportion of cells in AIM-V had already clustered into masses. When came to day 7, the clustering of T cells in OpTmizer was widely observed, even wider than the AIM-V group. On day 10, large cell masses were formed in the OpTmizer group. Smaller cell masses could also be easily observed among the AIM-V, X-VIVO, and 1640 groups. More individual cells with lower cell density were observed in the IMDM and SFEM group.
All our results suggested that in vitro human T cells have various proliferation abilities when cultured in different media with CD3/CD28 stimulation. In a short time durations (e.g., 3 d), T cells grew fastest in AIM-V system. When cells were cultured for longer time periods, OpTmizer became a better medium for T cell expansion. X-VIVO was proved to have good ability to support T cell proliferation as well. The capacity of IMDM and 1640 were relatively weaker while SFEM was observed to be an inappropriate medium for human T cell expansion. The 3 serum-free media (AIM-V was added with serum though) were proved to be better environments for human T cells proliferation.
Apoptosis of in vitro human T cells in various cultural media
Apoptosis assay was conducted to both CD3/CD28 and non-CD3/CD28 groups to determine their cell viability in different culture media, and the apoptosis rates increase over time from observation. When T cells were cultured under CD3/CD28 stimulation (Fig. 4b), the apoptosis rate was lowest in the AIM-V (10.4 ± 2.0% on day 3 and 16.8 ± 7.5% on day 7) and highest in the SFEM (41.2 ± 3.1% on day3 and 67.9 ± 2.7% on day 7), respectively, while the apoptosis rate was second-lowest in the OpTmizer group (14.3 ± 3.0% on day 3 and 27.2 ± 4.7% on day 7) and second-highest in the X-VIVO group (31.7 ± 3.0% on day3 and 44.3 ± 4.2% on day7). Therefore, we identify OpTmizer and AIM-V as better T cell culture environments compared with X-VIVO as T cells are proved to have higher viability in the first two media.
Apoptosis of in vitro human T cells in 1640, IMDM, OpTmizer, AIM-V, X-VIVO, and SFEM over time. The apoptosis rates of human T cells in the 6 media are shown on day 3 and day 7 with (b) and without (a) CD3/CD28 stimulation. Representative images of OpTmizer (c) and SFEM (d) are shown. Annexin V-FITC, Propidium iodine (PI)-PE.
When T cells were cultured without CD3/CD28 stimulation, the apoptosis rates significantly declined (Fig. 4a). All groups showed relatively low apoptosis rates (lower than 25%) except for the SFEM group on day 7. Taking the OpTmizer and SFEM groups as examples (Fig. 4c, d), their apoptosis rates increased significantly under CD3/CD28 stimulation. As we know, human T cells require CD3/CD28 co-stimulation, which is able to provide activation signal, and further promote proliferation for them. Based on these results, we infer that in vitro human T cells keep relatively resting status among non-CD3/CD28 groups, since they have weak proliferation and apoptosis abilities.
Phenotypes of in vitro human T cells
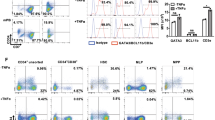
After figuring out various T cell functions in the 6 media, we focused on how the phenotypes of T cells shifted in different cultural environments over time. Similarly, when T cells were cultured in the 6 media, they were further divided into CD3/CD28 and non-CD3/CD28 groups, and cells were analyzed on day 0, day 3, and day 7, respectively. Firstly, a shift from a CD8 to a CD4 predominance was observed when in combination with CD3/CD28 stimulation at day 7 (Fig. 5a). To define specific subtypes of CD4 and CD8 T cells, we evaluated T cell maturation with markers CD45RA, CD45RO, and CCR7, finding that the phenotypes varied following a certain pattern after CD3/CD28 stimulation was added in, regardless of culture media. In CD4+ T cells, a large proportion of Tn was replaced by Tef and Tem, resulting in a significant decrease in Tn fraction under CD3/CD28 stimulation. The proportion of Tcm was observed to remain at a same level (Fig. 5b). Similar to CD4 + T cell group, the fraction of Tn also decreased and was replaced by Tem and Tef in CD8+ group. While Tcm cells only comprised a very low share in CD8+ group (Fig. 5c). The phenomenon represents T cell maturation when being activated with certain stimulations.
Phenotypes of in vitro human T cells in 1640, IMDM, OpTmizer, AIM-V, X-VIVO, and SFEM over time. (a) Proportions of CD4 and CD8 T cells are shown with and without CD3/CD28 stimulation on day 7. Maturation stages of CD4 (b) and CD8 (c) on day 7 are defined according to the following phenotype combinations: Tn (naïve T cells): CD3 + CD45RO-CD45RA + CCR7+, Tcm (central memory T cells): CD3+ CD45RO + CD45RA-CCR7+, Tem (effective memory T cells): CD3+ CD45RO + CD45RA-CCR7-, Tef (effective T cells): CD3+ CD45RO-CD45RA + CCR7-. Maturation stages of T cells in OpTmizer changed from baseline to day 3 and day 7 (d).
As the last step, we assessed the pattern of phenotype variation over our observation period. Take OpTmizer group as an example (Fig. 5d), Tn constantly differentiated into other subgroups with the observation proceeds, while the level of of CD4 and CD8 Tcm proportions maintained, which also indicated T cells maturation during culture duration.
Discussion
Immune system is capable of recognizing self and non-self to protect the human body, while tumor cells are often difficult to be recognized or attacked by the immune system as they come from our own bodies. This is also a combination of immune escape and immunosuppression abilities evolved by cancer cells. Nowadays, more powerful human immune system can be created by genetic modification to fight against cancers, relevant stunning results have been observed in relapsed/refractory hematological malignancies (Fesnak and June 2016). Therefore, human T cell adoptive immunotherapy is in a rapidly developing era. To prepare enough effective T cells for treatment use, their rapid expansion is favorable. Based on the background above, we dived into existing cultural environments aiming to find out an optimal method of T cell expansion.
Our study compared 6 commonly used cultural media for human T cells, including serum-containing media and serum-free media. In laboratory, T cells are usually cultured in RPMI 1640, IMDM, and other serum-containing media driven by economic factors. While during the immunotherapy process in which T cell culture was used, the culture media are generally required serum-free to avoid potential biological reactions (Lutz 2007). Besides, serum-free medium is more reliable due to its fixed components, while serum contains complex components and may interfere normal cell functionality. In a number of immunotherapy research institutions, T cells are cultured in serum-containing media for the first 2–3 generations, and then cultured in serum-free media for the rest generations for cost-saving purpose, and the influences on T cells are yet to be studied. Our study results suggest that serum-free media provide better proliferation environment for T cells, which are consistent with previous studies (Sato et al. 2009; Jeon and Lim 2010; Lu et al. 2016; Sutton et al. 2016). Whilst, T cells maintain similar phenotypes and functionality as compared to traditional serum-containing media. Among the 3 serum-free media we studied, OpTmizer was proved to be the best choice for in vitro human T cell proliferation. The reason why serum-free media can better facilitate T cell proliferation could be related to the specific supplements added into the media, which also makes serum-free media safer and widely applicable for cell immunotherapy.
In the presence of bead-bound CD3/CD28, T cells are significantly activated and then demonstrates the proliferate ability. In contrast, T cells remains in a relatively resting status when lacks CD3/CD28 stimulation, results in moderate proliferation and apoptosis capability and little variation in phenotype. CD3 and T cell receptor (TCR) together form CD3/TCR complex and provide activation signal for T cells, and only when being activated are T cells able to differentiate and proliferate (Moretta et al. 1989). The majority of naïve T cells differentiated into effective memory T cells and effective T cells under CD3/CD28 stimulation and CD4+ T cell predominance were observed, indicating the maturation of T cells during culture. When Tn are stimulated by pathogen antigens, they differentiate into Tef, and Tef will further differentiate into memory T cells. During re-exposure to the same antigen during the second immune response, memory T cells will undergo fast expansion and cause more effective and faster immune response against the primary immune response to eliminate the antigens (Golubovskaya 2016; Jameson 2009; Klaver et al. 2016; Sommermeyer et al. 2016; Turtle et al. 2016). Since Tef and Tem are short-lived cells while Tcm are long-lived cells, we conclude that impurity of the culture media caused slight and persistent stimulation to the T cells, which is able to induced T cells to differentiate into Tef and Tem, but not strong enough to differentiate into Tcm.
Efficient expansion of in vitro human T cells is the foundation of adoptive T cell immunotherapy. We are manipulating further studies via in vitro human T cell culture, in order to clarify the unsolved questions as mentioned above, and we look forward to illustrating the mechanisms of efficient adoptive T cell immunotherapy against hematological malignancies and solid tumors based on our sequential studies.
Conclusion
According to our study, among the 3 serum-free media, we identify OpTmizer and AIM-V as better T cell culture environments compared with X-VIVO as T cells are proved to have higher viability in the first two media. Besides, we found that in vitro human T cells keep relatively resting status among non-CD3/CD28 groups, since they have weak proliferation and apoptosis abilities. The phenotypes of T cells in different cultural environments over time indicate T cells maturation during culture duration. These results provide a firm foundation of adoptive T cell immunotherapy.
References
Bruserud O, Tronstad KJ (2005) In vitro culture of human osteosarcoma cell lines: a comparison of functional characteristics for cell lines cultured in medium without and with fetal calf serum. J Cancer Res Clin Oncol 131:377–384. https://doi.org/10.1007/s00432-004-0650-z
Chang ZL, Silver PA (2015) Identification and selective expansion of functionally superior T cells expressing chimeric antigen receptors. J Transl Med 13:161. https://doi.org/10.1186/s12967-015-0519-8
Dai H, Wang Y, Lu X (2016) Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst 108. https://doi.org/10.1093/jnci/djv439
Fesnak AD, June CH (2016) Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 16:566–581. https://doi.org/10.1038/nrc.2016.97
Golubovskaya V (2016) Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers (Basel) 8. https://doi.org/10.3390/cancers8030036
Jameson SC (2009) Diversity in T cell memory: an embarrassment of riches. Immunity 31:859–871. https://doi.org/10.1016/j.immuni.2009.11.007
Janetzki S, Price L, Britten CM et al (2010) Performance of serum-supplemented and serum-free media in IFNgamma Elispot assays for human T cells. Cancer Immunol Immunother 59:609–618. https://doi.org/10.1007/s00262-009-0788-2
Jeon MK, Lim JB (2010) Development of a serum-free medium for in vitro expansion of human cytotoxic T lymphocytes using a statistical design. BMC Biotechnol 10:70. https://doi.org/10.1186/1472-6750-10-70
Klaver Y, van Steenbergen SC, Sleijfer S et al (2016) T cell maturation stage prior to and during GMP processing informs on CAR T cell expansion in patients. Front Immunol 7:648. https://doi.org/10.3389/fimmu.2016.00648
Kochenderfer JN, Feldman SA, Zhao Y et al (2009) Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother 32:689–702. https://doi.org/10.1097/CJI.0b013e3181ac6138
Lu TL, Pugach O, Somerville R et al (2016) A rapid cell expansion process for production of engineered autologous CAR-T cell therapies. Hum Gene Ther Methods 27:209–218. https://doi.org/10.1089/hgtb.2016.120
Lutz MB (2007) Factors influencing the generation of murine dendritic cells from bone marrow: the special role of fetal calf serum. Immunobiology 212:855–862. https://doi.org/10.1016/j.imbio.2007.09.001
Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK (2017) Hypercholesterolemia enhances T cell receptor signaling and increases the regulatory T cell population. Sci Rep 7:15655. https://doi.org/10.1038/s41598-017-15546-8
Moretta A, Ciccone E, Pantaleo G et al (1989) Surface molecules involved in the activation and regulation of T or natural killer lymphocytes in humans. Immunol Rev 111:145–175
Sato K, Kondo M, Sakuta K et al (2009) Impact of culture medium on the expansion of T cells for immunotherapy. Cytotherapy 11:936–946. https://doi.org/10.3109/14653240903219114
Schiller CB, Braciak TA, Fenn NC et al (2016) CD19-specific triplebody SPM-1 engages NK and γδ T cells for rapid and efficient lysis of malignant B-lymphoid cells. Oncotarget 7:83392–83408. https://doi.org/10.18632/oncotarget.13110
Sommermeyer D, Hudecek M, Kosasih PL et al (2016) Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 30:492–500. https://doi.org/10.1038/leu.2015.247
Sutton KS, Dasgupta A, McCarty D et al (2016) Bioengineering and serum free expansion of blood-derived γδ T cells. Cytotherapy 18:881–892. https://doi.org/10.1016/j.jcyt.2016.04.001
Turtle CJ, Hanafi LA, Berger C et al (2016) CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126:2123–2138. https://doi.org/10.1172/JCI85309
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Wang, N., Cao, W. et al. Influence of various medium environment to in vitro human T cell culture. In Vitro Cell.Dev.Biol.-Animal 54, 559–566 (2018). https://doi.org/10.1007/s11626-018-0273-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-018-0273-3