Abstract
To understand the mechanism of muscle remodeling during Xenopus laevis metamorphosis, we examined the in vitro effect of insulin-like growth factor 1 (IGF-1) on growth and differentiation of three different-fate myogenic cell populations: tadpole tail, tadpole dorsal, and young adult leg muscle. IGF-1 promoted growth and differentiation of both tail and leg myogenic cells only under conditions where these cells could proliferate. Inhibition of cell proliferation by DNA synthesis inhibitor cytosine arabinoside completely canceled the IGF-1’s cell differentiation promotion, suggesting the possibility that IGF-1’s differentiation-promotion effect is an indirect effect via IGF-1’s cell proliferation promotion. IGF-1 promoted differentiation dose dependently with maximum effect at 100–500 ng/ml. RT-PCR analysis revealed the upregulation (11-fold) of ifg1 mRNA expression in developing limbs, suggesting that IGF-1 plays a role in promoting muscle differentiation during limb development. The combined effect of triiodo-l-thyronine (T3) and IGF-1 was also examined. In adult leg cells, IGF-1 promoted growth and differentiation irrespective of the presence of T3. In larval tail cells, cell count was 76% lower in the presence of T3, and IGF-1 did not promote proliferation and differentiation in T3-containing medium. In larval dorsal cells, cell count was also lower in the presence of T3, but IGF-1 enhanced proliferation and differentiation in T3-containing medium. This result is likely due to the presence among dorsal cells of both adult and larval types (1:1). Thus, IGF-1 affects only adult-type myogenic cells in the presence of T3 and helps accelerate dorsal muscle remodeling during metamorphosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During Xenopus laevis metamorphosis, muscle remodeling from larval to adult type is driven by the metamorphic hormone T3 (Nishikawa and Hayashi 1994). Tadpole dorsal muscles convert to adult type following an anterior–posterior axial gradient of adult-type muscle contractile protein expression. This process occurs by cell replacement, that is, the programmed cell death of larval cells and the new proliferation and differentiation of adult-type myogenic cells (Nishikawa and Hayashi 1995). A previous in vitro study of larval and adult-type myogenic cells, isolated from tadpole tails and adult leg muscles, respectively, clarified the following significant differences between larval and adult satellite cells: (1) cell size, (2) proliferation activity, (3) timing of myotube differentiation onset, and (4) thyroid hormone (T3) regulation of death-or-life fate and of myotube differentiation (Shibota et al. 2000).
It was previously suspected that tadpole dorsal muscles include a mixture of types of adult and larval myogenic cells. In order to prove this, cells were isolated from tadpole dorsal muscles, pre-cultured, and fractionated into two different types (“trunk 1” and “trunk 2”) by buoyant density centrifugation and gravity sedimentation. Both types of myogenic cells were found present at a 1:1 ratio. Cultures of these cells revealed that trunk 2 myogenic cells exhibited life fate as did adult leg cells, while trunk 1 exhibited death fate as did tail cells (Shimizu-Nishikawa et al. 2002).
Interestingly, myogenic cells isolated from the tail muscles also contain a small number of adult-type cells (tail 2) along with the more abundant larval cells (tail 1) (tail 1/tail 2 = 5:1). Yet, although adult-type myogenic cells exist in both dorsal and tail muscles, only dorsal muscles are converted from larval to adult type (adult myogenesis), whereas tail muscles never undergo this conversion. Therefore, we suspected that a mechanism inhibiting adult myogenesis was acting in the tail region. To investigate this possibility, analysis was performed using co-culture experiments focusing on cell–cell interaction between myogenic cells and non-myogenic cells (notochord and neural cells). The results suggested a cell interaction model in which the notochord cells abundant in the tail region suppress adult muscle differentiation in the tail by “notochord suppression,” while the spinal cord cells abundant in the dorsal region promote adult muscle differentiation in the dorsal muscles by “spinal cord promotion” (Yamane et al. 2011; Yamane and Nishikawa 2013).
It is evident that hormones and other cell-releasing factors are involved in the regulation of adult muscle formation during frog metamorphosis. We looked beyond these factors to focus on insulin-like growth factor 1 (IGF-1), a known growth and differentiation regulator. IGF-1 is also known as somatomedin (Daughaday et al. 1972) and is a protein with an insulin-like structure and a molecular weight of about 7600 (Rinderknecht and Humbel 1978). This protein is produced in the liver as an endocrine factor through the action of growth hormone (GH) (Mathews et al. 1986) but is also produced GH independently in various tissues as a local autocrine/paracrine factor (Perrini et al. 2010).
Many studies have shown that IGF-1 plays significant roles in skeletal muscle growth (Allen and Boxhorn 1989; Rosenthal and Cheng 1995; Engert et al. 1996; Adi et al. 2002; Machida et al. 2003) and muscle differentiation (Adams and Haddad 1996; Adams and McCue 1998; Semsarian et al. 1999; Jacquemin et al. 2004). Studies using rat muscle satellite cell cultures have revealed that IGF-1 stimulates cell proliferation in the early culture period and promotes myotube differentiation in the late culture period (Allen and Boxhorn 1989). This early effect on cell proliferation was found to be involved in the early upregulation of phosphorylation of the cell cycle regulator Rb (Rosenthal and Cheng 1995) and in bimodal regulation of ERK signaling (Adi et al. 2002). As regards the promotion of differentiation (muscle hypertrophy), IGF-1 was found to work through activating the PI3K/Akt pathways (Rommel et al. 2001). In studies with salmon primary muscle satellite cells and chicken primary cultures (Schmid et al. 1983; Duclos et al. 1991; McFarland et al. 1993), the IGF-1 pathway was also shown to be involved in the regulation of both proliferation and differentiation.
In frogs, little is known about IGF-1’s role in the regulation of proliferation and differentiation of myogenic cells, although a novel function for IGF signaling in regulating head formation (i.e., cell fate modulation) has been shown in X. laevis embryos (Richard-Parpaillon et al. 2002). Does IGF-1 signaling affect the regulation of X. laevis myogenesis? If so, does IGF-1 stimulate proliferation and differentiation of both types of myogenic cells (i.e., larval and adult type)? Given that, during metamorphosis, the myogenic system converts from larval to adult form, it is expected that IGF-1 preferentially promotes adult rather than larval myogenesis. Since it has been reported that GH increases during X. laevis metamorphosis (Huang et al. 2001), increased IGF-1 signaling may possibly promote proliferation and differentiation of myogenic cells during metamorphosis. In the present study, therefore, we used two (adult and larval) myogenic cell culture systems to examine the effect of IGF-1 on both cell proliferation and differentiation in order to determine whether IGF-1 signaling specifically promotes adult myogenesis during metamorphosis. The results clearly showed that IGF-1 promotes cell proliferation and differentiation (with somewhat different sensitivities) in both types of myogenic cells of X. laevis. Interestingly, in the presence of T3, IGF-1 was shown to promote muscle differentiation in adult-type myogenic cells but not in larval-type myogenic cells, indicating significant involvement of IGF-T3 cooperation in adult muscle differentiation during metamorphosis.
Materials and Methods
Animals
Tadpoles and froglets (2.5 cm in size) of X. laevis were grown from fertilized eggs in our laboratory and used for experiments according to the guidelines of the Department of Experimental Animals (Shimane University). The tadpoles were staged with the criterion described by Nieuwkoop and Faber (1967).
Primary culture of myogenic cells
Adult and larval (tail) myogenic cells were isolated each from young frog (2.5 cm) hind limb and tadpole (stage 54) tail muscles as described before (Shibota et al. 2000). Dorsal muscle myogenic cells were isolated from tadpole (stage 55) dorsal muscles as described by Shimizu-Nishikawa et al. (2002). Dissected muscles were minced, digested with 0.2% collagenase (90 min) and then 0.25% trypsin (15 min). The digested materials were gently mixed (30 times) using a Pasteur pipette, passed through nylon mesh (200-mesh), washed three times with culture medium, and used for cell cultures. Before cell inoculation, plastic culture plate (96-well, 6 mm in diameter) was pretreated with 0.01% gelatin (Sigma, St. Louis, MO, USA, G-1393, bovine skin) for 30 min, because our preliminary examination showed that gelatin treatment improved cell adhesion activity 1.5-fold as compared with control. Each myogenic cell was inoculated onto 6-mm wells at a density of 2 × 104 cells/well with 0.1 ml culture medium and cultured for 6 (or 7) d at 25°C in moisture atmosphere of 96% air/4% CO2. Dulbecco’s modified Eagle medium (DE) (which was diluted to 70%) supplemented with 10% fetal calf serum (FCS) was used as control culture medium. The FCS was pretreated with an anion exchange resin (AG-8, Bio-Rad, Hercules, CA) for deletion of endogenous serum thyroid hormones (Samuels et al. 1979), because thyroid hormone induces apoptotic cell death in larval myogenic cells (Shibota et al. 2000).
In experiments where the combined effect of IGF-1 (100 ng/ml) and serum concentration was examined (Figs. 3 and 4), the serum concentration used was 1%, 4%, and 10%. For all other control (Fig. 1) and IGF-1-added cultures (Figs. 2, 5, 7, 8, and 9) except for the Fig. 6 experiment, 10% FCS medium was used. In the case of the Fig. 6 experiment where the effect of various IGF-1 concentrations (0–500 ng/ml) on adult myogenic cells was examined, 4% FCS medium was used. This reduction in serum concentration from 10% to 4% was done because we found in a preliminary experiment that, in medium containing high serum (10%) and high IGF-1 (500 ng/ml), adult (but not larval tail) cells formed too many myotubes and overlapped in a complicated manner. This high density of complicatedly overlapped myotubes in adult cultures makes counting the number of nuclei very difficult and imprecise when assaying differentiation activity. To avoid this incorrect measurement, differentiation activity was totally diminished by decreasing the FCS concentration from 10% to 4% in the adult cell IGF concentration experiment (Fig. 6). However, in the case of larval cells in the same condition (10% FCS and 500 ng/ml IGF-1), no crowding or overlapping myotubes were observed. As such, a basic medium (10% FCS medium) was used for the larval cell IGF concentration experiment (Fig. 7). To prevent reader confusion, each experimental condition for the cell culture experiments is listed in Table 1.
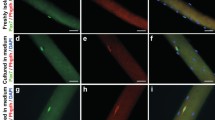
Cell growth and myotube differentiation of adult and larval myogenic cells in vitro. Adult and larval (tail) myogenic cells were cultured for 6 d. At days 1, 4, and 6, cultured cells were collected and their DNA content were measured. The values are shown in cell growth curves C (adult cells) and I (larval cells). Also at days 4 and 6, cells were fixed, stained with Giemsa’s solution. and photographed under microscope (A and B: adult cells; G and H: larval cells). Yellow arrowheads show myonuclei (nuclei in multinucleated myotubes). Red arrows show aligned myoblasts (A) or the myotubes consisting of many nuclei (B). After staining, myonuclei number/well was counted and differentiation activity was shown as myonuclei number/well (bar graph, D and J) and the ratio (%) of myonuclei number to total cell nuclei number (bar graph, E and K). D, E: adult cells; J, K: larval cells. F Immunohistochemical evidence for myotube differentiation in vitro. Cultured adult myogenic cells were fixed at day 6 and stained with anti-muscle myosin antibody. Stained (brown color) cells were myosin+-differentiated myotubes including single myocytes (yellow arrows) and fused multinucleated cells (red arrowheads). The bars in A, F, and G show 100 μm. Values (C–E and I–K) were the means from four wells of cultures, and vertical lines show the standard deviation.
Effect of IGF on cell growth and myotube differentiation of adult and larval myogenic cells in vitro. Adult (A, B) or larval (F, G) myogenic cells were cultured for 6 d with or without IGF-1 (100 ng/ml). (A, F) Control culture without IGF-1. (B, G) IGF-1 cultures. (A, B, F, G) Photomicrographs of cultured cells stained with Giemsa’s solution. Scale bars (A, F) = 100 μm. Arrowheads in A and B show multinucleated myotubes. Cell growth activity was estimated by DNA content/well and shown in bar graphs C (adult cells) and H (larval cells). Black columns show the value of attached cells at day 1. White column (control cultures) and gray column (IGF cultures) were values at day 6. Differentiation activity at day 6 was shown as myonuclei number/well (bar graph D) or myonuclei number/area (bar graph I). The ratio (%) of myonuclei number to total cell nuclei number was also shown in bar graphs E and J. D, E: adult cells; I, J: larval cells. White columns: control. Gray columns: IGF cultures. Values were the means from four wells of cultures (C–H) or from four areas (I and J), and vertical lines show the standard deviation. Double asterisk: significant difference (t value <0.05). Triple asterisks: very significant difference (t value <0.01)
Cell proliferation assay by DNA measurement with DAPI
The DNA content was measured with 4′, 6-diamidino-2-phenylindole (DAPI) (Brunk et al. 1979). Cultured cells were washed with phosphate-buffered saline (PBS), harvested by trypsinization, pelleted, and frozen at −30°C until use. Thawed cells were sonicated for 8 s in sonication buffer (0.1 M CH3COONa, pH 6.2) and added with 0.5 ml of DNA buffer (5 mM NaCl, 5 mM Hepes, pH 7) and 0.005 ml of 10 μg/ml DAPI solution. The fluorescence was measured at 360 nm excitation and 450 nm emission wavelengths. In each primary culture, DNA content of the initially isolated cells (2 × 104) was usually about 0.5 mg (25 pg/cell). Cell number per well at each culture day was estimated by using this value (25 pg/cell).
Myotube differentiation assay by counting myotubes and myotube nuclei number
Cultured myogenic cells were fixed with 70% ethanol and stained with Giemsa solution. The total numbers of myotube nuclei (myonuclei) in a culture well (or four to eight arbitrary areas of 4.7 or 9.4 mm2 in culture wells) were counted under a microscope. These values were used as parameters for myoblast terminal differentiation (experiments in Figs. 1, 2, 3, 4, 6, 7, and 9). In experiment with DNA synthesis inhibitor (Fig. 5), cell differentiation activity (myotube number and myonuclei number) was determined by using immunostained cultured cells with anti-muscle myosin antibody (MF20, DSHB, Iowa) as described below.
Immunostaining of cultured cells
Cells were washed with PBS (10 mM phosphate buffer, 0.1 M NaCl, pH 7.2), fixed with 70% ethanol for 5 min, washed three times with PBS, treated with 0.5% NP40 for 5 min, washed twice with PBS, treated with 3% H2O2 in MeOH for 5 min, washed twice with PBS, treated with 4% bovine serum albumin for 5 min, and then incubated for 18 h with MF20 (1:400) at 4°C. After three times washing with TPBS (0.05% Tween 20-PBS), the cells were incubated for 2 h with peroxidase-labeled secondary antibodies (Sigma, 1:100). Diaminobenzidine (DAB) was used as a peroxidase substrate.
DNA synthesis inhibitor experiment
Effect of AraC (experiment in Fig. 5A–E)
One day after cell inoculation, culture medium was changed to fresh one with the DNA synthesis inhibitor cytosine arabinoside (AraC, Sigma) (1 or 10 μg/ml) or without the inhibitor (control). Medium and inhibitor were changed on day 4 of culture. On days 4 and 6 of culture, cells (two wells from quadruplicate cultures) were collected by trypsinization as described above and processed for DNA content measurement. Another two wells from quadruplicate cultures were fixed with 70% ethanol and immunostained with muscle myosin as described above. Myonuclei number/area (eight arbitrary areas of 4.7 mm2 in culture wells) was counted under a microscope for determining differentiation activity.
Effect of AraC and IGF-1 (Fig. 5F-experiment)
Cells were cultured under various culture conditions (1–6). In conditions 1 and 2, cells were cultured throughout days 0–6 in control medium (condition 1) or control medium with IGF-1 (100 ng/ml) (condition 2). In conditions 3 and 4, cells were cultured for 3 d (during days 0–3) in control medium and further for 3 d (days 3–6) in medium with AraC (5 μg/ml) (condition 3) or medium with AraC (5 μg/ml) and IGF-1 (100 ng/ml) (condition 4). In conditions 5 and 6, cells were cultured throughout days 0–6 in medium with AraC (5 μg/ml) (condition 5) or medium with AraC (5 μg/ml) and IGF-1 (100 ng/ml) (condition 5). At the end of culture (at day 6), cells were fixed and processed for immunostaining with muscle myosin as described above. Myonuclei number/area (eight arbitrary areas of 4.7 mm2 in culture wells) was counted under a microscope for determining differentiation activity.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNAs were extracted from limb bud (stages 52–54) and limb (stages 56–60 and froglet) muscles by homogenizing with Isogen reagent (Nippon Gene, Tokyo, Japan). The RNA samples were treated with RNase-free DNase (RQ1, Promega, WI). The quantitative real-time RT-PCR was performed with the Thermal Cycler Dice Real-Time System TP800 (Takara, Kusatsu, Japan) and SYBR Premix ExTaq (Takara) as follows: 10 s at 95°C, followed by 40 cycles of two-step PCR (30 s at 94°C and 30 s at each annealing temperature). All PCR products showed a single peak on dissociation curves and had a single band of the expected size on electrophoretogram. The gapdh (accession, U41753.1; GI, 1136598) was used as a normalizer. Following sequences were used as PCR primers: X. laevis igf1 (NM_001163393.1; GI, 254281237) (5′-ACACTCTTCAGTTTGTCTGTGG-3′ and 5′-TACTTCCTTCTGGGCTTTTG-3′); X. laevis igf1r (NM_001088265.1; GI, 147904185) (5′-AGTCTGTCCCAGTGTTTGTG-3′ and 5′-CCCTCGTAGAAGTAGTGGTGA-3′); and X. laevis gapdh (5′-ATAGCGGCAAAGTTCAAGTCG-3′ and 5′-ACATACACGGCACCAGCATC-3′).
Statistical analysis of the data
For cell proliferation (DNA content) analysis, each points of DNA content data (bar or line graphs) were shown by mean values of three to six wells and their standard deviations (n = 3–6) were shown by vertical lines. For cell differentiation (myotube number) analysis, each points of data (bar graphs) were shown by mean values of four wells (or four to eight areas of microscopic field, one field = 4.7 or 9.4 mm2) and their standard deviations (n = 4–8) were shown by vertical lines. For PCR analysis, three independent experiments were performed and the mean values were shown with their standard deviations (n = 3).
To evaluate the statistical significance of difference between mean values, Student’s t test values were shown on open parentheses in the graph by triple asterisk (very significant difference; t value <0.01), double asterisk (significant difference; t value <0.05), or single asterisk (no significant difference; t value >0.05).
Results
Growth and differentiation in control cultures
In the first of our experiments, we examined the growth and differentiation patterns of adult myogenic cells under control conditions (Fig. 1 A–E). On day 4 of culture, myoblast fusion started to occur (red arrows in Fig. 1A) and a few multinucleated myotubes were observed (yellow arrowheads show myotube nuclei; Fig. 1 A). On day 6, both the number of myonuclei per myotube and the width of the myotubes increased, with large thick myotubes appearing (10–30 nuclei/myotube, red arrows in Fig. 1 B). Cell growth activity was also monitored (Fig. 1 C): cell count increased 1.6-fold from day 1 to day 4 and 1.4-fold from day 4 to day 6. Differentiation activity was estimated by counting all myonuclei in multinucleated myotubes (Fig. 1 D). The mean number of myonuclei per well was about 100 on day 4 and increased 24-fold (from 100 to 2370) from day 4 to day 6. The ratio of myonuclei number/total nuclei number (Mn/Tn) was very low (0.64%) on day 4 but relatively high (10.7%) on day 6 (Fig. 1 E). These results indicate that the primary event in the early culture period (days 1–4) is cell proliferation (not differentiation). Myotube differentiation, in contrast, starts around day 4 and active fusion begins during the late culture period (days 4–6).
To confirm that the multinucleated cells are differentiated myotubes, day 6 samples were stained with anti-muscle myosin antibody (MF20) (Fig. 1 F). The results clearly showed that all multinucleated cells (Fig. 1 F, arrowheads) were muscle myosin-positive (brown color) differentiated myotubes. Some single cells (Fig. 1 F, arrows) also expressed muscle myosin. As a simple means of determining the total number of myonuclei, we employed Giemsa staining and counted all myonuclei in the myotubes with more than two nuclei. This assay system enabled us to measure the activity of the final differentiation process after myoblast fusion.
In the second stage of our experiments, we examined the growth and differentiation patterns of larval myogenic cells under control conditions (Fig. 1 G–K). Cell growth activity was lower in larval cells than in adult cells (2.2-fold increase in adult cells but 1.6-fold increase in larval cells during days 1–6; compare subpanels C and I of Fig. 1). As reported previously (Shibota et al. 2000), larval myoblasts started to fuse earlier (days 2–3 after inoculation) than adult myogenic cells did (day 4). The arrowheads in Fig. 1 G show nuclei in multinucleated myotubes. Some myotubes were observed to contain more than seven nuclei on day 4. The ratio of Mn/Tn was 0.74% on day 4 and had increased to 6.2% by day 6 (Fig. 1 K). Earlier onset of myotube formation is a unique feature of larval myogenic cells and is not seen in adult myogenic cells.
Effect of IGF-1 on adult and larval myogenic cells
The effect of IGF-1 (100 ng/ml) on cultured adult myogenic cells was examined first (Fig. 2 A–E). On day 4, myoblasts started to line up and fuse themselves in both control and IGF-1 cultures. On day 6, the myotubes observed in IGF-1 cultures were longer and thicker than those in control cultures (Fig. 2 A, B). Furthermore, myotubes with many nuclei were observed in IGF-1 cultures (an arrowhead in Fig. 2 B) but not in control cultures (arrowheads in Fig. 2 A). A comparison of cell growth activity (Fig. 2 C) revealed that, during days 1–6, cell number increased 2-fold in control cultures and 2.8-fold in IGF-1 cultures, indicating that IGF-1 has a cell growth-promoting effect (significance: t test, p = 0.010). Differentiation activity was compared by counting the number of myotube nuclei/well (Mn/w) on day 6 (Fig. 2 D). The Mn/w value was 910 in control cultures and 2031 in IGF-1 cultures (2.2-fold), indicating that IGF-1 has a differentiation-promoting effect as well (significance: t test, p = 0.0003 < 0.001). Percent differentiation (Mn/Tn) on day 6 was calculated and compared between control and IGF-1 cultures (Fig. 2 E). The Mn/Tn value was 4.5% in control and 7.6% in IGF-1 cultures (1.7-fold), indicating that IGF-1 has a strong “fusion-promoting” effect (significance: t test, p = 0.017 < 0.05).
Next, the effect of IGF-1 (100 ng/ml) on cultured larval (tail) myogenic cells was examined (Fig. 2 F–J). During days 1–6, attached larval cells proliferated 1.74-fold in control cultures and 1.98-fold in IGF-1 cultures (Fig. 2 H), indicating that IGF-1 has a cell growth-promoting effect on larval cells (significance: t test, p = 0.007 < 0.01). In addition, the presence in culture of IGF-1 increased myotube length and width (as measured by increased myonuclei numbers and thickness) (Fig. 2 F, G). The myonuclei number/area (9.4 mm2) was 398 in control cultures and 668 in IGF-1 cultures (1.7-fold) (Fig. 2 I), indicating that IGF-1 has an intense differentiation-promoting effect on larval cells (significance: t test, p = 0.012 < 0.05). The Mn/Tn value was 6.5% in control and 9.5% in IGF-1 cultures (1.5-fold) (Fig. 2 J), indicating that IGF-1 also has a fusion-promoting effect on larval cells (significance: t test, p = 0.028 < 0.05).
These data show that IGF-1 promotes the growth and differentiation of both adult and larval Xenopus myogenic cells in vitro.
Effect of IGF-1 and serum concentration on adult and larval myogenic cells
A high growth rate of myogenic cells is usually observed in media with high serum concentrations (e.g., 10–20% FCS) but in vitro myotube differentiation occurs under low serum concentrations (e.g., 2–5% horse serum) (Rando and Blau 1994). To explore the relationship between IGF-1’s growth-promoting and differentiation-promoting effects, we examined IGF-1’s effect on cultured Xenopus myogenic cells under various serum concentrations (Figs. 3 and 4).
Effect of IGF-1 and serum concentration on cell growth and myotube differentiation of adult myogenic cells in vitro. Adult cells were cultured for 6 d with or without IGF-1 (100 ng/ml) under various serum concentration. (A–F) Photomicrographs of cultured cells stained with Giemsa’s solution at day 6. (A–C) Control cultures. (D–F) IGF-1 cultures. (A, D) Cultures with medium containing 1% FCS. (B, E) Cultures with 4% FCS medium. (C, F) Cultures with 10% FCS medium. Scale bar in A = 100 μm. (G) Cell growth curve estimated by DNA content/well. White and gray columns show DNA content/well at day 6 of control and IGF-1 cultures, respectively. Black column: DNA content/well of attached cells at day 1. (H) Differentiation activity estimated by myonuclei number/well. White columns: control cultures. Gray columns: IGF-1 cultures. The abscissa shows the serum concentration (%) in culture medium. Values (G and H) were the means from four to six wells of cultures, and vertical lines show the standard deviation. Single asterisks (*): no significant difference (p > 0.05). Double asterisks (**): significant difference (p < 0.05). Triple asterisks (***): very significant difference (p < 0.01).
Effect of IGF-1 and serum concentration on cell growth and myotube differentiation of larval myogenic cells in vitro. Larval cells were cultured for 6 d with or without IGF-1 (100 ng/ml) under various serum concentration. (A–F) Photomicrographs of cultured cells stained with Giemsa’s solution at day 6. (A–C) Control cultures. (D–F) IGF-1 cultures. (A, D) Cultures with medium containing 1% FCS. (B, E) Cultures with 4% FCS medium. (C, F) Cultures with 10% FCS medium. Scale bar in A = 100 μm. (G) Cell growth curve estimated by DNA content/well. White and gray columns show DNA content/well at day 6 of control and IGF-1 cultures, respectively. Black column: DNA content/well of attached cells at day 1. (H) Differentiation activity estimated by myonuclei number/area. White columns: control cultures. Gray columns: IGF-1 cultures. The abscissa shows the serum concentration (%) in culture medium. Values (G and H) were the means from four wells (or four areas) of cultures, and vertical lines show the standard deviation. Single asterisks (*): no significant difference (p > 0.05). Double asterisks (**): significant difference (p < 0.05). Triple asterisks (***): very significant difference (p < 0.01).
First, the effect of IGF-1 (100 ng/ml) at various serum concentrations (1%, 4%, and 10%) was examined in adult cells (leg cells) (Fig. 3). At the lowest serum concentration (1%), cell growth did not occur during days 1–6 (Fig. 3 G) and very few myotubes were observed on day 6 in control cultures (Fig. 3 A). The presence of IGF-1 (100 ng/ml) in 1% serum cultures during days 1–6 did not increase cell number (DNA content/well) or myotube number (Fig. 3 D, G, and H). At serum concentrations greater than 4%, however, the cell number increased in control cultures during days 1–6, reaching 1.88-fold in 4% serum and 2.02-fold in 10% serum (Fig. 3 G). The presence of IGF-1 (100 ng/ml) promoted this increase in cell number only at high serum concentrations (10%): 1.4-fold growth promotion was seen in 10% FCS (red arrows in Fig. 3 G) but no promotion was seen under low serum conditions (1–4%). As for differentiation, IGF-1 increased the Mn/w value in media containing 4% serum (1.95-fold) and 10% serum (1.88-fold) (Fig. 3 H, yellow arrows). In 1% serum cultures, however, IGF-1 did not increase the Mn/w value (Fig. 3 H). These data suggest that the differentiation-promoting effect of IGF-1 occurs only when cell proliferation is ongoing (i.e., when cells are cultured in a medium with serum concentration greater than 4%).
Next, the effect of IGF-1 at various serum concentrations was examined in larval tail cells (Fig. 4), and the results were very similar to those seen in adult cells. At the lowest serum concentration (1%), cell growth did not occur (Fig. 4 G) and very few myotubes were observed on day 6 in control culture (Fig. 4 A). The presence of IGF-1 (100 ng/ml) in 1% serum cultures did not increase cell numbers or myotube nuclei numbers (Fig. 4 D, G, and H). At serum concentrations greater than 4%, however, cell number increased in control cultures, reaching 1.2-fold in 4% serum and 1.7-fold in 10% serum (Fig. 4 G). Although the cell numbers increased somewhat more slowly, the serum concentrations effective for stimulating the growth of larval cells were the same as those for adult cells. The presence of IGF-1 (100 ng/ml) promoted cell number increase only at high serum concentrations (10%): 1.13-fold growth promotion was seen at 10% FCS (a red arrow in Fig. 4 G) but no promotion was seen in cultures with lower serum concentrations (1–4%). As for differentiation, IGF-1 increased myonuclei number/area in media containing 4% serum (3.4-fold) and 10% serum (1.7-fold) (Fig. 4 H, yellow arrows). In 1% serum cultures, however, IGF-1 did not increase the myonuclei number/area (Fig. 4 H). These findings suggest that, in larval cells as in adult-type cells, the differentiation-promoting effect of IGF-1 occurs only during cell proliferation.
Effect of AraC (DNA synthesis inhibitor) and AraC plus IGF-1 on cell proliferation and differentiation
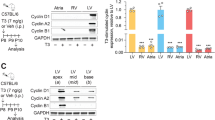
To elucidate the relation between cell proliferation and differentiation potentials, we at first examined whether cell cycle arrest by the DNA synthesis inhibitor AraC influenced differentiation activity (myotube formation) of primary cultures of Xenopus myogenic cells. The results (Fig. 5 A–D) showed that AraC concentrations greater than 1 μg/ml strongly suppressed cell number increase in leg myogenic cells during days 1–6 of culture (Fig. 5 D) and strongly decreased the number of muscle myosin+-differentiated myotubes (including single myocytes) (compare Fig. 5 A with Fig. 5 B, C). The quantitated data showed that differentiation activity was diminished by AraC-mediated cell cycle arrest, decreasing to 1/5 of control value in the presence of 1 μg/ml AraC and 1/7 in the presence of 10 μg/ml AraC (Fig. 5 E), suggesting that differentiation is strongly dependent on cell proliferation process.
Effect of the DNA synthesis inhibitor AraC on muscle differentiation. Adult myogenic cells were inoculated with control medium. One day after inoculation, medium was changed with control (white circle) or AraC (white up-pointing triangle, 1 μg/ml; black circle, 10 μg/ml)-containing medium and culture was continued. At fourth (or sixth) d in culture, cells were collected for DNA content analysis or fixed for immunostaining with anti-muscle myosin antibody. (A–C) Photographs of immunostained cells on day 6. (A) Control. (B) AraC (1 μg/ml). C AraC (10 μg/ml). The bar = 100 μm. (D, E) Changes of DNA content/well (D) and myonuclei/area (E) during culture period under control (white circle) or AraC (white up-pointing triangle, 1 μg/ml; black circle, 10 μg/ml)-containing medium. Each point shows the mean value from four wells (or four areas), and vertical lines show the standard deviation. (F) Effect of IGF-1 and AraC on muscle differentiation. Adult myogenic cells were cultured under various culture conditions (conditions 1–6). Conditions 1 and 2: Cells were cultured for 6 d in control medium (condition 1) or control medium plus IGF-1 (condition 2). Conditions 3 and 4: Cells were cultured for 3 days in control medium and then for 3 d in medium containing AraC (5 μg/ml) (condition 3) or medium containing AraC (5 μg/ml) and IGF-1 (100 ng/ml) (condition 4). Conditions 5 and 6: Cells were cultured for 6 d in medium containing AraC (5 μg/ml) (condition 5) or medium containing AraC (5 μg/ml) and IGF-1 (100 ng/ml) (condition 6). Differentiation activity at day 6 (myonuclei/area) was shown by white columns (without IGF-1; conditions 1, 3, and 5) or gray columns (with IGF-1; conditions 2, 4, and 6). Values were the mean from eight areas of cultures, and horizontal lines show the standard deviation. Triple asterisks (***): very significant difference (p < 0.01).
To confirm the idea that IGF-1’s differentiation promotion occurs via IGF-1’s cell proliferation promotion, we next examined whether IGF-1 can promote cell differentiation under complete cell cycle arrest by AraC (Fig. 5 F). The results showed that, in the presence of AraC for days 3–6 (conditions 3 and 4) or days 0–6 (conditions 5 and 6), IGF-1 had no promotion effect at all on myogenic differentiation. From these results, we concluded that the differentiation promotion effect of IGF-1 is an indirect effect via proliferation promotion by IGF-1.
Effect of IGF-1 concentration on adult and larval myogenic cells
To identify the differences between larval and adult myogenic cells’ responses to IGF-1, we examined the effects of various IGF-1 concentrations on cultured larval and adult myogenic cells (Figs. 6 and 7).
Effect of IGF-1 concentration on myotube differentiation of adult myogenic cells in vitro. Adult cells were cultured for 6 d in medium with 4% serum and various concentration of IGF-1 (0, 0.8, 4, 20, 100, and 500 ng/ml). (A) Giemsa-stained cells at day 6. Scale bar in A = 100 μm. B Differentiation activity (myonuclei number/area) at day 6. Values (B) were the means from eight areas of cultures, and vertical lines show the standard deviation. Single asterisks (*): no significant difference (p > 0.05). Double asterisks (**): significant difference (p < 0.05). Triple asterisks (***): very significant difference (p < 0.01).
Effect of IGF-1 concentration on cell growth and myotube differentiation of larval myogenic cells in vitro. Larval cells were cultured for 6 d in medium with 10% serum and various concentration of IGF-1 (0, 0.8, 4, 20, 100, and 500 ng/ml). (A) Giemsa-stained cells at day 6. Scale bar in A = 100 μm. (B) Differentiation activity (myonuclei number/well) at day 6. (C) Cell growth activity during 1–6 d (DNA content (μg)/well). Black column: DNA content/well of attached cells at day 1. Gray columns: DNA content/well of IGF cultures at day 6. Values (B and C) were the means from four wells of cultures, and vertical lines show the standard deviation. Double asterisks (**): significant difference (p < 0.05). Triple asterisks (***): very significant difference (p < 0.01).
First, we examined the effect of various concentrations (0, 0.8, 4, 20, 100, and 500 ng/ml) of IGF-1 on adult (leg) cells (Fig. 6). At concentrations greater than 20 ng/ml, IGF-1 increased myonuclei number/area on day 6 (Fig. 6 A, B). The maximum effect (a six- to sevenfold increase) was observed at concentrations of 100–500 ng/ml. In this condition (4% FCS medium), as shown by Fig. 3, there was no cell proliferation promotion by IGF-1 (data not shown).
Next, we examined the effect of various concentrations (0–500 ng/ml) of IGF-1 on larval myogenic cells (tail cells) (Fig. 7). IGF-1 at all concentrations (0.8–500 ng/ml) increased the Mn/w value on day 6; the maximum effect on myotube differentiation (a tenfold increase) was observed at a concentration of 500 ng/ml (Fig. 7 A, B). The effect of IGF-1 on cell growth was small but was observed in this condition (10% FCS medium) at all concentrations from 0.8 to 500 ng/ml: it increased the cell number (DNA content/well) by 1.05-fold (at 0.8–4 ng/ml), 1.15-fold (at 20 ng/ml), or 1.2-fold (at 100–500 ng/ml) as compared with control (Fig. 7 C).
Taken together, the evidence shows that adult and larval myogenic cells respond differently to various IGF-1 concentrations in terms of IGF-1’s effect on differentiation: adult cells required more than 20 ng/ml of “added” IGF-1 for differentiation promotion, whereas in larval cells, the addition of any amount of IGF-1 (0.8 ng/ml or more) was enough to enhance differentiation, although we should consider that serum usually contains some quantity of IGF-1 (see the “Discussion” section).
Effect of IGF-1 plus T3 on myogenic cells from adult leg, larval tail, and larval dorsal muscles
Each of our muscle cell types has a different fate during metamorphosis: the leg muscles are newly formed, the larval tail muscles are programmed to die, and the tadpole dorsal muscles, which contain two types of myogenic cells (larval and adult), are remodeled through larval-type cell death and adult-type muscle formation. To determine the effect of IGF-1 on each of these regions in the presence or absence of T3, the combined effects of IGF-1 (100 ng/ml) and T3 (10−8 M) were examined in these three types of myogenic cells (Figs. 8 and 9).
Combined effect of IGF-1 and T3 on three different myogenic cells isolated from larval tail and dorsal muscles and adult leg muscles. Each type of myogenic cell was cultured in 10% FCS medium (control), 10% FCS medium supplemented with IGF-1 (100 ng/ml), 10% FCS medium supplemented with T3 (10−8 M), and 10% FCS medium supplemented with both T3 (10−8 M) and IGF-1 (100 ng/ml). (A–L) Photomicrographs of Giemsa-stained cells at day 6. (A–D) Adult cells. (E–H) Larval tail cells. (I–L) Larval dorsal muscle cells. (A, E, I) Control cultures. (B, F, J) IGF-1 cultures. (C, G, K) T3 cultures. (D, H, L) T3+IGF cultures. Scale bar in A = 100 μm.
Quantitative analysis of combined effect of IGF-1 and T3 on three different myogenic cells (adult leg, larval tail, and larval dorsal cells). (A–C) Cell growth during 1–6 d estimated by DNA content (μg/well). Black column: DNA content/well of attached cells at day 1. Gray columns: DNA content/well (at day 6) of cells cultured with various combinations of factors. (A) Adult leg cells. (B) Larval tail cells. (C) Larval dorsal muscle cells. (D–F) Differentiation activity estimated by myonuclei number/area at day 6. The ordinate shows myonuclei number/area and the abscissa shows the culture conditions. (D) Adult leg cells. (E) Larval tail cells. (F) Larval dorsal muscle cells. Values were the means from three to four wells (A–C) or eight areas (D–F) of cultures, and vertical lines show the standard deviation. Single asterisks (*): no significant difference (p > 0.05). Double asterisks (**): significant difference (p < 0.05). Triple asterisks (***): very significant difference (p < 0.01).
In adult limb cells, T3 had no effect on myotube formation (compare subpanels A and C of Fig. 8), while IGF-1 enhanced myotube differentiation in both control and T3 cultures (Fig. 8 B, D) during days 1–6. In larval tail cells, T3 diminished myotube formation (compare subpanels E and G of Fig. 8), while IGF-1 enhanced myotube formation in control cultures (Fig. 8 F) but not in T3 cultures (Fig. 8 H). In larval dorsal muscle cells, T3 diminished myotube formation (compare subpanels I and K of Fig. 8), while IGF-1 enhanced myotube formation in both control and T3 cultures (Fig. 8 J, L).
Quantified data are shown in Fig. 9. In adult limb cells, T3 had no effect on cell growth during days 1–6 of culture with or without IGF-1. IGF-1’s growth-promoting effect was observed in both the presence and the absence of T3: IGF-1 increased DNA content 1.27-fold in “cont-IGF” cultures and 1.29-fold in “T3-IGF” cultures (Fig. 9 A). The presence or absence of T3 (10−8 M) also had no effect on the myonuclei number/area on day 6. IGF-1’s differentiation-promoting effect was evident but was almost unaffected by the presence or absence of T3: IGF-1 increased the myonuclei number/area 4.3-fold in “cont-IGF” cultures and 5.4-fold in “T3-IGF” cultures (Fig. 9 D). These results clearly show that T3 does not disturb IGF-1’s effect on growth and differentiation of adult-type myogenic cells.
In larval tail cells, IGF-1 increased cell number (1.13-fold at day 6; compare “cont” and “cont-IGF” in Fig. 9 B), while T3 had a strong suppressive effect on both cell growth and differentiation. T3 decreased the cell number (DNA content/well at day 6) to 0.24-fold of that seen in control cultures (compare “cont” and “T3” in Fig. 9 B), and IGF-1 was not able to increase the cell number in the presence of T3 (compare “T3” and “T3-IGF” Fig. 9 B). As for differentiation, IGF-1 increased the myonuclei number/area 3.4-fold in cultures without T3 (compare “cont” and “cont-IGF” in Fig. 9 E), but in cultures with T3, the myonuclei number/area actually decreased 0.09-fold (compare “cont” and “T3” in Fig. 9 E), and IGF-1’s differentiation-promoting effect was completely eradicated (compare “T3” and “T3-IGF” in Fig. 9 E). T3 was thus found to suppress IGF-1’s growth-promoting effect and to inhibit the differentiation of larval-type myogenic cells.
In larval dorsal myogenic cells, a response combining the characteristics of larval and adult cell responses was expected given that the dorsal region contains both types of cells (Shimizu-Nishikawa et al. 2002). The results were exactly as expected: without IGF-1, T3 decreased cell number 0.39-fold (compare “cont” and “T3” in Fig. 9 C); when IGF-1 was present, however, it increased cell number 1.4-fold even in the presence of T3 (compare “T3” and “T3-IGF” Fig. 9 C). As for differentiation, T3 alone decreased the myonuclei number/area 0.07-fold (compare “cont” and “T3” in Fig. 9 F), but IGF-1 enhanced it 8.5-fold even in the presence of T3 (compare “T3” and “T3-IGF” in Fig. 9 F). It is conceivable that T3’s suppressive effect on cell number and myotube number (i.e., differentiation) is due to the response of larval-type dorsal cells, whereas IGF-1’s positive effects (i.e., increases in cell and myotube numbers even in the presence of T3) are due to the response of adult-type dorsal cells.
These different responses to various combinations of IGF-1 and T3 by larval and adult-type myogenic cells seem to be a critical key point for the adult myogenic cell-specific mechanism of growth and differentiation acceleration during metamorphosis.
Expression of igf1 and igf1r mRNAs in limb buds during metamorphosis
To see whether IGF-1 signaling is really involved in adult myogenesis during Xenopus metamorphosis, expression of mRNAs of igf1 and igf1r (IGF-1 receptor) was examined in developing limb buds by qRT-PCR (Fig. 10). The results showed that the expression of igf1 mRNA started from an early stage (stage 52; undifferentiated limb buds, limb length/limb width = 2:1), increased to maximum level at the middle stage of metamorphosis (stage 56; growing limbs with differentiated muscle fibers) (11-fold increase; compare stages 52 and 56 in Fig. 10 A), and thereafter decreased to about 37% of its stage 56 value in the late metamorphic stage (stages 58 and 60; well-grown limbs). In adulthood, the ifg1 mRNA level was further downregulated. As for igf1r mRNA, maximum expression was observed during the early limb bud stage (stage 52) (Fig. 10 B). During middle-to-late metamorphosis (stages 56–60), the expression decreased first to 16% of the maximum (stage 56) and then to 2% (stages 58–60). In adulthood, the igf1r mRNA level was further downregulated.
Quantitative RT-PCR analysis of igf1 (A) and igf1r (B) expressions in developing limbs during Xenopus laevis metamorphosis. The x-axis represents developmental stages, and the y-axis shows relative mRNA content (arbitrary unit). Error bars show the standard deviations of triplicate experiments. Single asterisks (*): no significant difference (p > 0.05). Double asterisks (**): significant difference (p < 0.05). Triple asterisks (***): very significant difference (p < 0.01).
These results show that igf1 and igf1r expression actually occurred in vivo in both undifferentiated and differentiated limbs and suggested that IGF-1 signaling may make a meaningful contribution toward promoting adult muscle proliferation and differentiation during X. laevis metamorphosis.
Discussion
To the best of our knowledge, this is the first report on IGF-1’s effect on the differentiation of cultured amphibian myogenic cells. In a study using urodele myoblast cultures, IGF-1 was shown to promote the progression of these cells into S-phase, but the effect of IGF-1 on cell differentiation was not examined (Lo et al. 1993). In the present study, the effect of IGF-1 on growth and differentiation of amphibian myogenic cells was studied in detail. The major findings can be summarized as follows:
-
1.
IGF-1 promoted cell proliferation and differentiation of both larval (tail) and adult (froglet leg) myogenic cells of X. laevis. IGF-1’s effects in X. laevis cells were very similar to those in mammalian cells.
-
2.
IGF-1 promoted differentiation of cultured larval and adult myogenic cells of X. laevis only when cell proliferation was occurring, which suggests that its differentiation-promoting effect is dependent on the cell proliferation process.
-
3.
In the presence of T3, IGF-1 promoted proliferation and differentiation in adult (leg) myogenic cells but not in larval (tail) myogenic cells.
-
4.
In cell cultures with tadpole dorsal myogenic cells, T3 suppressed differentiation while IGF-1 plus T3 had the opposite effect, promoting differentiation. These results suggest the importance of the coordinated actions of IGF-1 and T3 for adult-type-specific promotion of muscle differentiation during metamorphosis.
-
5.
igf1 and igf1r mRNA expression levels were upregulated in developing hind limb buds during X. laevis metamorphosis.
These findings are discussed below in detail from the standpoint that, in cooperation with T3, IGF-1 signaling regulates the proliferation and differentiation of larval and adult myogenic cells and efficiently promotes larval-to-adult muscle conversion during X. laevis metamorphosis.
Basic properties of larval and adult myogenic cells
In control cultures, proliferation activity was higher in adult cells than in larval cells (Fig. 1 C, I), but larval cells started differentiation earlier in the cell culture period than adult cells did (compare subpanel A with G of Fig. 1). Why do these differences exist? To answer this, it is essential to examine cell division cycle number, which must reach a certain level before differentiation can begin. Larval stem cells might enter a state of cell fusion upon completing fewer cell cycles than are required for adult stem cells to begin cell fusion. Considering the results of a study using rat cells that revealed a close relationship between suppression of cell cycling and induction of terminal differentiation (Guo et al. 1995), it is likely that the timing of early differentiation in larval cell cultures is involved in their low proliferation activity and thus that an early termination of the cell cycle would occur in larval cell cultures. Clonal analysis of the processes of cell proliferation and differentiation of larval and adult myogenic cells in vitro would provide more precise information about a possible difference in the degree of stemness between them.
Aside from the differences already pointed out, the two cell types exhibited almost identical behavior in control cultures. In medium containing 1% FCS, there was no cell proliferation and almost no myotube formation in either cell type. In medium containing 10% FCS, cell proliferation occurred in both cell types, and the ratio (%) of myotube-forming nuclei (% differentiation: Mn/Tn) greatly increased (6–10%) on the sixth day (Fig. 1 E, K). Furthermore, under conditions permitting cell proliferation (serum concentration greater than 4%), both types of cells can differentiate into myotubes at a high ratio (Figs. 3 and 4). These results suggest the possibility that isolated Xenopus cells (larval and adult myogenic cells) keep their “undifferentiated single-cell” status without undergoing differentiation into myotubes if they do not enter into the cell proliferation stage and thus withdraw from the cell cycle. In relation to this possibility, our experiment using the DNA synthesis inhibitor AraC showed that treatment with sufficient AraC to completely inhibit cell proliferation of Xenopus myogenic cells (10 μg/ml) strongly suppressed myotube differentiation activity (1/7 reduction) during days 1–6 (Fig. 5E). From this result, it is concluded that cell proliferation in primary cultures of Xenopus myogenic cells contributes to the production of large quantities of terminally differentiated “myocytes” and “myotubes.”
IGF-1 promotes proliferation and differentiation of both larval (tail) and adult (leg) myogenic cells in vitro
In the present study, IGF-1 was shown to promote proliferation and differentiation of both larval and adult myogenic cells in primary cultures. IGF-1’s promotion of proliferation and differentiation has also been reported in various other vertebrate culture systems, including the rat cell line L6E9 (Rosenthal and Cheng 1995; Adi et al. 2002) and rat, human, and chicken primary muscle stem cell cultures ( Schmid et al. 1983; Allen and Boxhorn 1989; Jacquemin et al. 2004). It has also been found in fish (rainbow trout) satellite cells (Díaz et al. 2009; Gabillard et al. 2010). These results suggest that IGF-1’s roles in regulating growth and differentiation of muscle stem cells are well conserved among vertebrate species. In the present study with Xenopus myogenic cells, dual effects (promotion of both cell proliferation and myotube differentiation) were found only in cultures with more than 4% serum, where cell proliferation was possible. Therefore, in Xenopus, IFG-1’s differentiation-promoting effect may be brought about through the promotion of cell proliferation by IGF-1. In relation to this point, the Fig. 5 F experiment clearly showed that under cell cycle-arrested conditions, there is no IGF-1 effect on cell differentiation. This indicates that the differentiation-promoting effect of IGF-1 is an indirect one via IGF-1’s promotion of the cell proliferation step.
The IGF-1 concentration required to promote differentiation was different between larval and adult satellite cells (Figs. 6 and 7): the effective “added” IGF-1 concentration was more than 20 ng/ml for adult cells but was only 0.8 ng/ml for larval cells. If we consider the quantity of endogenous serum IGF-1 in control medium (about 4–10 ng/ml) as a baseline (Honegger and Humbel 1986), the effective minimum “medium” IGF-1 concentration for differentiation promotion is estimated to be about 24 ng/ml for adult cells and about 11 ng/ml for larval cells. This indicates that adult cells require at least twofold higher IGF-1 concentration for promotion of differentiation than larval cells do and suggests the possibility that increasing IGF-1 concentration during metamorphosis functions as a switch that turns on adult muscle differentiation.
The earlier onset of myotube formation in larval cells than in adult myogenic cells (Shibota et al. 2000) and the more rapid increase in the differentiation ratio in larval cells at day 4 (Fig. 1 E, K) suggests a difference in the degree of stemness between the two types of Xenopus myogenic cells. A recent mouse study revealed the existence of an intermediate state (GAlert stage) of muscle satellite cells between the quiescent stage (G0) and the activated stage (G1) and that activation from this intermediate stage requires less time than activation from G0 does (Rodgers et al. 2014). If this mechanism is also present in X. laevis, larval cells might go into GAlert stage earlier than adult cells do. Otherwise, cells isolated from larval tail muscles may contain many GAlert cells from the beginning. Rodgers et al. (2014) also showed that mTORC1 activity and cMet-mediated signaling are necessary for the G0–GAlert transition. Therefore, analysis of these molecules in the Xenopus system may yield more precise molecular level information about the difference in degree of stemness between larval and adult myogenic cells.
Metamorphic hormone (T3) effectively stimulates larval-to-adult dorsal muscle conversion by limiting the target of IGF-1’s cell growth-promoting effect to adult-type myogenic cells only
IGF-1’s cell growth-promoting effect was found to be limited to adult-type cells only in the presence of both T3 and IGF-1 (Figs. 8 and 9). Even in the presence of T3, IGF-1 could promote adult (leg) cell proliferation and differentiation (Fig. 9 A, D). In larval (tail) cell cultures, on the other hand, the differentiation of cells that survived under T3 condition was not enhanced by IGF-1 (Fig. 9 B, E). The eradication of IGF-1’s differentiation-promoting effect in tail cell cultures under T3 (Fig. 9 E) may be caused by T3-mediated cell cycle suppression (or inhibition of DNA synthesis) (Shibota et al. 2000). This is because myotube differentiation occurs under conditions permitting cell proliferation (Figs. 3 and 4), and the experimental suppression of cell proliferation by a DNA synthesis inhibitor (AraC, 10 μg/ml) greatly diminished cell differentiation activity (1/7-fold) (Fig. 5 E). In contrast, the persistence of IGF-1’s differentiation-promoting effect in adult (leg) cells even in the presence of T3 may be due to the absence of any growth-inhibiting effect on adult myogenic cells in the presence of T3 (Shibota et al. 2000). We can conclude, therefore, that the differential effect of T3 on cell proliferation in adult and larval myogenic cells plays a key role in limiting IGF-1’s promotion of myotube differentiation to adult-type cells only.
Interestingly, in cultures of larval dorsal muscle myogenic cells, the presence of T3 alone diminished total cell number and myotube number, but the presence of both T3 and IGF-1 increased cell number 1.4-fold and myotube number 8.5-fold (Fig. 9 C, F). These results may be linked to the presence of both types of myogenic cells (larval and adult) within the dorsal muscles (Shimizu-Nishikawa et al. 2002). Our previous study confirmed the presence of both types in tadpole dorsal muscles at a 1:1 ratio, whereas tail myogenic cells from same-stage tadpoles contained both types at a less balanced ratio (larval: adult = 5:1) (Shimizu-Nishikawa et al. 2002). This differential anteroposterior distribution of adult myogenic precursor cells along the axial muscles seems to play a role in the region-specific regulation of adult muscle differentiation during metamorphosis. In tadpole dorsal muscles, which contain abundant adult-type cells, adult myogenesis is strongly promoted by IGF-1 even in the presence of T3 during metamorphosis. In tadpole tail muscles, however, which contain mainly larval-type myogenic cells, proliferation is suppressed, half of the cells die due to the presence of T3 during metamorphosis, and IGF-1 does not promote differentiation of the cells that survive T3. The small numbers of adult-type myogenic cells present in the tail are prevented from entering into adult muscle formation by notochord suppression in tail region during metamorphosis (Yamane et al. 2011; Yamane and Nishikawa 2013).
The present study suggests that the cooperation of T3 and IGF-1 plays an important role in promoting larval-to-adult conversion of dorsal muscles during metamorphosis. Accordingly, we analyzed the expressions of igf1 and igf1r (IGF-1 receptor) in limb buds throughout metamorphosis. The results showed that both igf1 and igf1r expressions really occurred in limb buds and that the relative igf1r expression intensity was higher before the onset of myotube differentiation (stages 52–54; Fig. 10 B). This suggests that IGF-1 signaling is particularly influential at the proliferation stage in adult myogenic cells in the undifferentiated limb buds. In contrast, ifg1 expression was not as high as in the early limb buds but it increased rapidly during stages 54–56 (Fig. 10 A). This increase in igf1 expression might be induced by an increase in GH during metamorphosis. This is because blood GH, which is released from the liver, is known to induce tissue IGF-1 expression (Mathews et al. 1986) and because blood GH level is reported to increase in X. laevis during metamorphosis (Huang et al. 2001). A previous study has shown that, during X. laevis limb myogenesis, early (stage 54) myotubes express a larval phenotype of muscle contractile proteins (i.e., larval pattern of tropomyosin (TM) isoform; α-TM) but then switch rapidly during stages 54–57 to an adult expression pattern (i.e., 1:1 expression of α- and β-TM isoforms) (Nishikawa and Hayashi 1994). The timing of this isoform conversion is almost identical to that of the rapid upregulation of igf-1 expression in the limb buds during stages 54–56 (Fig. 10 A). Therefore, igf-1 upregulation in X. laevis limb buds seems to be required for myotube maturation into the adult type. In a future study, it would be useful to examine the differences in gene expression patterns between larval and adult myogenic cells in order to uncover the mechanism of stem cell conversion during metamorphosis.
References
Adams GR, Haddad F (1996) The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol 81:2509–2516
Adams GR, McCue SA (1998) Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84:1716–1722
Adi S, Bin-Abbas B, NY W, Rosenthal SM (2002) Early stimulation and late inhibition of extracellular signal-regulated kinase 1/2 phosphorylation by IGF-I: a potential mechanism mediating the switch in IGF-I action on skeletal muscle cell differentiation. Endocrinology 143:511–516
Allen RE, Boxhorn LK (1989) Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138:311–315
Brunk CF, Jones KC, James TW (1979) Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem 92:497–500
Daughaday WH, Hall K, Raben MS, Salmon WD, Van Den Brande JL, Van Wyk JJ (1972) Somatomedin: proposed designation for sulphation factor. Nature 235:107
Díaz M, Vraskou Y, Gutiérrez J, Planas JV (2009) Expression of rainbow trout glucose transporters GLUT1 and GLUT4 during in vitro muscle cell differentiation and regulation by insulin and IGF-I. Am J Physiol Regul Integr Comp Physiol 296:R794–R800
Duclos MJ, Wilkie RS, Goddard C (1991) Stimulation of DNA synthesis in chicken muscle satellite cells by insulin and insulin-like growth factors: evidence for exclusive mediation by a type-I insulin-like growth factor receptor. J Endocrinol 128:35–42
Engert JC, Berglund EB, Rosenthal N (1996) Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol 135:431–440
Gabillard JC, Sabin N, Paboeuf G (2010) In vitro characterization of proliferation and differentiation of trout satellite cells. Cell Tissue Res 342:471–477
Guo K, Wang J, Andre V, Smith R, Walsh K (1995) Myod-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol 15:3823–3829
Honegger A, Humbel RE (1986) Insulin-like growth factors I and II in fetal and adult bovine serum. J Biol Chem 261:569–575
Huang H, Cai L, Remo BF, Brown DD (2001) Timing of metamorphosis and the onset of the negative feedback loop between the thyroid gland and the pituitary is controlled by type II iodothyronine deiodinase in Xenopus laevis. Proc Natl Acad Sc USA 98:7348–7353
Jacquemin V, Furling D, Bigot A, Butler-Browne GS, Mouly V (2004) IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Exp Cell Res 299:148–158
Lo DC, Allen F, Brockes JP (1993) Reversal of muscle differentiation during urodele limb regeneration. Proc Natl Acad Sci U S A 90:7230–7234
Machida S, Spangenburg EE, Booth FW (2003) Forkhead transcription factor FoxO1 transduces insulin-like growth factor’s signal to p27Kip1 in primary skeletal muscle satellite cells. J Cell Physiol 196:523–531
Mathews LS, Norstedt G, Palmiter RD (1986) Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci U S A 83:9343–9347
McFarland DC, Pesall JE, Gilkerson KK (1993) The influence of growth factors on Turkey embryonic myoblasts and satellite cells in vitro. Gen Comp Endocrinol 89:415–424
Nieuwkoop PD, Faber J (1967) Normal table of Xenopus laevis (Daudin). North-Holland Publishing Co, Amsterdam
Nishikawa A, Hayashi H (1994) Isoform transition of contractile proteins related to muscle remodeling with an axial gradient during metamorphosis in Xenopus laevis. Dev Biol 165:86–94
Nishikawa A, Hayashi H (1995) Spatial, temporal and hormonal regulation of programmed muscle cell death during metamorphosis of the frog Xenopus laevis. Differentiation 59:207–214
Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F (2010) The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol 205:201–210
Rando TA, Blau HM (1994) Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125:1275–1287
Richard-Parpaillon L, Héligon C, Chesnel F, Boujard D, Philpott A (2002) The IGF pathway regulates head formation by inhibiting Wnt signaling in Xenopus. Dev Biol 244:407–417
Rinderknecht E, Humbel RE (1978) The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem 253:2769–2776
Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, Goodell MA, Rando TA (2014) mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G Alert. Nature 510:393–396
Rommel C, Bodine S, Clarke B, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013
Rosenthal SM, Cheng ZQ (1995) Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc Natl Acad Sci U S A 92:10307–10311
Samuels HH, Stanley F, Casanova J (1979) Depletion of L-3, 5, 3′-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology 105:80–85
Schmid C, Steiner T, Froesch ER (1983) Preferential enhancement of myoblast differentiation by insulin-like growth factors (IGF I and IGF II) in primary cultures of chicken embryonic cells. FEBS Lett 161:117–121
Semsarian C, Sutrave P, Richmond DR, Graham RM (1999) Insulin-like growth factor (IGF-I) induces myotube hypertrophy associated with an increase in anaerobic glycolysis in a clonal skeletal-muscle cell model. Biochem J 339:443–451
Shibota Y, Kaneko Y, Kuroda M, Nishikawa A (2000) Larval-to-adult conversion of a myogenic system in the frog, Xenopus laevis, by larval-type myoblast-specific control of cell division, cell differentiation, and programmed cell death by triiodo-L-thyronine. Differentiation 66:227–238
Shimizu-Nishikawa K, Shibota Y, Takei A, Kuroda M, Nishikawa A (2002) Regulation of specific developmental fates of larval- and adult-type muscles during metamorphosis of the frog Xenopus. Dev Biol 251:91–104
Yamane H, Nishikawa A (2013) Differential muscle regulatory factor gene expression between larval and adult myogenesis in the frog Xenopus laevis: adult myogenic cell-specific myf5 upregulation and its relation to the notochord suppression of adult muscle differentiation. In Vitro Cell Dev Biol Anim 49:524–536
Yamane H, Ihara S, Kuroda M, Nishikawa A (2011) Adult-type myogenesis of the frog Xenopus laevis specifically suppressed by notochord cells but promoted by spinal cord cells in vitro. In Vitro Cell Dev Biol Anim 47:470–483
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Miyata, S., Yada, T., Ishikawa, N. et al. Insulin-like growth factor 1 regulation of proliferation and differentiation of Xenopus laevis myogenic cells in vitro. In Vitro Cell.Dev.Biol.-Animal 53, 231–247 (2017). https://doi.org/10.1007/s11626-016-0099-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-016-0099-9