Abstract
The aim of this study was to assess in vitro meiosis resumption and nuclear maturation of Rattus norvegicus oocytes after vitrification with different cryoprotective solutions. Cumulus-oocyte complexes (COCs) were exposed to an equilibration solution for 4 min placed in cryoprotective solutions for 1 min and vitrified in open pulled straws. Cryoprotective solutions were prepared with 15% ethylene glycol + 15% dimethyl sulfoxide + 0.5 M sucrose and different supplements, to form the following groups: G1, 20% fetal bovine serum in modified phosphate-buffered saline (mPBS); G2, 0.4% bovine serum albumine in mPBS; G3, 1% hyaluronic acid in mPBS; and G4, 0.4% polyvinyl alcohol in mPBS. Seven days after vitrification, the COCs from G1 to G4 were warmed and in vitro matured for 30 h along with the control group. Hoechst staining was performed to assess meiosis resumption and nuclear maturation rates. Control group showed higher meiosis resumption (77.88%) and nuclear maturation rates (55.75%) compared to all vitrified groups. Among the vitrified COCs, G3 showed the highest meiosis resumption and nuclear maturation rates (G1, 26.5 and 15.38%; G2, 22.12 and 11.54%; G3, 34.55 and 20%; G4, 20.17 and 9.24%). Supplementation of the vitrification solution with 1% hyaluronic acid provided better results, compared to the other supplements. Hyaluronic acid can be useful to vitrify rat COCs associated with other cryoprotectant agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitrification of embryos has been successfully applied in many species, including humans. However, oocyte cryopreservation still fails to meet expected results (Mahmoud, et al. 2010; Shahedi, et al. 2013; Wu, et al. 2013). This is mainly due to adverse effects that damage ooplasmic structures and are correlated with low meiosis resumption and nuclear maturation rates, as well as low fertilization and developmental rates (Mahmoud, et al. 2010; Shahedi, et al. 2013; Wu, et al. 2013). The main adverse effects caused by cryopreservation of female gametes include cortical granule exocytosis, leading to zona hardening; increased parthenogenetic activation; damage to cytoskeletal elements; and disruption of the meiotic spindle (Pornwiroon, et al. 2006).
The combination of different types of cryoprotectants, as well as supplementation with macromolecules such as bovine serum albumin (BSA) and fetal bovine serum (FBS), is often used in vitrification solutions to increase viscosity and glass transition temperature as well as to reduce toxicity (Saragusty and Arav 2011). Hyaluronic acid (HA) is a linear high molar mass glycosaminoglycan found in extracellular and pericellular matrix and soft connective tissues (Kogan, et al. 2007). In reproductive biotechnology, HA has been used as a supplement for embryo culture media in bovines (Furnus, et al. 1998; Franco and Hansen 2006; Block, et al. 2009) and as sperm HA binding selection before intracytoplasmic sperm injection in humans (Choe, et al. 2012; Parmegiani, et al. 2012). Also, HA production by cumulus cell has been correlated with cumulus expansion and oocyte nuclear maturation in bovines (Marei, et al. 2012). Due to the vast array of physiological properties and of viscoelastic characteristics of HA, this molecule can be potentially beneficial as a supplement for cryoprotective solutions.
In experimental embryology and assisted reproduction research, the mouse is commonly used as a model, while the rat (Rattus norvegicus) has been under-explored, although this model is consistently used in several other areas (Hedrich 2000). Despite the fact that rats and mice are phenotypically similar, it has been suggested that the rat genome more closely resembles the human than the mice genome (Downs 2011). Furthermore, significant ultrastructure and meiotic differences between mouse and rat oocytes have been reported, with special regard to polar body formation and energy requirements (Sotelo and Porter 1959; Weakley 1968; Zeilmaker and Verhamme 1974).
To the best of our knowledge, there are no experiments comparing different supplements added to vitrification solutions and their effects on in vitro maturation (IVM) of rat cumulus-oocyte complexes (COCs). Based on these facts, in our experiments, we assessed the rates of meiosis resumption and in vitro nuclear maturation of R. norvegicus COCs cryopreserved with vitrification solutions supplemented with FBS, BSA, HA, and polyvinyl alcohol (PVA).
Animals
Seventy-four female Wistar rats obtained from the Animal Facility of Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA, Porto Alegre, Brazil) were used for the experiments. All rats were housed in groups (five rats per cage) in polypropylene cages (38 × 32 × 16 cm) under standard conditions (mean temperature 22 ± 2°C, 50 ± 10% relative humidity, 12/12-h light-dark cycle) on wood shaving bedding (VetSul Ltda., Porto Alegre, Brazil). They were given a commercial pelletized diet (Nuvilab CR1, Nuvital Nutrientes S/A, Colombo, Brazil) and tap water ad libitum. All animal experimental protocols were performed with the approval of the Animal Care Committee of UFCSPA, through the approval 113/12. Furthermore, all the experiments were conducted according to the Brazilian law 11794/2008 for animal experiments.
Materials and Methods
Media and reagents.
All the reagents used in this study were purchased on Sigma-Aldrich (St. Louis, MO), unless otherwise indicated on the text. Modified phosphate-buffered saline (mPBS) (Whittingham 1975) was used for transportation of the ovaries and selection of COCs. Further manipulation and washing of the COCs were performed in M2 medium (Quinn, et al. 1982), and in vitro maturation was performed in M16 medium (Whittingham 1971).
Cumulus-oocyte complexes recovery.
Female rats, 4 to 6 wk old, were treated with intraperitoneal injection of 20 IU eCG (Folligon, Intervet, Boxmeer, Netherlands). After 48 h, they were killed by cervical displacement. Ovaries were then immediately removed from the abdominal cavity and maintained at 37°C in modified PBS supplemented with 1% (v/v) FBS, upon ovarian slicing. The ovaries were transferred to a plastic petri dish containing mPBS, and their cortexes were sliced using a scalpel blade to release the COCs into the medium. After this procedure, the dish containing the COCs was analyzed under stereomicroscope (Meiji EMZ 13TR, Meiji Techno Co, Saitama, Japan) for further morphological selection. This selection took into consideration the homogeneity of the ooplasm and the number of layers and degree of compaction of the surrounding cumulus cells. We classified the COCs in four main groups: (I) COCs that presented homogeneous ooplasm and various layers of surrounding cumulus cells which showed a high degree of compaction; (II) COCs with homogeneous ooplasm, showing fewer layers and less compacted cumulus cells; (III) COCs that might present more heterogeneous ooplasm, with some degree of granulation, very few or no layers of surrounding cumulus cells with no degree of compaction; and (IV) COCs with unusual shapes and very heterogeneous ooplasm, presenting no layers of surrounding cumulus cells. COCs classified as I and II were considered better suited for in vitro maturation and, thus, were selected and placed in a dish containing M2 medium.
Vitrification and warming.
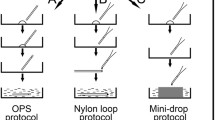
The selected COCs were randomly divided into five groups of 10 to 15 COCs. Groups 1 to 4 were exposed to the vitrification process, while group 5 was not vitrified (control group). Groups 1 to 4 were exposed to an equilibration solution composed of 7.5% ethylene glycol (EG) + 7.5% dimethyl sulfoxide (DMSO) + 0.4% BSA in mPBS for 4 min. After this period, the COCs were exposed to different vitrification solutions, according to the experimental groups: G1, cryoprotective solution (CS) composed of 15% EG + 15% DMSO+ 0.5 M sucrose supplemented with 20% FBS diluted in mPBS; G2, CS supplemented with 0.4% BSA diluted in mPBS; G3, CS supplemented with 1% HA (w/v; 10 mg/mL; Sigma 53747) diluted in mPBS; and G4, CS supplemented with 0.4% PVA diluted in mPBS. After 1 min of exposure to the vitrification solutions, the COCs from groups G1 to G4 were held at the tip of a short-diameter straw like an Open Pulled Straw (OPS) by capillarity and immediately plunged directly into liquid nitrogen. Five to ten COCs were stored per straw, which were kept in the liquid nitrogen container for 7 days before warming.
The doses of the cryoprotectants were decided based on previous studies using rats or other mammalian species. In our experiments, we used the concentration of 15% EG, 15% DMSO, and 0.5 M sucrose, since these doses were successfully applied for the vitrification of rat oocytes in a recent study by Fujiwara et al. (2010). The dose of FBS used in our experiments was 20% since this concentration is widely applied in most of the vitrification protocols in several species (Naitana et al. 1997; Asada et al. 2002; Fujiwara et al. 2010; Sanchez-Osorio et al. 2010). Similarly, the concentration of 0.4% BSA is also well established for most species (Joly et al. 1992; Sharma and Purohit 2008; Succu et al. 2011; El-Shahat and Hammam 2014). Regarding the concentration of PVA, most of the authors use concentrations varying between 0.1 and 0.5% for vitrification of embryos and oocytes (Naitana et al. 1997; Checura and Seidel 2007; Sanchez-Osorio et al. 2010; Villamil et al. 2011). When it comes to hyaluronic acid, to the best of our knowledge, there are only two studies that apply it as a supplement for cryoprotective solutions (Joly et al. 1992; Palasz et al. 1993). Both of these studies were performed in bovine embryos, and the authors applied slow freezing or deep freezing techniques instead of vitrification, which require lower concentrations of cryoprotectants. The authors have used 0.1% hyaluronic acid, whereas in our experiments, we decided to use 1% hyaluronic acid, since the vitrification technique requires higher concentrations of cryoprotectants in order to successfully achieve the vitreous state and to avoid the formation of intracellular ice crystals. Also, hyaluronic acid has high viscosity and the concentration of 1% was the maximum concentration that was suitable for adequate manipulation of the solution.
To produce the OPS, 0.25-mL straws were elongated using a warmed platform. The straws were warmed and pulled manually until the internal diameter and the thickness of the central wall decreased to half the initial size, so the diameter decreased from 1.7 mm to approximately 0.8 mm and the thickness decreased from 0.15 mm to approximately 0.07 mm (Vajta, et al. 1998).
For warming, the OPSs were removed from the liquid nitrogen container and kept in air for 5 s and the tip of the OPS capillary was placed in a 400-μL microdrop containing mPBS supplemented with 0.5 M sucrose. This procedure released the COCs in the medium. COCs were kept into the medium for 5 min before IVM. The G5 (control group) was maintained in M2 medium during vitrification and warming of the COCs, so all the groups were placed in maturation medium at the same time.
In vitro maturation and developmental competence assessment.
To perform IVM, COCs were initially removed from 0.5 M sucrose solution, washed three times in M2 medium, and transferred to microdrops containing 100 μL of maturation medium (M16 supplemented with 10% FBS, 0.1 IU LH, and 5 μg/mL FSH) covered by mineral oil. Ten to 15 COCs were placed in each microdrop, and the plates were maintained in an incubator with 5% CO2 atmosphere, 100% humidity, and 37°C for 30 h. At the end, COCs were placed in a 100-μL microdrop containing M2 supplemented with 0.1% hyaluronidase and incubated for 5 min to detach and remove cumulus cells. Cumulus cells were completely removed by gentle pipetting, using an elongated glass capillary.
Hoechst staining was performed by placing the oocytes for 3 min into a 100 μL microdrop containing M2 supplemented with 5.0 μg/mL Hoechst 33342. After incubation, the oocytes were observed in an inverted fluorescence microscope (Olympus IX-51-I, Olympus Co., Tokyo, Japan), in a dark room, to assess the disposal of genetic material. Nuclear maturation criterion was the extrusion of the first polar body which indicated the end of meiosis I, or further second metaphase plate formation (Alcoba, et al. 2013). Meiosis resumption was assumed when the oocyte presented germinal vesicle breakdown, metaphase plate, polar body, or both (Alcoba, et al. 2013).
Statistical analysis.
The influence of different supplementation (FBS, BSA, HA, and PVA) in the cryoprotective solution on COCs meiosis resumption and nuclear maturation rates was evaluated using the chi-square test, supplemented by adjusted residual calculation. Statistically significant differences were considered as p ≤ 0.05.
Results
The average age of the 74 female rats was 36.5 ± 8.08 (mean ± SD) days old. The experiments were repeated 18 times, until we obtained at least 100 COCs per group. Therefore, 563 COCs were used at the end of all experiments.
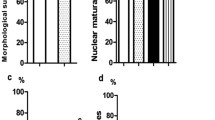
Regarding meiosis resumption, the control group presented statistically higher rates, compared to the other groups (77.88%, 88/113). Among the groups exposed to vitrification, G3 had statistically higher results compared to the other vitrified groups (34.55%, 38/110).
Also, the control group had statistically higher nuclear maturation rates, compared to the other groups (55.75%, 63/113). Again, among the groups exposed to vitrification, only G3 had statistically higher results, compared to the other vitrified groups (20.00%, 22/110).
We also compared the number of COCs that resumed meiosis with those that presented nuclear maturation, obtaining a maturation/resumption ratio. There were no statistically significant differences among any of the groups. The results of meiosis resumption and nuclear maturation rates, as well as maturation/resumption ratio, are summarized in Table 1.
We performed an observational evaluation regarding the expansion of COCs after in vitro maturation for both control and vitrified groups. The COCs from control group presented normal expansion of cumulus cells, and we could observe that in some cases, there was attachment of cumulus cells to the culture plate after maturation. However, COCs from the vitrified groups generally presented very few or no cumulus cells attached to the oocyte after maturation.
Discussion
The present study was the first to compare meiosis resumption and nuclear maturation of rat COCs after vitrification with the supplements FBS, BSA, HA, and PVA added to cryoprotective solutions. Our results showed that the COCs vitrified with 1% HA presented meiosis resumption and nuclear maturation rates after warming higher than the other groups vitrified with FBS, BSA, and PVA.
It is known that HA solutions have pronounced viscoelastic properties and that HA behaves as a stiffened random coil in solution, due to hydrogen bonding between adjacent saccharides, combined with some effect from the mutual electrostatic repulsion between carboxyl groups and chains self-association (Hardingham 2004). In the vitrification solution supplemented with HA, we observed high viscosity, which may be the main reason why this group showed better results, compared to the other vitrified groups. Three factors can affect the probability of successful vitrification: viscosity of the sample, cooling and warming rates, and sample volume (Yavin and Arav 2007). Therefore, increasing the viscosity or the cooling/warming rates, or decreasing the volume will each independently increase the probability of vitrification. However, trying to influence the properties of a vitrification solution by using very high concentrations of permeable cryoprotectants is not advisable, because they tend to have both toxic and hypertonic effects when used at concentrations that are effective for successful vitrification; therefore, it is crucial to restrict cryoprotectant concentration to a minimum (Yavin and Arav 2007).In our experiments, we observed that HA was the main factor responsible for the higher viscosity of the medium. Thus, this probably led to high vitrification rates and minimum toxic effects, since HA is a natural molecule, widely distributed in extracellular matrix (Hardingham 2004).
Some authors have used HA to cryopreserve different types of cells, and obtained promising results (Pena, et al. 2004; Bucak, et al. 2007; Ujihira, et al. 2010; Turner, et al. 2012). Pena et al. (2004) have used different concentrations of HA to cryopreserve boar sperm and assessed sperm motility and lipid membrane architecture after cryopreservation. They concluded that, compared to controls, sperm samples supplemented with HA to 500 and 1000 mg/mL showed dose-dependent increases in post-thaw motility and increases in linear motility (Pena, et al. 2004). Ujihira et al. (2010) assessed the availability of low molecular weight HA at concentrations of 0, 0.5, and 5% as a protectant for human dermal fibroblast freezing and found that cell survival increased with HA concentration. Moreover, the membrane integrity of the cells after freeze-thaw increased with higher concentrations of low molecular weight HA (Ujihira, et al. 2010). Similarly, Turner, et al. (2012) evaluated different isotonic media with and without the presence of HAs as a cryoprotective agent and observed improved survival of human endodermal stem cells and progenitors after cryopreservation. Cells frozen in media supplemented with 0.05 or 0.1% hyaluronan exhibited the best viabilities after thawing.
In our experiment, the control group (not exposed to vitrification) presented statistically higher meiosis resumption and nuclear maturation rates, compared to the vitrified groups. Other authors observed similar results. Mahmoud et al (2010) compared the application of different combinations of cryoprotectants during vitrification of immature buffalo COCs and found that the percentage of oocytes that reached telophase and metaphase II was lower in cryopreserved groups, compared to the control group. Diez et al. (2012) suggest that the low maturation rates found after most of the vitrification protocols are probably related to a compromised developmental competence of the oocytes and functional and morphological damages caused by the process of vitrification.
Currently, most solutions used for oocyte vitrification contain a macromolecule plus FBS or BSA, or simply one of them as the macromolecular component (Checura and Seidel 2007). The advantage of using FBS as a supplement for vitrification solutions is that this component prevents the conversion of the zona pellucida proteins to a state known as zona hardening, although it does not prevent premature release of cortical granules (Checura and Seidel 2007). Succu, et al(2011) reported that replacement of FBS with BSA during sheep mature oocyte cryopreservation, warming, and rehydration has a negative influence on subsequent developmental competence. Similarly, Tan, et al. (2009) reported that decrease of FBS concentration from 15 and 20% to 5 and 10% in mouse mature oocyte vitrification medium also decreased developmental competence after warming. In our experiments, the group exposed to vitrification with cryoprotective solution containing 20% FBS did not show significant differences in meiosis resumption and nuclear maturation rates, compared to the other vitrified groups. We hypothesize that the discrepancies found between our results and those from other authors may be explained by two main factors: (1) There are morphological and metabolic differences between the rat oocyte and those from other species used in those studies (Sotelo and Porter 1959; Weakley 1968; Zeilmaker and Verhamme 1974; Boja, et al. 2005), and (2) structural and functional differences between mature and immature oocytes cryopreserved with or without cumulus cells.
The addition of macromolecules to cryoprotective solutions is commonly used in most vitrification protocols in several species; however, there are no studies comparing these additives on vitrification of R. norvegicus immature COCs. Many cryoprotective solutions were tested in other species (Mingoti, et al. 2011), although structural and metabolic differences between the rat oocytes and those from other species were reported by several authors (Downs 2011; Sotelo and Porter 1959; Weakley 1968; Zeilmaker and Verhamme 1974; Boja, et al. 2005). Weakley (1968) analyzed ultrastructural characteristics of oocytes from hamsters, rats, mice, and guinea pig and reported differences in the rat oocyte, such as more clearly delineated lamellas, associated with ribosomes and mitochondria; no concentric endoplasmic reticulum, and presence of cisternae that encircle masses of ribosomes; and round membrane-bound bodies. Moreover, the composition of rat zona pellucida is different from that of other species, mainly the mouse (Boja, et al. 2005). While mouse zona pellucida is composed of only three glycoproteins (ZP1, ZP2, and ZP3), the rat zona pellucida contains three homologous proteins (ZP1–ZP3) and a fourth glycoprotein (ZP4/ZPB), similarly to human zona (Boja, et al. 2005). Furthermore, the way in which polar body is formed and metabolic requirements for its formation are different in the rat and mouse oocytes (Zeilmaker and Verhamme 1974). Downs (2011) reported several differences in maintenance of meiotic arrest and responsiveness to hormones and other agents between rat and mouse oocytes. In arrested mouse oocytes, raising pyruvate concentration promoted higher maturation rates, whereas glucose had an opposite, inhibitory influence when added to the medium (Downs 2011). On the other hand, identical manipulation produced opposite responses in rat oocytes (Downs 2011). These differences define the importance of studying the rat as a model in reproductive biotechnology research and indicate that results obtained with other species cannot be applied to the rat, without in-depth investigation.
Bovine serum albumin has been used in several cryopreservation solutions, due to its apparent ability to prevent changes in zona pellucida, similarly to FBS (Carroll, et al. 1993). However, this component has the same disadvantages as FBS, such as variations between different batches and preparations and risk of carrying infectious agents, due to its animal origin (Carroll, et al. 1993). In our experiments, we did not obtain expressive results using 0.4% BSA as an additive to cryopreservation solutions. Our results showed that there were no significant differences in meiosis resumption and nuclear maturation rates of this group, compared to the other vitrified groups. Several authors used concentrations of BSA ranging from 0.4 to 6% (Carroll, et al. 1993; Dinnyes, et al. 1999; Dobrinsky, et al. 2000) for vitrification of mouse, bovine, and pig oocytes and embryos and obtained different results. Vitrification solutions containing 3 or 6% fatty-acid-free BSA (FAF-BSA) to cryopreserve bovine oocyte showed very low rates of blastocyst when compared to other macromolecules, such as FBS (Checura and Seidel 2007). However, lower concentrations of BSA, such as 0.3%, provided better results, compared to the high concentrations (Checura and Seidel 2007). Similarly, cryopreservation of mouse oocytes with cryoprotective solutions supplemented with crystalline or Fraction V BSA provided lower fertilization rates than that of oocytes cryopreserved in solutions containing FBS (Carroll, et al. 1993). One possible explanation for these results is that an unknown interaction between BSA and other cryoprotectant, or the existence of toxic effects related to residues left during the process of purification of the BSA may be acting as well (Checura and Seidel 2007). Yet, more research is needed to determine the reasons for these controversial results obtained for different types of BSA (Checura and Seidel 2007).
In order to produce chemically defined medium, PVA and polyvinylpirrollidone (PVP) have been used as substitutes for serum in bovine oocytes and embryos culture media (Pugh, et al. 2000; Asada, et al. 2002), but PVA has lower toxicity than PVP (Fuku, et al. 1995; Asada, et al. 2002). Polyvinyl alcohol is reported to protect cell membrane during cryopreservation and, in blastocysts from domestic animals, it has been used as a substitute for serum in vitrification and warming solutions (Vajta, et al. 1998; Pugh, et al. 2000; Asada, et al. 2002). Checura and Seidel (2007) reported that 0.3% PVA supplementation to vitrification solution was inappropriate for vitrification of bovine oocytes, although the authors have argued that the problem could be an insufficient concentration of the macromolecule, because the results with 1% PVA were the highest in the experiment and did not differ from those of non-vitrified oocytes. Our results show that addition of 0.4% PVA to cryoprotective solution did not provide significant differences in meiosis resumption and nuclear maturation rates from the groups vitrified with FBS and BSA. This is an interesting result, since PVA is a synthetic molecule and still provided comparable results to FBS and BSA supplementation. Similarly, Asada, et al. (2002) reported no significant differences in morula stage and blastocyst development after bovine oocyte vitrification with cryoprotective solution supplemented with 0.1% PVA or 20% FBS (Asada, et al. 2002).Hence, the authors reported that PVA could substitute FBS in vitrification solution for bovine oocyte (Asada, et al. 2002). However, more studies are necessary to elucidate the efficiency of PVA as a substitute for FBS and BSA in rat oocytes.
In order to evaluate if the maturation medium used in our experiments was responsible for the lower maturation rates between the groups, we performed the nuclear maturation/resumption of meiosis ratio as an indication that morphological damages caused by vitrification are probably the main cause for the lower rates of maturation. Our results showed that there were no statistical differences in this parameter between the experimental groups, indicating that, probably, the maturation conditions provided to the COCs did not influence the differences obtained among the experimental groups. Therefore, it is possible to deduce that the morphological and functional alterations in oocytes that led to decreased maturation rates were caused by other factors, such as vitrification, not by the maturation medium used.
In our experiments, we evaluated the rate of degeneration among the COCs exposed to vitrification and the control group. The control group presented statistically lower rates of degeneration when compared to the vitrified groups, although, among the vitrified groups, there were no statistically significant differences. The degeneration rates of oocytes after vitrification found on the literature are highly variable due, mainly, to differences between species, the stage of maturation of the oocytes, and the vitrification protocol used. In rat mature oocytes, Fujiwara et al. (2010) achieved 10.1% degeneration rate in their best protocol. However, most of their protocols provided degeneration rates as high as 40%, which was very similar to our results. Similarly, Cocchia et al. (2010) evaluated the viability of immature cat oocytes after vitrification using OPS and found that 36% of the oocytes were not viable after vitrification. With this in mind, we can say that although we had significantly higher degeneration rates among the groups exposed to vitrification compared to the control group, our results were similar to those found on the literature when it comes to vitrification of oocytes.
One way to evaluate influences of experimental conditions on the viability of oocytes is determining their ability to resume meiosis and reach nuclear maturation. Maturation of the cumulus-oocyte complex is one of the most important factors determining the progress to metaphase II followed by fertilization and embryo development (Kempisty, et al. 2011). It has been suggested that maturation of the COC is the most important and critical stage for future embryo growth (Kempisty, et al. 2011). Some authors have used nuclear maturation rate as a marker of oocyte competence with no subsequent evaluation of embryo development, such as Mahmoud et al. (2010) who vitrified immature buffalo oocytes with different combinations of cryoprotectants and evaluated the effects of these conditions by in vitro maturation assessment. However, the authors did not perform subsequent embryo development. Besides, several other studies have evaluated the effects of oocyte cryopreservation methods by assessing nuclear maturation and meiosis resumption rates with no subsequent embryo development, obtaining equally important results (Mohsenzadeh, et al. 2012; Rao, et al. 2012; Yazdanpanah, et al. 2013).
It is well known that cumulus cells play important roles on oocyte maturation, fertilization, and subsequent embryo development, mainly due to communications between oocytes and cumulus cells through gap junctions (Li and Albertini 2013). Thus, the impairment of cell communication between oocyte and cumulus cells may cause a negative impact on paracrine activity and disturbance to biophysical properties of the oocytes cortex, leading to decreased ability to use and store factors required for later development (Li and Albertini 2013). With this in mind, it is very likely that one of the main reasons why COCs exposed to vitrification presented significantly lower meiosis resumption and nuclear maturation rates is the loss of communication between cumulus cells and oocyte.
Considering the results obtained in our experiments, it is possible to conclude that vitrification of R. norvegicus cumulus-oocyte complexes using the tested vitrification solution supplemented with 1% HA provides better meiosis resumption and nuclear maturation rates after warming, when compared to 20% FBS, 0.4% BSA, and 0.4% PVA supplementation. Besides, the addition of 0.4% PVA to the vitrification solution provided similar meiosis resumption and nuclear maturation rates that 20% FBS and 0.4% BSA supplementation.
As stated above, the laboratory rat is widely applied as an animal model in several research areas, allowing the development of biotechnologies and providing essential scientific data. However, it has been increasingly accepted that studies should be focused on the refinement of techniques, as well as the reduction and replacement of these animals, in order to preserve their welfare and allow ethical scientific development. With this in mind, vitrification of immature COCs can be used for maintaining the germplasm of this species, allowing long-term storage for further use in experimentation. Also, cryopreservation of COCs may lead to reduction of the number of animals killed, since there would be more control over the number of offspring generated for experimentation.
Hence, our study was able to demonstrate that the use of hyaluronic acid as a supplement for cryoprotective solutions provided better meiosis resumption and nuclear maturation rates of R. norvegicus COCs in comparison to FBS, BSA, and PVA. Although more studies are necessary to determine ideal conditions for vitrification of rat COCs, our data were essential in order to establish initial insights regarding cryopreservation of rat COCs.
References
Alcoba DD, da Rosa Braga BL, Sandi-Monroy NL, Proenca LA, Felix Lopes RF, de Oliveira AT (2013) Selection of Rattus norvegicus oocytes for in vitro maturation by brilliant cresyl blue staining. Zygote 21:238–245
Asada M, Ishibashi S, Ikumi S, Fukui Y (2002) Effect of polyvinyl alcohol (PVA) concentration during vitrification of in vitro matured bovine oocytes. Theriogenology 58:1199–1208
Block J, Bonilla L, Hansen PJ (2009) Effect of addition of hyaluronan to embryo culture medium on survival of bovine embryos in vitro following vitrification and establishment of pregnancy after transfer to recipients. Theriogenology 71:1063–1071
Boja ES, Hoodbhoy T, Garfield M, Fales HM (2005) Structural conservation of mouse and rat zona pellucida glycoproteins Probing the native rat zona pellucida proteome by mass spectrometry. Biochemistry 44:16445–16460
Bucak MN, Atessahin A, Varisli O, Yuce A, Tekin N, Akcay A (2007) The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen Microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology 67:1060–1067
Carroll J, Wood MJ, Whittingham DG (1993) Normal fertilization and development of frozen-thawed mouse oocytes: protective action of certain macromolecules. Biol Reprod 48:606–612
Checura CM, Seidel GE Jr (2007) Effect of macromolecules in solutions for vitrification of mature bovine oocytes. Theriogenology 67:919–930
Choe SA, Tae JC, Shin MY, Kim HJ, Kim CH, Lee JY, Hwang D, Kim KC, Suh CS, Jee BC (2012) Application of sperm selection using hyaluronic acid binding in intracytoplasmic sperm injection cycles: a sibling oocyte study. J Korean Med Sci 27:1569–1573
Cocchia N, Ciani F, Russo M, El Rass R, Rosapane I, Avallone L, Tortora G, Lorizio R (2010) Immature cat oocyte vitrification in open pulled straws (OPSs) using cryoprotectant mixture. Cryobiology 60(2):229–234
Diez C, Munoz M, Caamano JN, Gomez E (2012) Cryopreservation of the bovine oocyte: current status and perspectives. Reprod Domest Anim 47(Suppl 3):76–83
Dinnyes A, Lonergan P, Fair T, Boland MP, Yang X (1999) Timing of the first cleavage post-insemination affects cryosurvival of in vitro-produced bovine blastocysts. Mol Reprod Dev 53:318–324
Dobrinsky JR, Pursel VG, Long CR, Johnson LA (2000) Birth of piglets after transfer of embryos cryopreserved by cytoskeletal stabilization and vitrification. Biol Reprod 62:564–570
Downs SM (2011) Mouse versus rat: Profound differences in meiotic regulation at the level of the isolated oocyte. Mol Reprod Dev 78:778–794
El-Shahat KH, Hammam AM (2014) Effect of different types of cryoprotectants on developmental capacity of vitrified-thawed immature buffalo oocytes. Anim Reprod 11(4):543–548
Franco M, Hansen PJ (2006) Effects of hyaluronic acid in culture and cytochalasin B treatment before freezing on survival of cryopreserved bovine embryos produced in vitro. In Vitro Cell Dev Biol Anim 42:40–44
Fujiwara K, Sano D, Seita Y, Inomata T, Ito J, Kashiwazaki N (2010) Ethylene glycol-supplemented calcium-free media improve zona penetration of vitrified rat oocytes by sperm cells. J Reprod Dev 56:169–175
Fuku EJ, Liu J, Downey BR (1995) In vitro viability and ultrastructural changes in bovine oocytes treated with a vitrification solution. Mol Reprod Dev 40:177–185
Furnus CC, de Matos DG, Martinez AG (1998) Effect of hyaluronic acid on development of in vitro produced bovine embryos. Theriogenology 49:1489–1499
Hardingham T (2004) Chemistry and biology of hyaluronan, 1st edn. Elsevier, Oxford
Hedrich HD (2000) History, strains and models. In: Krinke GJ (ed) The laboratory Rat. Academic Press, San Diego, pp 3–16
Joly T, Nibart M, Thibier M (1992) Hyaluronic acid as a substitute for proteins in the deep-freezing of embryos from mice and sheep an in vitro investigation. Theriogenology 37(2):473–480
Kempisty B, Bukowska D, Piotrowska H, Zawierucha P, Sniadek P, Walczak R, Dziuban J, Antosik P, Jaskowski J, Brussow KP, Nowicki M, Zabel M (2011) Selected molecular and microfluidic aspects of mammalian oocyte maturation-perspectives: a review. Vet Med 56:367–378
Kogan G, Soltes L, Stern R, Gemeiner P (2007) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29:17–25
Li R, Albertini DF (2013) The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol 14(3):141–152
Mahmoud KG, Scholkamy TH, Ahmed YF, Seidel GE Jr, Nawito MF (2010) Effect of different combinations of cryoprotectants on in vitro maturation of immature buffalo (Bubalus bubalis) oocytes vitrified by straw and open-pulled straw methods. Reprod Domest Anim 45:565–571
Marei WF, Ghafari F, Fouladi-Nashta AA (2012) Role of hyaluronic acid in maturation and further early embryo development of bovine oocytes. Theriogenology 78:670–677
Mingoti GZ, Castro VS, Meo SC, Sa Barretto LS, Garcia JM (2011) The effects of macromolecular and serum supplements and oxygen tension during bovine in vitro procedures on kinetics of oocyte maturation and embryo development. In Vitro Cell Dev Biol Anim 47:361–367
Mohsenzadeh M, Khalili MA, Nazari S, Jahromi VH, Agharahimi A, Halvaei I (2012) Effect of vitrification on morphology and in-vitro maturation outcome of human immature oocytes. Ital J Anat Embryol 117:190–198
Naitana S, Ledda S, Loi P, Leoni G, Bogliolo L, Dattena M et al (1997) Polyvinyl alcohol as a defined substitute for serum in vitrification and warming solutions to cryopreserve ovine embryos at different stages of development.Animal. Reprod Sci 48(2-4):247–256
Palasz A, Alkemade S, Mapletoft J (1993) The use of sodium hyaluronate in freezing media for bovine and murine embryos. Cryobiology 30:172–178
Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Taraborrelli S, Arnone A, Maccarini AM, Filicori M (2012) Comparison of two ready-to-use systems designed for sperm-hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs. Sperm Slow: a prospective, randomized trial. Fertil Steril 98:632–637
Pena FJ, Johannisson A, Wallgren M, Rodriguez-Martinez H (2004) Effect of hyaluronan supplementation on boar sperm motility and membrane lipid architecture status after cryopreservation. Theriogenology 61:63–70
Pornwiroon S, Kunathikom S, Makemaharn O, Huanaraj R (2006) Vitrification of mouse oocyte using open pulled straws compared with needles. J Med Assoc Thai 89:2015–2020
Pugh PA, Tervit HR, Niemann H (2000) Effects of vitrification medium composition on the survival of bovine in vitro produced embryos, following in straw-dilution, in vitro and in vivo following transfer. Anim Reprod Sci 58:9–22
Quinn P, Barros C, Whittingham DG (1982) Preservation of hamster oocytes to assay the fertilizing capacity of human spermatozoa. J Reprod Fertil 66:161–168
Rao BS, Mahesh YU, Charan KV, Suman K, Sekhar N, Shivaji S (2012) Effect of vitrification on meiotic maturation and expression of genes in immature goat cumulus oocyte complexes. Cryobiology 64:176–184
Sanchez-Osorio J, Cuello C, Gil MA, Parrilla I, Maside C, Alminana C et al (2010) Vitrification and warming of in vivo-derived porcine embryos in a chemically defined medium. Theriogenology 73(3):300–308
Saragusty J, Arav A (2011) Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 141:1–19
Shahedi A, Hosseini A, Khalili MA, Norouzian M, Salehi M, Piriaei A, Nottola SA (2013) The effect of vitrification on ultrastructure of human in vitro matured germinal vesicle oocytes. Eur J Obstet Gynecol Reprod Biol 167:69–75
Sharma A, Purohit GN (2008) Vitrification of immature bubaline cumulus oocyte complexes by the open-pulled straw and conventional straw methods and their subsequent in vitro fertilization. Vet Med 53(8):427–433
Sotelo JR, Porter KR (1959) An electron microscope study of the rat ovum. J Biophys Biochem Cytol 5:327–342
Succu S, Berlinguer F, Leoni GG, Bebbere D, Satta V, Marco-Jimenez F, Pasciu V, Naitana S (2011) Calcium concentration in vitrification medium affects the developmental competence of in vitro matured ovine oocytes. Theriogenology 75:715–721
Tan X, Song E, Liu X, You W, Wan F (2009) Factors affecting the survival, fertilization, and embryonic development of mouse oocytes after vitrification using glass capillaries. In Vitro Cell Dev Biol Anim 45:420–429
Turner RA, Mendel G, Wauthier E, Barbier C, Reid LM (2012) Hyaluronan-supplemented buffers preserve adhesion mechanisms facilitating cryopreservation of human hepatic stem/progenitor cells. Cell Transplant 21:2257–2266
Ujihira M, Iwama A, Aoki M, Aoki K, Omaki S, Goto E, Mabuchi K (2010) Cryoprotective effect of low-molecular-weight hyaluronan on human dermal fibroblast monolayers. Cryo Letters 31:101–111
Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, Callesen H (1998) Open Pulled Straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev 51:53–58
Villamil PR, Ongaratto FL, da Silva DS, Rodrigues BA, Rodrigues JL (2011) Survival rates of mouse blastocyst vitrified in dimethylformamide based solutions associated with ethylene glycol or 1–2 propanediol. Ciênca Rural 41(11):1985–1990
Weakley BS (1968) Comparison of cytoplasmic lamellae and membranous elements in the oocytes of five mammalian species. Z Zellforsch Mikrosk Anat 85:109–123
Whittingham DG (1971) Culture of mouse ova. J Reprod Fertil Suppl 14:7–21
Whittingham DG (1975) Survival of rat embryos after freezing and thawing. J Reprod Fertil 43:575–578
Wu G, Jia B, Mo X, Liu C, Fu X, Zhu S, Hou Y (2013) Nuclear maturation and embryo development of porcine oocytes vitrified by cryotop: effect of different stages of in vitro maturation. Cryobiology 67:95–101
Yavin S, Arav A (2007) Measurement of essential physical properties of vitrification solutions. Theriogenology 67:81–89
Yazdanpanah F, Khalili MA, Eftekhar M, Karimi H (2013) The effect of vitrification on maturation and viability capacities of immature human oocytes. Arch Gynecol Obstet 288:439–444
Zeilmaker GH, Verhamme CM (1974) Observations on rat oocyte maturation in vitro: morphology and energy requirements. Biol Reprod 11:145–152
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Paim, L.M.G., Gal, L.L., Lopes, R.F.F. et al. Vitrification of Rattus norvegicus immature cumulus-oocyte complexes using hyaluronic acid. In Vitro Cell.Dev.Biol.-Animal 51, 995–1002 (2015). https://doi.org/10.1007/s11626-015-9940-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9940-9