Abstract
The aim of this study was to develop adequate in vitro conditions for the differentiation of bovine skeletal muscle cells. Therefore, satellite cells isolated from the left foreleg of a Holstein-Friesian fetus at 4.5 mo of gestation were seeded on 24-well plates coated with extracellular matrix gel. Cells were cultured for 5 d in growth medium containing 10% fetal bovine serum. After reaching confluence, several differentiation media were tested for inducing myotube formation. The highest fusion rate of approximately 30% was achieved with a serum-free medium containing 1 μM dexamethasone, 1 μg/ml linoleic acid, and 0.1 μM insulin after a differentiation phase of 72 h. Two different culture conditions (serum-free and serum-containing) appropriate for bovine skeletal muscle cell differentiation are described in detail which allow the investigation of bovine skeletal muscle cell proliferation and differentiation in general as well as in response to bioactive compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Typically, cell lines from mouse (C2C12) or rat (L6) are used for investigating the growth and differentiation of skeletal muscle. However, although primary cultures derived from skeletal muscles are often mixed populations of muscle cells and non-myogenic cells such as adipocytes, immune cells (e.g., macrophages), and particularly fibroblasts (Baquero-Perez et al. 2012), they are more suitable to describe developmental processes in farm animals as they represent a model that is closer to the in vivo situation. Insights into skeletal muscle growth and differentiation were gained from studies on murine and avian myoblast cultures (Hembree et al. 1991). Concerning meat-producing animals, a number of different protocols for the cultivation of myogenic cells derived from sheep or cattle (Dodson et al. 1987; Roe et al. 1989; Johnson et al. 1998) and pig (Mau et al. 2008) exist. Bovine myogenic satellite cells were firstly isolated and cultured by Dodson et al. (1987). In this study, different factors influencing myotube formation, e.g., serum type and coating of cell culture ware, were investigated to optimize the differentiation of bovine myoblasts. Up to now, most of the studies employing bovine cell culture models use serum-containing media to induce the differentiation of myoblasts to multinucleated myotubes (Cassar-Malek et al. 1999; Kamanga-Sollo et al. 2004; Kook et al. 2006; Montoya-Flores et al. 2011; Ge et al. 2012; Lapin et al. 2013; Ronning et al. 2013; Van Ba and Inho 2013; Lee et al. 2014) instead of defined serum-free culture conditions (Muroya et al. 2005). Unfortunately, the detailed composition of the serum-free medium used in the study of Muroya et al. (2005) was not described. Ronning et al. (2013) showed that a special coating with a combination of glycosaminoglycans and fibrous proteins improves early differentiation of bovine primary skeletal muscle cells. Cassar-Malek et al. (1999) investigated the insulin- and T3-regulated proliferation and differentiation of bovine satellite cells. The existing in vitro models for bovine skeletal muscle cells are difficult to compare as they differ in muscle type, breed, and age of the animals used for satellite cell isolation.
Since the components present in serum are not well characterized, this study was undertaken to find a better defined medium and optimized cell culture conditions for bovine primary myoblast cultures. We developed and present here a protocol which is partly based on a protocol established for the cultivation of proliferating and differentiating porcine myoblasts (Mau et al. 2008).
Bovine satellite cells were obtained from skeletal muscle tissue of the left foreleg of a Holstein-Friesian fetus at 4.5 mo of gestation. Animal husbandry and slaughter followed the guidelines set by the Animal Care Committee of the State Mecklenburg-Western Pomerania, Germany, based on the German Law of Animal Protection. Due to the little amount of tissue (9.74 g), no specific muscle was taken. The tissue was minced with scissors in phosphate-buffered saline (PBS; 144 mM NaCl, 5.4 mM KCl, 25 mM glucose, 14 mM sucrose, 5 mM Na2HPO4, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 1 μg/ml phenol red, adjusted to pH 7.4 at 22°C). Cells were dissociated by digestion with 0.3% trypsin (Invitrogen, Karlsruhe, Germany) in PBS for 1 h at 37°C with continuous shaking in a water bath. Digestion process was stopped by 20% fetal bovine serum (FBS; Invitrogen). Then, the cell suspension was filtered through three layers of sterile nylon mesh (2 × 63 μm, 1 × 20 μm pore size), diluted 1:1 in PBS, and centrifuged at 250×g for 10 min at 4°C. The resulting cell pellet was resuspended in PBS, and cell number was determined with a Neubauer counting chamber after trypan blue staining. After another centrifugation step, cells were again resuspended and seeded at approximately 105 cells/cm2 on 100-mm Primaria plastic petri dishes (Falcon, Becton Dickinson, Heidelberg, Germany) in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 0.02 M glutamine (Serva, Heidelberg, Germany), 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (FBS). Cell incubation was performed at 37°C under a humidified atmosphere of 6% CO2 in air. After 24 h, cells were washed once with PBS, and new medium (DMEM + 10% FBS) was added. After 48 h, cell monolayers were harvested using a trypsin/EDTA solution (0.05%/0.02%, Roth, Karlsruhe, Germany) in PBS. After cell counting, 2 ml aliquots with 1.08 × 106 cells/ml were frozen in liquid nitrogen using DMEM containing 10% dimethyl sulfoxide (DMSO) and 20% FBS until required for the experiments.
FormalPara Cultivation of Differentiating Bovine MyoblastsCell incubation was performed at 37°C under a humidified atmosphere of 6% CO2 (CO2 incubator: Binder GmbH, Tuttlingen, Germany). Cells were thawed and seeded in extracellular matrix (ECM)-gel coated (Sigma-Aldrich, Schnelldorf, Germany) 24-well plates at a density of 5 × 103 cells/well and cultivated in growth medium 1 (GM1; DMEM/F12 PAN-Biotech, Aidenbach, Germany) supplemented with 0.02 M glutamine (Serva), 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% FBS for 5 d. Medium was changed every other day except for the first 48 h. At reaching confluence, several differentiation media were used to induce myotube formation: (1) serum-free differentiation medium (SFDM) supplemented according to Doumit et al. (1996); (2) serum-free medium consisting of DMEM/F12 supplemented with 1 μM dexamethasone, 1 μg/ml linoleic acid, and 0.1 μM insulin (DLI medium) adapted from a protocol of Allen et al. (1985); and (3) DMEM/F12 supplemented with 2% FBS, 1 μM insulin, and 1 μM cytosine arabinoside (DM). The detailed media compositions are presented in Fig. 1. When using SFDM, cells were incubated with GM1 only for 4 d followed by treatment with growth medium 2 (GM2; DMEM/F12 + 10% FBS + 1 μM insulin) for 24 h to prepare the cells for differentiation. Cultivation in differentiation media occurred for 48, 72, or 96 h. In Fig. 2, the differentiation process of bovine myoblasts to multinucleated myotubes is shown. To determine the degree of differentiation, differentiated cells were stained for desmin and DNA according to a protocol described for porcine muscle cells by Mau et al. (2008), which itself represents a modification of a protocol given by Yablonka-Reuveni et al. (1999). At the end of the experiments, culture medium was removed and cells were washed once with pre-warmed PBS before being fixed in 100% methanol for 10 min at −20°C and stored in PBS at 4°C until staining. Then, cells were incubated in sterile Tris-buffered saline (TBS)-goat serum (GS) for 1 h at room temperature (RT) to block nonspecific binding of the secondary antibody. Thereafter, cells were washed twice with TBS-Tw20 before being incubated with the primary antibody overnight at 4°C. The mouse monoclonal antibody against desmin from pig stomach (mAb DE-U-10, ascites fluid; Sigma-Aldrich) was diluted 1:100 in TBS-GS. Negative control was represented by cells not incubated with the primary antibody. Next day, cells were washed four times with TBS-Tw20 and incubated for 1 h with the secondary antibody (goat anti-mouse ALEXA 488; Invitrogen) diluted 1:500 in TBS-GS. After that, cells were washed four times with TBS-Tw20 followed by nuclei staining with Hoechst 33258 (Sigma-Aldrich) in PBS (1 μg/ml) for 5 min at RT. After a final washing step with TBS-Tw20, cells were stored in PBS until microscopic evaluation. Cells were analyzed using the blue and UV excitation and green and blue emission filters, respectively, of the Leica DM 24000 fluorescence microscope (Leica Microsystems, Wetzlar, Germany). In 5–8 visual fields (0.84 mm2), the number of myotubes, the number of nuclei within myotubes, and the total number of nuclei per well were counted using the QWin software (Leica Microsystems). Degree of differentiation (fusion percentage) was determined as the number of myotube nuclei in relation to the total number of nuclei. The highest fusion percentage of 30.92 ± 3.43% was reached after 72 h incubation in DLI medium (Table 1, Fig. 3). However, all used differentiation media were suitable to induce myotube formation of bovine skeletal muscle cells. The degree of differentiation of bovine myotubes cultured in SFDM (19.90 ± 4.43%) or DM (22.39 ± 11.72%) was almost the same.
Media compositions and design of experiments for the differentiation of primary bovine myoblasts. GM1/GM2 growth medium 1/growth medium 2, SFDM serum-free differentiation medium, DLI differentiation medium with dexamethasone, linoleic acid, and insulin, DM differentiation medium, FBS fetal bovine serum, BSA bovine serum albumin.
Phase-contrast images of bovine myoblasts differentiating to multinucleated myotubes. Proliferating myoblasts cultured in DMEM/F12 + 10% FBS on (a) day 2 and (b) day 4. Short myotubes are visible (c) after 24 h in serum-free differentiation medium (SFDM). Myotube formation progresses on (d) day 2 of differentiation. Numerous and thicker myotubes are apparent (e) after 72 h.
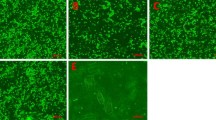
Fluorescence image of bovine myoblast cultures differentiated for 72 h in (a, b) serum-free differentiation medium (SFDM), (c, d) DLI (1 μM dexamethasone, 1 μg/ml linoleic acid, 0.1 μM insulin), or (e, f) differentiation medium with 2% FBS, 1 μM insulin, and 1 μM cytosine arabinoside (DM). (a, c, e) Detection of desmin using a mouse monoclonal antibody against desmin from pig stomach and a secondary antibody goat anti-mouse ALEXA 488. (b, d, f) Merged image of desmin (green) and nuclear staining with Hoechst 33258. Note that multinucleated myotubes are desmin positive.
The described culture system provides a good in vitro model for studying differentiation processes of bovine skeletal muscle cells. Furthermore, it is beneficial to investigate the response of proliferating and differentiating cultures to various bioactive compounds under defined conditions using serum-free medium with a known composition. In former studies, we observed that the effects of compounds like adipokines on the proliferation of primary porcine skeletal muscle cells largely depend on the present culture conditions, as serum components can interact with the tested compound (Will et al. 2012, 2013). If the experimental setup does not require strictly serum-free conditions, these can be modified and replaced by serum contents up to 5% FBS. With 2% FBS in the medium, a differentiation degree of about 20% still can be achieved (Table 1, Fig. 3).
References
Allen RE, Dodson MV, Luiten LS, Boxhorn LK (1985) A serum-free medium that supports the growth of cultured skeletal muscle satellite cells. In Vitro Cell Dev Biol 21:636–640
Baquero-Perez B, Kuchipudi SV, Nelli RK, Chang KC (2012) A simplified but robust method for the isolation of avian and mammalian muscle satellite cells. BMC Cell Biol 13:16. doi:10.1186/1471-2121-13-16
Cassar-Malek I, Langlois N, Picard B, Geay Y (1999) Regulation of bovine satellite cell proliferation and differentiation by insulin and triiodothyronine. Domest Anim Endocrinol 17:373–388
Dodson MV, Martin EL, Brannon MA, Mathison BA, McFarland DC (1987) Optimization of bovine satellite-derived myotube formation in vitro. Tissue Cell 19:159–166
Doumit ME, Cook DR, Merkel RA (1996) Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology 137:1385–1394
Ge X, Yu J, Jiang H (2012) Growth hormone stimulates protein synthesis in bovine skeletal muscle cells without altering insulin-like growth factor-I mRNA expression. J Anim Sci 90:1126–1133
Hembree JR, Hathaway MR, Dayton WR (1991) Isolation and culture of fetal porcine myogenic cells and the effect of insulin, IGF-I, and sera on protein turnover in porcine myotube cultures. J Anim Sci 69:3241–3250
Johnson BJ, Halstead N, White ME, Hathaway MR, DiCostanzo A, Dayton WR (1998) Activation state of muscle satellite cells isolated from steers implanted with a combined trenbolone acetate and estradiol implant. J Anim Sci 76:2779–2786
Kamanga-Sollo E, Pampusch MS, Xi G, White ME, Hathaway MR, Dayton WR (2004) IGF-I mRNA levels in bovine satellite cell cultures: effects of fusion and anabolic steroid treatment. J Cell Physiol 201:181–189
Kook SH, Choi KC, Son YO, Lee KY, Hwang IH, Lee HJ, Chang JS, Choi IH, Lee JC (2006) Satellite cells isolated from adult Hanwoo muscle can proliferate and differentiate into myoblasts and adipose-like cells. Mol Cells 22:239–245
Lapin MR, Gonzalez JM, Johnson SE (2013) Substrate elasticity affects bovine satellite cell activation kinetics in vitro. J Anim Sci 91:2083–2090
Lee EJ, Lee HJ, Kamli MR, Pokharel S, Bhat AR, Lee YH, Choi BH, Chun T, Kang SW, Lee YS, Kim JW, Schnabel RD, Taylor JF, Choi I (2014) Depot-specific gene expression profiles during differentiation and transdifferentiation of bovine muscle satellite cells, and differentiation of preadipocytes. Genomics 100:195–202
Mau M, Oksbjerg N, Rehfeldt C (2008) Establishment and conditions for growth and differentiation of a myoblast cell line derived from the semimembranosus muscle of newborn piglets. In Vitro Cell Dev Biol Anim 44:1–5
Montoya-Flores D, Mora O, Tamariz E, González-Dávalos L, González-Gallardo A, Antaramian A, Shimada A, Varela-Echavarría A, Romano-Muñoz JL (2011) Ghrelin stimulates myogenic differentiation in a mouse satellite cell line and in primary cultures of bovine myoblasts. J Anim Physiol Anim Nutr (Berl) 96:725–738
Muroya S, Nakajima I, Oe M, Chikuni K (2005) Effect of phase limited inhibition of MyoD expression on the terminal differentiation of bovine myoblasts: no alteration of Myf5 or myogenin expression. Dev Growth Differ 47:483–492
Roe JA, Harper JM, Buttery PJ (1989) Protein metabolism in ovine primary cultures derived from satellite cells—effects of selected peptide hormones and growth factors. J Endocrinol 122:565–571
Ronning SB, Pedersen ME, Andersen PV, Hollung K (2013) The combination of glycosaminoglycans and fibrous proteins improves cell proliferation and early differentiation of bovine primary skeletal muscle cells. Differentiation 86:13–22
Van Ba H, Inho H (2013) Significant role of μ-calpain (CANP1) in proliferation/survival of bovine skeletal muscle satellite cells. In Vitro Cell Dev Biol Anim 49:785–797
Will K, Kalbe C, Kuzinski J, Lösel D, Viergutz T, Palin MF, Rehfeldt C (2012) Effects of leptin and adiponectin on proliferation and protein metabolism of porcine myoblasts. Histochem Cell Biol 138:271–287
Will K, Kuzinski J, Kalbe C, Palin MF, Rehfeldt C (2013) Effects of leptin and adiponectin on the growth of porcine myoblasts are associated with changes in p44/42 MAPK signaling. Domest Anim Endocrinol 45:196–205
Yablonka-Reuveni Z, Seger R, Rivera AJ (1999) Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem 47:23–42
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Will, K., Schering, L., Albrecht, E. et al. Differentiation of bovine satellite cell-derived myoblasts under different culture conditions. In Vitro Cell.Dev.Biol.-Animal 51, 885–889 (2015). https://doi.org/10.1007/s11626-015-9916-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9916-9