Abstract
In the present study, purified human cord blood stem cells were co-cultivated with murine hepatic alpha mouse liver 12 (AML12) cells to compare the effect on endodermal stem cell differentiation by either direct cell-cell interaction or by soluble factors in conditioned hepatic cell medium. With that approach, we want to mimic in vitro the situation of preclinical transplantation experiments using human cells in mice. Cord blood stem cells, cultivated with hepatic conditioned medium, showed a low endodermal differentiation but an increased connexin 32 (Cx32) and Cx43, and cytokeratin 8 (CK8) and CK19 expression was monitored by reverse transcription polymerase chain reaction (RT-PCR). Microarray profiling indicated that in cultivated cord blood cells, 604 genes were upregulated 2-fold, with the highest expression for epithelial CK19 and epithelial cadherin (E-cadherin). On ultrastructural level, there were no major changes in the cellular morphology, except a higher presence of phago(ly)some-like structures observed. Direct co-culture of AML12 cells with cord blood cells led to less incisive differentiation with increased sex-determining region Y-box 17 (SOX17), Cx32 and Cx43, as well as epithelial CK8 and CK19 expressions. On ultrastructural level, tight cell contacts along the plasma membranes were revealed. FACS analysis in co-cultivated cells quantified dye exchange on low level, as also proved by time relapse video-imaging of labelled cells. Modulators of gap junction formation influenced dye transfer between the co-cultured cells, whereby retinoic acid increased and 3-heptanol reduced the dye transfer. The study indicated that the cell-co-cultured model of human umbilical cord blood cells and murine AML12 cells may be a suitable approach to study some aspects of endodermal/hepatic cell differentiation induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cells vary in their differentiation potential. Adult human umbilical cord blood stem cells (UCBC) showed preferably a lineage-specific haematopoietic differentiation (Chularojmontri and Wattanapitayakul 2009), but some reports described a differentiation potential towards other cell types in vitro and in vivo, including models for tissue regeneration (Lagasse et al. 2000; Jang et al. 2004; Saez-Lara et al. 2006; Minamiguchi et al. 2008). However, the results remain controversial, depending on the target cell and the methods applied (Saez-Lara et al. 2006; Chen et al. 2008; Hombach-Klonisch et al. 2008).
Endodermal/hepatic differentiation in vitro is a multistep process. Each step is controlled by different cytokines. Differentiated cells are characterized by specific marker profiles (Lavon and Benvenisty 2005; Dong et al. 2009; Song et al. 2010). In most of the published protocols, soluble factors were used to induce a non-haematopoietic differentiation of haematopoietic stem cells (Chen and Zeng 2011). For hepatic differentiation in vitro, UCBC were treated for instance with hepatocyte growth factor (HGF) or epidermal growth factor and oncostatin M (Wang et al. 2003; Yan et al. 2009). Differentiation was analyzed mainly by the induction of endodermal/hepatic marker expression, morphology and functional properties. For hepatic differentiation of UCBC, conditioned medium from injured murine hepatocytes in transwell chambers was found to be efficient to induce differentiation measured by liver-specific phenotype and functional changes (Jang et al. 2004).
Direct co-culture of UCBC with stromal feeder cells was found to be optimal for haematopoiesis and superior to cytokine treatment (Zhang et al. 2006). In vivo, transplanted cord blood cells into liver-injured mice were described to be functionally integrated into the organ, as measured by albumin production (Kakinuma et al. 2003; Fujino et al. 2007). Further adult stem cells isolated from cord blood and human foetal liver were intrahepatically transplanted in irradiated NOD scid gamma (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) immunodeficient mice strain (NSG) mice and lead to an efficient development of human haematopoietic and human hepatocyte-like cells in vivo (Chen et al. 2013).
In this paper, we applied both a co-culture approach and hepatic conditioned medium of murine alpha mouse liver 12 (AML12) cells for an in vivo investigation of the endodermal/hepatic differentiation potential of adult UCBC. We used the murine hepatic AML12 cells for co-culturing because they express a high level of messenger RNA (mRNA) for serum and gap junction proteins like albumin, alpha-1 antitrypsin, transferrin and connexin 32 (Cx32). Alpha-1 antitrypsin stimulates the HGF. Transferrin is essential for cell growth and differentiation of a variety of cells in vitro. With that approach, we wanted to simulate a mouse-like micro-environment for potential preclinical in vivo studies. Differentiation was evaluated by gene expression, using real-time reverse transcription polymerase chain reaction (RT-PCR), microarray analysis and on the ultrastructural level by electron microscopy. As a main result, we found that direct co-culture but not conditioned medium induced an increase of cytoskeleton markers like cytokeratin 8 (CK8) and CK19, which could be an evidence for a transition from mesenchymal to epithelial cell type as described in literature.
Materials and Methods
Isolation and purification of human umbilical cord blood stem cells—Cluster of differentiation 34 + (CD34 +) cells.
UCBC were isolated with the MACS cell isolation kit from Miltenyi Biotec (Bergisch Gladbach, Germany) according to the manufacturer’s instruction. Fluorescence-activated cell sorting (FACS) analysis with anti-CD34 phycoerythrin (PE) antibody (Becton Dickinson, Heidelberg, Germany) was performed to determine the percentage of the positive fraction. The freshly isolated cells were cultured according to in-house developed in vitro protocols (Wulf-Goldenberg et al. 2008).
Cell culture.
All cell culture experiments were performed in a 5% CO2 incubator at 37°C.
Murine AML12 cell culture and production of hepatic conditioned medium.
Murine AML12 cells (ATCC-Nr.CRL-2254) were cultured according to the manufacturer’s instruction (www.atcc.org). For production of hepatic conditioned medium, serum-free Sautin medium (Sautin et al. 2001) (Biochrom, Berlin, Germany) was used. Confluent AML12 cells were cultivated with Sautin medium for 1, 3 or 7 d to produce hepatic conditioned medium. Medium supernatant was collected and sterile filtrated.
Culture of UCBC with hepatic conditioned medium.
5 × 104 cells/cm2 UCBC were seeded in Matrigel-coated wells and were cultured with 1 d, 3 d or 7 d hepatic conditioned medium supplemented with 100 ng/ml stem cell factor (SCF) and 20 ng/ml HGF. Cells were cultured for 7 d. After 4 d, half of the medium was changed. Samples were taken at day 1 and 7 for gene expression analysis.
Co-culture of murine AML12 cells with UCBC.
For co-culture, 5 × 104 cells/cm2 UCBC were seeded on a confluent AML12 cell layer in Sautin medium supplemented with 100 ng/ml SCF and 20 ng/ml HGF. Medium was changed after 4 d. Samples were taken at day 1 and 7 for gene expression analysis. UCBC cells were displaced from the AML12 cell layer by carefully rinsing with PBS. Efficiency was microscopically controlled, and the rinsing step was repeated until all UCBC were detached from the AML12 cell layer. Collected UCBC were counted and analyzed with human-specific primers. Murine AML12 cells were used as negative control.
Reverse transcription and real-time polymerase chain reaction.
Total RNA was prepared from UCBC and AML12 cells using the RNeasy Micro and Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Quantification of RNA was performed by spectrophotometry using Nanodrop device (PeqLab, Erlangen, Germany).
RT-PCR of RNA (200 ng) was realized using TaqMan RT reagent (Applied Biosystems, Darmstadt, Germany), real-time PCR using TaqMan Universal PCR Master Mix (Applied Biosystems, Darmstadt, Germany) and human specific primer assays (Applied Biosystems, Darmstadt, Germany), which are listed as well as the PCR conditions in Table S1 (supplementary data).
Relative quantification (ΔC T method) was used to analyze the gene expression level. Cycle threshold (C T) value was normalized to those of housekeeping gene glycerinaldehyd-3-phosphat dehydrogenase (GAPDH). The following scoring system was used:
ΔC T | Score | Expression level |
>16.0 | − | No detectable expression |
12.1–16.0 | + | Low expression |
8.1–12.0 | ++ | Moderate expression |
4.1–8.0 | +++ | High expression |
0–4.0 | ++++ | Very high expression |
Electron microscopy.
Freshly isolated UCBC and cells treated with hepatic conditioned medium were pelletized and fixed with 2% formaldehyde and 1% glutaraldehyd in 0.1 M phosphate buffer for 2 h.
Murine AML12 cells as adherent cells and co-culture of AML12 cells with human stem cells were performed on lumox™ dishes (Greiner Bio-one, Frickenhausen, Germany) as described above, washed two times with phosphate buffer and fixed like the UCBC. All samples were stained with 1% Osmiumtetroxid (OSO4) for 2 h, dehydrated in a graded ethanol series and propylene oxide and embedded in Poly/BedR 812 (Polysciences, Inc., Eppelheim, Germany). Ultrathin sections were contrasted with uranyl acetate and lead citrate.
Sections were examined with a FEI Morgagni electron microscope, and digital images were taken with a Morada CCD camera and the iTEM software (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
Dye transfer.
UCBC were labelled with pkH26 (Sigma-Aldrich, Munich, Germany) and murine AML12 cells with Calcein-AM (Sigma-Aldrich, Munich, Germany) according to the manufacturer’s instructions. The cells were co-cultured for at least 48 h, and the uptake of calcein acteoxymethylester (Calcein-AM) in co-cultured Paul Karl Horan-26 (pkH26)-labelled UCBC was measured by FACS. Influences on dye transfer were investigated by using 10 μM retinoic acid (Sigma-Aldrich, Munich, Germany) on the one hand and 1 mM 3-heptanol (Sigma-Aldrich, Munich, Germany) on the other hand (microarray analysis (supplementary data); video-imaging (supplementary data)).
Results
UCBC cultivated with hepatic conditioned medium.
Hepatic conditioned medium from murine AML12 cells was harvested on day 1, 3 and 7 and added to UCBC. After 7 d of cultivation with hepatic conditioned medium, the cell number of UCBC cells was 1.2-fold increased with 90% cell viability and a more adherent appearance, compared to cells cultivated in stem cell medium only (data not shown).
Electron microscopy.
The cultivated cells were analyzed by electron microscopy for morphological changes. As shown in Fig. 1, untreated UCBC are characterized by high nucleus-to-plasma ratio, low microvilli numbers on cell surface and spare cell-to-cell contacts. In contrast, in most of the UCBC, cultivated for 7 d with hepatic conditioned medium, large numbers of microvilli per cell and sporadically cell protrusions with characteristic features of kinocilia or stereocilia are visible. Tight cell-to-cell contacts and in some cases membrane fusion were detectable. At both time points, electron-dense gap junction (GJ) structures were not found. In addition, in about 50% of the cells, structures with phagolysosomic appearance are detectable, associated occasionally with degradation of cell organelles. The percentage of phagolysosomic structures in treated cells was higher in comparison to cultivation with 1 d and 3 d hepatic conditioned medium (data not shown), suggesting few apoptotic processes during longer conditioning time.
Electron micrographs of UCBC (CD34+ stem cells). Morphological comparison between (a) freshly isolated UCBC and (b, c) after cultivation with conditioned medium for 7 d. Untreated UCBC show no distinct microvilli formation and hardly cell-to-cell contacts, whereas (b) treated stem cells developed few cell protrusions with characteristic features of kinocilia or stereocilia and (c) membrane fusions. Scale bars at (a) 1 μm and (b, c) 500 nm.
Real-time RT-PCR.
Expression pattern of UCBC was characterized by real-time RT-PCR using a panel of human specific primer sets of early endodermal and hepatic markers, CK, Cx and epithelial cadherin (E-cadherin). Untreated UCBC showed a high level of CD34 transcripts, with moderate CCAAT/enhancer binding protein alpha (C/EBPα) and E-cadherin expression (Table 1). Typical endodermal and hepatic markers (alpha fetoprotein (AFP), albumin (ALB) or GATA binding protein to DNA sequence 5′-(A/T)GATA(A/G)-3′ (GATA4)) were determined at low levels or not detectable. Cx43 and Cx32 were low expressed in UCBC, a result which is in agreement with immunohistochemical staining (data not shown). Expression pattern of hepatic conditioned medium treated UCBC was analyzed after a cultivation time of 1 d and 7 d, respectively. Due to cultivation, the CD34 and E-cadherin expression is reduced, but still detectable. CK8, CK19 and Cx32 and Cx43 expression increased to a moderate or high level, whereas the endodermal marker AFP and hepatic markers ALB and hepatocyte nuclear factor 4alpha (HNF4α) remained unchanged.
However, cultivation of UCBC in stem cell medium supplemented with cytokines revealed no comparable significant changes in expression levels. The growth factor addition of HGF and SCF into stem cell media had no effect on the CK19 expression.
Microarray analysis.
Whole genome microarray profiling was performed to confirm and analyze additional genes, involved in cellular changes induced by hepatic conditioned medium. Compared to untreated cells, 604 genes were upregulated more than 2-fold and 1,126 genes were downregulated in UCBC cultivated with hepatic conditioned medium for 10 d. Genes involved in endodermal and hepatic differentiation were diversely upregulated, E-cadherin (CDH1) with the highest increase up to 16-fold and CK19 up to 5-fold. Gene expression data are summarized in Table 2. Besides the selected endodermal hepatic genes, genes of the erythroid differentiation (NUSAP1, MAFB) and for blood vessel formation (ANPEP, DHCR7, JAG1, PSEN1) were upregulated. In contrast, transcripts for Cx43 (GJA1) and CD34+ were expressed to a lesser extent.
UCBC in co-culture with AML12 cells.
UCBC were seeded on a confluent AML 12 cells layer and co-cultivated for 7 d.
Dye transfer and live cell imaging.
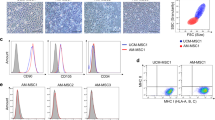
To quantify the cell-cell communication, UCBC were labelled with pkH26, murine AML12 cells with Calcein-AM and then co-cultured. After a co-cultivation time of 48 h, the number of pkH26/Calcein-AM-positive UCBC was estimated by FACS analysis using quadrant statistic analysis (Fig. 2). As demonstrated, in the absence of AML12 cells, pkH26-labelled cells showed a homogenous cell population of 98% positive cells with a middle fluorescence intensity (mFI) of 800 (Fig. 2a, b ). Forty-eight hours after co-cultivation, about 7.18% of the UCBC were found to be Calcein-AM-positive (Fig. 2c ). Modulators of gap junction formation and interference with connexins were tested in the UCBC/AML12 co-culture model. For that, cells were cultured in the presence of retinoic acid and 3-heptanol for 48 h. Retinoic acid increased the number of pkH26/Calcein-AM-positive UCBC cells to 15.32% (Fig. 2d ), whereas the inhibitor 3-heptanol decreased the number to 1.19% (Fig. 2e ). The results demonstrated the functionality of the applied dye transfer method for measuring cell-cell communication and suggest the participation of connexins.
Flow cytometry analysis of dye transfer between AML12 cells (calcein labelled) and UCBC (pkH26 labelled) in co-culture. At 0 h (a) and 48 h (b) after labelling of UCBC with pkH26, no distinct changes in Calcein-AM mediated fluorescence intensity measurable. (c) After 48 h in co-culture with Calcein-AM labelled AML12 cells, 7.18% of UCBC took up Calcein-AM and were double-positive. (d) Treatment with retinoic acid increased uptake of Calcein-AM and double-positive cells to 15.32%. (e) In contrast, incubation with 3-heptanol for 48-h reduced uptake and double-positive cells to 1.19%.
Cell-cell interaction of labelled UCBC and confluent AML12 cells was visualized by time relapsed video-imaging over a time period of 16 h (Fig. S1 supplementary data). As demonstrated on adherent, murine AML12 cells, UCBC possess high motility with a sporadic cell contact to the murine cells. However, most of the UCBC get no direct contact with AML12 cells for a longer time period. This result may explain the low level of communicating cells, measured as well by dye transfer.
Electron microscopy.
Co-culture systems of murine AML12 and UCBC were investigated on ultrastructural level at days 1 and 7. As demonstrated in Fig. 3a , no distinct morphological changes of UCBC were detectable at day 1. Compared to freshly isolated UCBC (see Fig. 1a ), they maintained their round morphology with high nucleus-to-plasma ratio, only few microvilli and no dense cell-to-cell contacts. At day 7 of co-culture, microvilli structures and a flattened cell phenotype appeared more frequently (Fig. 3b ). Occasionally, tight UCBC-to-AML12 cell contacts along the plasma membrane are present (Fig. 3c ).
Fine structure of co-culture between UCBC (CD34+ stem cells) and AML12 cells disclosed (a) 1 d after co-cultivation hardly any cell-to-cell contacts, and no morphological changes are visible. (b, c) After 7 d, a flattened cell phenotype of UCBC is detectable, and close cell contacts between AML12 cells (down) and UCBC (above) occur. (c) Higher magnification of the region gated in (b). Scale bar at 1 μm.
Real-time RT-PCR.
Expression pattern of UCBC in co-culture with murine AML12 cells was investigated at day 1 and 7 (Table 3) by real-time PCR. Besides a slight reduction of CD34 marker expression, seven different RNA transcripts were upregulated in a time-dependent manner. The transcripts for CK8, CK18 and CK19 were elevated to a moderate or high level. The expressions of Cx32 and Cx43 were also upregulated from a low to moderate rate during cultivation. The higher expression of sex-determining region Y-box 17 (SOX17) in co-cultivated UCBC suggests an early endodermal differentiation, whereas forhead box A2 genename (Hepatocyte nuclear factor 3beta (HNF3β)) (FOXA2), AFP and ALB were not affected, suggesting no endodermal or hepatic differentiation. For comparison, human HepG2 cells are characterized by a high expression of these transcripts (Table 3).
Discussion
The aim of this study was to demonstrate the in vitro endodermal/hepatic differentiation potential of human UCBC with two different methods. The UCBC were freshly isolated, and an enrichment of CD34-positive cells was performed with common bead technology. We compared the differentiation induction potential of murine AML12 cells used either in direct co-culture with UCBC or as donor for conditioned medium. We intended to investigate the mechanism of cell-cell interaction for endodermal/hepatic induction of these adult stem cells.
The murine AML12 cells were used as the source for production of conditioned medium and effector cells in direct co-culture as they express high levels of mRNA for serum albumin, alpha 1 antitrypsin, transferrin and the gap junction proteins Cx26 and Cx32. We found an increased expression of epithelial markers like CK8 and CK19 as well as markers for gap junctions like Cx32 and Cx43 as revealed by real-time PCR analysis in UCBC cultured with conditioned medium. The endodermal/hepatic marker expression profile of SOX17, FOXA2, AFP and HNF4α was not significantly changed on cells cultivated with hepatic conditioned medium compared to untreated UCBC. The results of microarray analysis revealed the diverse upregulation from genes involved in endodermal differentiation, like E-cadherin up to an 16-fold increase and CK19- up to a 5-fold increase. Further, we determined changes in gene profile for blood vessel formation and erythroid differentiation. The results of both mRNA methods agree essentially. Protein expression was analyzed on cultured stem cells exemplarily. The expression of CD34 and CD45 was considerably reduced on stem cells after cultivation with hepatic conditioned medium or in co-culture, respectively (data not shown). Slight changes on mRNA level of cultured stem cells could be noticed due to the high sensitivity and specificity of the used mRNA methods. These changes are not distinguishable on protein level. On ultrastructural level, some phenotypic changes appear, like large numbers of microvilli per cell and structures with phagolysosomic appearance.
In direct co-culture approach of UCBC with murine cells, we found that the transcripts for CK8, CK18 and CK19 and connexins were upregulated to a moderate or high level. Further, the higher expression of SOX17 in co-cultivated UCBC suggests an early endodermal differentiation. In electron microscopy images taken after 7 d co-culturing, we found frequent microvilli structures and a flattened cell phenotype and occasionally tight UCBC-to-AML12 cell contacts along the plasma membrane. Cell-cell interaction of labelled UCBC and confluent AML12 cells was visualized by time-relapsed video-imaging. Here, we found a high motility of UCBC with a sporadic cell contact to the murine cells. Also, a low level of communicating cells was measured by dye transfer of UCBC co-cultured with murine AML12 cells.
The endodermal/hepatic in vitro differentiation of adult UCBC (CD34+ stem cells) was described in several studies (Lysy et al. 2008; Esrefoglu 2013). Different in vitro protocols, mainly on the basis of combinations of chemicals, growth and differentiation factors, were used in order to induce multistep hepatic cell differentiation (Fiegel et al. 2003; Kakinuma et al. 2003; Teramoto et al. 2005; Ishii et al. 2008; Lavon 2010; Chen and Zeng 2011). For UCBC, combinations of growth and differentiation factors are known to contribute to the in vitro proliferation and differentiation of hepatic progenitor cells in the rodent and human liver (Fiegel et al. 2003). Further, a defined medium containing HGF induced albumin and CK19 expression which was accompanied by increased cell proliferation (Fiegel et al. 2003). In another study, HGF in combination with SCF, leukaemia inhibitory factor (LIF), fibroblast growth factor-1 (FGF-1) and FGF-2 induced ALB+ cells with an expression of markers from hepatocyte lineage, like CK19, CK18 and AFP in human cord blood cells. These cells had the same phenotype as bipotential hepatic progenitor cells (Kakinuma et al. 2003). The addition of HGF and SCF into stem cell media had no effect on the CK19 expression, but both factors increased the CK8 and CK18 expression of cultured cells (Wulf-Goldenberg et al. 2008). We used in our study SCF and HGF for the induction of the endodermal differentiation. Our results demonstrated a slight early endodermal differentiation which was more intense after co-culture approach compared with the use of conditioned medium. The differences, compared to the results of literature reports, may be due to the use of other stem cell types and/or the inclusion of injured primary mouse liver cells as effector cells, suggesting an involvement of the injured microenvironment. In a published transwell co-culture system using bone marrow-derived cells and injured murine liver cells, epithelial CK18 expression, albumin, GATA4, HNF4α and liver enzyme transcripts on protein and RNA level were found. These cells were converted into viable hepatocytes after transplantation into liver-injured mice (Jang et al. 2004). Coding proteins for cell-cell communication and GJ proteins like the connexins were discussed to play an important role during development and differentiation of stem cells (Elias and Kriegstein 2008; Kihara et al. 2008; Choi et al. 2010). However, their precise involvement and the contribution of connexins in these processes remain speculative. In our study, undifferentiated UCBC showed low Cx32 and Cx43 transcript expression. Both connexin transcripts were upregulated by cultivation with hepatic conditioned AML12 medium. GJ structures were not detectable by electron microscopy, but tight cell-cell contacts were visualized. Further, microvilli formation and sporadic cell protrusions with kinocilia- and stereocilia-like structures of cultivated cells could be observed. In the present study, this may suggest either low expression of connexins at protein level or a short half-life of the proteins, which is described at least for Cx43 (Simek et al. 2009; Solan and Lampe 2009). In haematopoietic stem cells, upregulation of Cx43 was discussed as a response to events that call for active division of stem cells, perhaps enabling them to divide (Rosendaal et al. 1994). In our study, upregulation of the connexins apparently was not clearly associated with induction of endodermal differentiation. Expression of hepatic cell-associated Cx32 in cultivated UCBC was only sparsely reported. In one study, cytokine-supported cultivation of umbilical cord stem cells led to the expression of Cx32 (Campard et al. 2008). This result is in comparison to our findings on co-culture with AML12 cells. Further, in direct co-culture of UCBC with AML12 cells, the expression of transcripts for CK8 and CK19, as well as for Cx32 and Cx43, was higher compared to cells cultivated in hepatic conditioned medium. In addition, induction of SOX17 expression suggests an early endodermal cell differentiation obtained in direct co-culture. In hepatic differentiation protocols for stem cells, SOX17 expression was identified as an early marker of endodermal cell differentiation (Chen et al. 2006; Cho et al. 2008; Rolletschek et al. 2010). Mature hepatocytes and the human HepG2 cell line express SOX17 at low level (Matsui et al. 2006). The connexins process a pivotal function on the maintenance of the differentiated cell function, including albumin and bile secretion (Fladmark et al. 1997; Kojima et al. 2003; Naiki-Ito et al. 2010). Using dye transfer, the effects of stimulators (retinoic acid) and inhibitors (3-heptanol) of GJ-committed cell-cell interaction suggested in our experiments the participation of connexins. However, also, direct pharmacological drug effects cannot be excluded. Functional studies using small interfering ribonucleic acid (siRNA) inhibition are intended. No GJ formation was visible by electron microscopy in co-culture of UCBC with AML12 cells. But, tight cell-cell contact between neighbouring cells and infrequent cell fusions was detected. According to literature, the endodermal/hepatic differentiation potential of UCBC (CD34+ cells) is more restricted, compared to embryonic stem cells (Wagers et al. 2002; Wang et al. 2003; Masson et al. 2004; Agarwal et al. 2008; Basma et al. 2009; Esrefoglu 2013).
References
Agarwal S, Holton KL, Lanza R (2008) Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells 26(5):1117–1127
Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, Muirhead D, Navarro-Alvarez N, Wong RJ, Roy-Chowdhury J, Platt JL, Mercer DF, Miller JD, Strom SC, Kobayashi N, Fox IJ (2009) Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 136(3):990–999
Campard D, Lysy PA, Najimi M, Sokal EM (2008) Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 134(3):833–848
Chen X, Zeng F (2011) Directed hepatic differentiation from embryonic stem cells. Prot Cell 2(3):180–188
Chen Y, Soto-Gutierrez A, Navarro-Alvarez N, Rivas-Carrillo JD, Yamatsuji T, Shirakawa Y, Tanaka N, Basma H, Fox IJ, Kobayashi N (2006) Instant hepatic differentiation of human embryonic stem cells using activin A and a deleted variant of HGF. Cell Transplant 15(10):865–871
Chen Y, Cai H, Tan W (2008) [Research on in vitro differentiation of human umbilical cord blood-derived CD34+ cells into hepatocyte-like cells]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 22(6):747–752
Chen Q, Khoury M, Limmon G, Choolani M, Chan JK, Chen J (2013) Human fetal hepatic progenitor cells are distinct from, but closely related to, hematopoietic stem/progenitor cells. Stem Cells 31(6):1160–1169
Cho CH, Parashurama N, Park EY, Suganuma K, Nahmias Y, Park J, Tilles AW, Berthiaume F, Yarmush ML (2008) Homogeneous differentiation of hepatocyte-like cells from embryonic stem cells: applications for the treatment of liver failure. FASEB J 22(3):898–909
Choi YS, Dusting GJ, Stubbs S, Arunothayaraj S, Han XL, Collas P, Morrison WA, Dilley RJ (2010) Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J Cell Mol Med 14(4):878–889
Chularojmontri L, Wattanapitayakul SK (2009) Isolation and characterization of umbilical cord blood hematopoietic stem cells. J Med Assoc Thai 92(Suppl 3):S88–S94
Dong XJ, Zhang GR, Zhou QJ, Pan RL, Chen Y, Xiang LX, Shao JZ (2009) Direct hepatic differentiation of mouse embryonic stem cells induced by valproic acid and cytokines. World J Gastroenterol 15(41):5165–5175
Elias LA, Kriegstein AR (2008) Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci 31(5):243–250
Esrefoglu M (2013) Role of stem cells in repair of liver injury: experimental and clinical benefit of transferred stem cells on liver failure. World J Gastroenterol 19(40):6757–6773
Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR (2003) Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells 21(1):98–104
Fladmark KE, Gjertsen BT, Molven A, Mellgren G, Vintermyr OK, Doskeland SO (1997) Gap junctions and growth control in liver regeneration and in isolated rat hepatocytes. Hepatology 25(4):847–855
Fujino H, Hiramatsu H, Tsuchiya A, Niwa A, Noma H, Shiota M, Umeda K, Yoshimoto M, Ito M, Heike T, Nakahata T (2007) Human cord blood CD34+ cells develop into hepatocytes in the livers of NOD/SCID/gamma(c)null mice through cell fusion. FASEB J 21(13):3499–3510
Hombach-Klonisch S, Panigrahi S, Rashedi I, Seifert A, Alberti E, Pocar P, Kurpisz M, Schulze-Osthoff K, Mackiewicz A, Los M (2008) Adult stem cells and their trans-differentiation potential—perspectives and therapeutic applications. J Mol Med (Berl) 86(12):1301–1314
Ishii T, Fukumitsu K, Yasuchika K, Adachi K, Kawase E, Suemori H, Nakatsuji N, Ikai I, Uemoto S (2008) Effects of extracellular matrixes and growth factors on the hepatic differentiation of human embryonic stem cells. Am J Physiol Gastrointest Liver Physiol 295(2):G313–G321
Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ (2004) Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 6(6):532–539
Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K, Yasumizu T, Teraoka H (2003) Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells 21(2):217–227
Kihara AH, Paschon V, Akamine PS, Saito KC, Leonelli M, Jiang JX, Hamassaki DE, Britto LR (2008) Differential expression of connexins during histogenesis of the chick retina. Dev Neurobiol 68(11):1287–1302
Kojima T, Yamamoto T, Murata M, Chiba H, Kokai Y, Sawada N (2003) Regulation of the blood-biliary barrier: interaction between gap and tight junctions in hepatocytes. Med Electron Microsc 36(3):157–164
Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6(11):1229–1234
Lavon N (2010) Generation of hepatocytes from human embryonic stem cells. Methods Mol Biol 640:237–246
Lavon N, Benvenisty N (2005) Study of hepatocyte differentiation using embryonic stem cells. J Cell Biochem 96(6):1193–1202
Lysy PA, Campard D, Smets F, Najimi M, Sokal EM (2008) Stem cells for liver tissue repair: current knowledge and perspectives. World J Gastroenterol 14(6):864–875
Masson S, Harrison DJ, Plevris JN, Newsome PN (2004) Potential of hematopoietic stem cell therapy in hepatology: a critical review. Stem Cells 22(6):897–907
Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y (2006) Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci 119(Pt 17):3513–3526
Minamiguchi H, Ishikawa F, Fleming PA, Yang S, Drake CJ, Wingard JR, Ogawa M (2008) Transplanted human cord blood cells generate amylase-producing pancreatic acinar cells in engrafted mice. Pancreas 36(2):e30–e35
Naiki-Ito A, Asamoto M, Naiki T, Ogawa K, Takahashi S, Sato S, Shirai T (2010) Gap junction dysfunction reduces acetaminophen hepatotoxicity with impact on apoptotic signaling and connexin 43 protein induction in rat. Toxicol Pathol 38(2):280–286
Rolletschek A, Schroeder IS, Schulz H, Hummel O, Huebner N, Wobus AM (2010) Characterization of mouse embryonic stem cell differentiation into the pancreatic lineage in vitro by transcriptional profiling, quantitative RT-PCR and immunocytochemistry. Int J Dev Biol 54(1):41–54
Rosendaal M, Green CR, Rahman A, Morgan D (1994) Up-regulation of the connexin43+ gap junction network in haemopoietic tissue before the growth of stem cells. J Cell Sci 107(Pt 1):29–37
Saez-Lara MJ, Frecha C, Martin F, Abadia F, Toscano M, Gil A, Fontana L (2006) Transplantation of human CD34+ stem cells from umbilical cord blood to rats with thioacetamide-induced liver cirrhosis. Xenotransplantation 13(6):529–535
Sautin YY, Crawford JM, Svetlov SI (2001) Enhancement of survival by LPA via Erk1/Erk2 and PI 3-kinase/Akt pathways in a murine hepatocyte cell line. Am J Physiol Cell Physiol 281(6):C2010–C2019
Simek J, Churko J, Shao Q, Laird DW (2009) Cx43 has distinct mobility within plasma-membrane domains, indicative of progressive formation of gap-junction plaques. J Cell Sci 122(Pt 4):554–562
Solan JL, Lampe PD (2009) Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419(2):261–272
Song L, Wang H, Gao X, Shen K, Niu W, Qin X (2010) Proliferation and differentiation potential of mouse adult hepatic progenitor cells cultured in vitro. Acta Biochim Biophys Sin (Shanghai) 42(2):122–128
Teramoto K, Asahina K, Kumashiro Y, Kakinuma S, Chinzei R, Shimizu-Saito K, Tanaka Y, Teraoka H, Arii S (2005) Hepatocyte differentiation from embryonic stem cells and umbilical cord blood cells. J Hepatobiliary Pancreat Surg 12(3):196–202
Wagers AJ, Sherwood RI, Christensen JL, Weissman IL (2002) Little evidence for developmental plasticity of adult hematopoietic stem cells. Sci 297(5590):2256–2259
Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M (2003) Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nat 422(6934):897–901
Wulf-Goldenberg A, Eckert K, Fichtner I (2008) Cytokine-pretreatment of CD34(+) cord blood stem cells in vitro reduces long-term cell engraftment in NOD/SCID mice. Eur J Cell Biol 87(2):69–80
Yan L, Cai H, Chen Y, Tan W (2009) [Effect of hepatocyte-like cells induced by CD34+ cells in vitro on the repair of injured hepatic tissues of mice in vivo]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 23(10):1235–1240
Zhang Y, Chai C, Jiang XS, Teoh SH, Leong KW (2006) Co-culture of umbilical cord blood CD34+ cells with human mesenchymal stem cells. Tissue Eng 12(8):2161–2170
Acknowledgments
The skilful technical assistance of Mrs. Vannauer is gratefully acknowledged. We thank the HELIOS-Clinics GmbH in Berlin-Buch and the Vivantes Clinics in Berlin for collecting and providing the umbilical cord blood. The work was supported by a grant from the Federal Ministry of Education and Research (BMBF; 01GN0528)
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Stecklum, M., Wulf-Goldenberg, A., Purfürst, B. et al. Cell differentiation mediated by co-culture of human umbilical cord blood stem cells with murine hepatic cells. In Vitro Cell.Dev.Biol.-Animal 51, 183–191 (2015). https://doi.org/10.1007/s11626-014-9817-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-014-9817-3