Abstract
The objective of this study was morphological and functional characterization of cells from the primary cell culture developed from lactating goat mammary gland, focusing on distribution of lineage-specific markers. Primary cells were grown on a thin layer of basement membrane matrix, a growth surface that resembles in vivo conditions. The cells in adherent conditions rapidly proliferated and showed cobblestone morphology, typical for epithelial cells. Under non-adherent conditions, goat mammary cells formed spherical, acini-like structures that resembled alveoli of lactating mammary gland. Immunofluorescence and RNA sequencing were employed to determine expression of lineage-specific markers. Presence of markers cytokeratin 14 and 18, integrin alpha 6, vimentin, estrogen receptor, smooth muscle actin, and cytokeratin 5 was detected using immunofluorescence. The greatest expression was observed for markers typical for myoepithelial cells, luminal cells, and mesenchymal cells. Based on our characterization, we can conclude that established primary culture was composed of mainly epithelial and stromal cells. These findings demonstrate that primary mammary cells express some of the most important functional and biochemical markers needed for their characterization. First, they grow in the characteristic cobblestone morphology of epithelial cells. Second, they express classical cytoplasmic network of cytokeratin fibers. Third, they express markers typical of mammary parenchyma and stroma. The established cell culture represents a good in vitro model for studies of mammary gland development, differentiation, and lactation. We suggest that herein revealed lineage markers are suitable for characterization of mammary cells of goat and possibly other mammalian species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development of the mammary gland occurs in defined stages that are connected to sexual development and reproduction (Hennighausen and Robinson 2001). The gland is composed of alveoli, ducts, and stromal compartment. The functional unit of mammary gland consists of two epithelial cell types; the milk secreting luminal cells and the contractile myoepithelial cells, which form an outer basal layer. This myoepithelial layer in turn sits on the basement membrane (Pitelka et al. 2009). Mammary secretion mechanism in caprine is apocrine, whereas in bovine is merocrine. Furthermore, the mammary gland regression and milk stasis is associated with decreased number of mammary epithelial cells in goat and loss of differentiated function and minimal decrease in cell number in cows (Hurley 1989; Knight and Peaker 1984). Adequate species-specific in vitro model, mimicking the function of the mammary gland, would be of great benefit to study physiological, biochemical, and immunologic functions of the mammary gland, without the need for experiments on animals. There is a considerable potential to use cell cultures or 3D cell culture-based models instead of tissues from live animals. Mammary cell culture-based models were successfully used to study cell differentiation, innate immune response, and hormonal induction of lactogenesis in mammary epithelial cells (Barcelloshoff et al. 1989; Rainard and Riollet 2006; Stiening et al. 2008). For research purposes, several ruminant immortalized cell lines such as MAC-T (Huynh et al. 1991) and BME-UV (Zavizion et al. 1996) have been established by stable integration of the simian virus large T-antigen (SV40LTA). However, because it is not clear how modifications in immortalized cell lines alter pathways of transformed cells, the use of primary cell cultures is much more representative of the in vivo state, maintaining organ-specific functions and signal transduction pathways (Pantschenko et al. 2000b).
In the recent years, several primary cultures of bovine and goat cells have been established by enzymatic dissociation of mammary tissue, and cells were mainly plated directly onto plastic flasks or on collagen-coated surfaces (Keys et al. 1997; Pantschenko et al. 2000b; Hu et al. 2009; Sorg et al. 2012; Tong et al. 2012); Jedrzejczak and Szatkowska 2013. It is impossible to compare the growth conditions of primary goat mammary epithelial cells (pgMEC) previously cultured, because the media used were of different compositions, having RPMI1640, DMEM, and DMEM/F12 as their bases with variety of added growth factors. Primary cultures were also prepared as explant cultures grown from a fragment of goat tissue (Liu et al. 2012) and were transfected to produce EGFP (Zheng et al. 2010). The pgMEC were, for instance, infected with small ruminant lentiviruses (Lerondelle et al. 1999; Milhau et al. 2005) and used to explore the function of PPARγ (Shi et al. 2013), to produce cloned goat offspring (Yuan et al. 2009), and to study glycosylation of recombinant human erythropoietin (Sanchez et al. 2007). Mammary epithelial cell (MEC) cultures and lines have been established from glands of several other species. A primary culture of mammary epithelial cells isolated from lactating sows was used to study gene transfer and expression in vitro (Sun et al. 2005). Mammary epithelial cell lines from sow and buffalo spontaneously became immortal (Sun et al. 2006; Anand et al. 2012). Ovine MEC lines have also been characterized (Duchler et al. 1998; Ilan et al. 1998) and were used to study their in vitro response to Staphylococcus aureus infection (Bonnefont et al. 2012). But the research has been mainly focused on their morphology, growth rate and karyotype, not focusing on lineage-marker distribution.
Conditions that do not allow the adherence of MEC lead to anoikis of differentiated cells. On the contrary, undifferentiated mammary stem/progenitor cells are able to survive and can form spherical structures (i.e., mammospheres; Dontu et al. 2003). It has been previously recognized that the assembly of polarized tissue-like acinus structure, reminiscent of alveoli in vivo, was induced by basement membrane, which facilitates cell rounding and drives cell–cell interactions (Petersen et al. 1992). Riley and colleagues used gene expression profiling during mammosphere formation of bovine MEC on basement membrane matrix, by high-density microarray analysis, to showcase that mammospheres underwent similar molecular and cellular processes to developing alveoli in the mammary gland (Riley et al. 2010).
Therefore, primary cell culture from lactating goat mammary gland was established and morphologically and functionally characterized. We hypothesized that markers of lineage and cell fate determination will be expressed and that primary cells cultivated under low-attachment conditions will be able to form 3D structures, resembling alveoli of lactating mammary gland. Herein, we report that the established mammary cell culture is composed of typical epithelial cells with cobblestone morphology and spindle shaped stromal cells. Based on protein and RNA expression, detected by immunocytochemistry and RNA sequencing, markers associated with luminal, myoepithelial, and stromal cells were expressed.
Materials and Methods
Establishment of MECs in culture.
Mammary tissue was aseptically removed from the mammary gland of lactating Saanen goat (Capra hircus), at its peak of lactation, immediately after slaughter. Sample collection was performed with the authorization and supervision of representatives of the Veterinary Services of the Slovenian National Health Service branch of the Ministry of Health. Pieces of tissue were washed in Hank’s Buffered Salt Solution (HBSS) containing penicillin (200 μg/mL), streptomycin (200 μg/mL), gentamicin (200 μg/mL), ampicillin (200 μg/mL), and amphotericin B (10 μg/mL). Processed tissue was digested in 100 mL solution of HBSS with Hepes, supplemented with collagenase (Type IV, Biochrom AG, Berlin, Germany) and hyaluronidase (Sigma-Aldrich, Munich, Germany; 400 U/mL of each) at 37°C with gentle shaking. The digests were collected after 60, 120, and 180 min and filtered through a steel mesh. The filtrates were transferred to 50 mL tubes and washed several times with HBSS and centrifuged at 200×g for 5 min. The pellets were collected and washed several times with HBSS. Finally, the cell suspensions were filtered through 40 μm meshes, centrifuged, re-suspended, and plated or frozen in 90% FBS and 10% DMSO in liquid nitrogen.
Aliquots of cell suspensions were plated in RPMI 1640 supplemented with fetal bovine serum (FBS, 5%), insulin (1 μg/mL), hydrocortisone (1 μg/mL), prolactin (1 μg/mL), 100 U/mL penicillin, and 100 μg/mL streptomycin (all Sigma-Aldrich). Number of primary cells seeded ranged from 0.5 to 1 million per mL of growth medium. Primary cells were grown on a thin layer of basement membrane matrix (Geltrex (GT), Invitrogen, Gibco, Carlsbad, CA), which was prepared according to the manufacturer’s guidelines. Geltrex is a soluble form of basement membrane extracted from murine Engelbreth–Holm–Swarm tumors. The major components of Geltrex matrix include laminin, collagen IV, entactin, and heparin sulfate proteoglycans. The fibroblast fraction was partially removed by decanting the unattached cells after one hour, into another flask in which epithelial cells arose. The epithelial cells were further enriched by differential trypsinisation, namely, fibroblasts detach much faster than cells of epithelial origin. Cells were incubated in an incubator at 37°C, 5% CO2, and saturated humidity. The medium was changed every 2 to 3 d. To evaluate mycoplasmal contamination PCR based detection of mycoplasma-specific DNA sequences was performed, using 16S ribosomal RNA universal primers as previously described (Johansson et al. 1998).
Immunofluorescent staining.
Attached cells were fixed in a mixture of acetone and methanol (dilution 1:1) at room temperature. After fixation cells were kept in Tris buffer (TBS, 0.1 M Tris HCl, 0.14 M NaCl, pH 7.6, all Sigma-Aldrich) containing 10% goat serum for one hour at room temperature, and then for one hour with one or two primary antibodies in antibody dilution buffer (Tris buffer with 1 g of sodium azide and 10 g of BSA per liter, all Sigma-Aldrich). Primary antibodies against cytokeratin (CK) 14 (polyclonal AF-64, Covance, Emeryville, CA), CK18 (C-04, sc-51582), CK5 (H-40, sc-66856), integrin alpha 6 (CD49f, H-87, sc-10730), vimentin (2Q1035, sc-73262), estrogen receptor (ER, H-184, sc-7207), smooth muscle α-actin (SMA, 0.N.5, sc-58669; latter six Santa Cruz Biotechnology, Santa Cruz, CA), and beta-casein (CSN2, #250558, Abbiotec, San Diego, CA) were used. Cells were then washed with TBS and incubated in a solution of secondary antibodies AlexaFluor 488-labeled goat anti-rabbit IgG and AlexaFluor 594-labeled goat anti-mouse IgG (both Invitrogen) for 1 h, washed with TBS, and counterstained with 4′,6-diamino-2-phenylindole (DAPI, Sigma-Aldrich) for 5 to 10 min. After final washing, the TBS was added, and the staining was observed under an inverted fluorescent microscope (Nikon Eclipse TE, 2000). Negative controls were performed for each antigen by replacing the primary antibody with a suitable isotype antibody (normal mouse IgG or normal rabbit IgG from Santa Cruz Biotechnology) at the same dilutions.
Oil red O staining.
Medium was aspired and the cells fixed in 4% paraformaldehyde for 15 min. Oil Red O (0.5 g, Sigma-Aldrich) was dissolved in 50 mL isopropanol and diluted with water (3:2), left for 10 min and filtered through a 20-μm filter. Cells were briefly washed with isopropanol (60%) and incubated with solution of Oil red O for 15 min at room temperature. The cells were then rinsed with isopropanol and washed under tap water. The formation of lipid droplets was observed under bright field microscope.
3D model.
Mammosphere formation was performed in ultralow attachment 6-well plates (Nunc, Sigma-Aldrich) with DMEM/F12 medium supplemented with EGF (human, 20 ng/mL), bFGF (human, 20 ng/mL), heparin (4 μg/mL), cholera toxin (10 ng/mL), hydrocortisone (0.5 μg/mL; all from Sigma-Aldrich), and B27 supplement (2%, Invitrogen). After mammary tissue dissociation, 80,000 of single cells were seeded per well. Six days after seeding mammospheres were measured, and their mean size was calculated.
MEC were also grown in human Epicult-B medium (Stem Cell Technology, Vancouver, Canada) containing GT (5%), FBS (5%), EGF (10 ng/mL), bFGF (20 ng/mL), and heparin (4 mg/mL; all Sigma-Aldrich). Cells were seeded at 50,000 per well in ultralow attachment 6-well plates (Nunc). The average size of spheres was assessed 7 d after seeding.
3D structures were stained by immunofluorescence in suspension or were first fixed in 4% paraformaldehyde, embedded in paraffin, and 5 μm sections were stained.
mRNA sequence acquisition and analysis.
First passage pgMECs were grown to approximately 80% confluency. Total RNA was extracted from three dishes of primary cell cultures using Tri-Reagent (Ambion, Life Technologies, Grand Island, NY) and miRNeasy kit (Qiagen, Hilden, Germany) in accordance to manufacturer’s instructions. Contaminating genomic DNA was removed from the RNA samples by DNase I digestion (Thermo Scientific, Schwerte, Germany). Sequencing library was prepared (Illumina, protocol version 1004898 Rev. D, San Diego, CA) and sequenced in a total of five lanes in three flow cells on an Illumina Genome Analyzer (version IIx, SCS2.5/RTA). The Burrows-Wheeler Aligner (ver. 0.5.7, http://bio-bwa.sourceforge.net/) (Li and Durbin 2009) was used to map the 50 bp sequences against the Bos taurus reference sequences (NCBI, Btau 3.1, ftp://ftp.ncbi.nih.gov/refseq/B_taurus/). An expression matrix was prepared by summing the reads mapping to individual RefSeq accessions in the R statistical environment (version 2.11.0, http://www.R-project.org).
Relative expression of CSN2 in pgMECs.
The expression of CSN2 was analyzed in over confluent pgMECs, using reverse transcription quantitative PCR (RT-qPCR). Housekeeping gene ACTB was used as a reference gene to compare the expression values with CSN2. The primers were: CSN2 forward 5′-ACAGCCTCCCACAAAACATC-3′, reverse 5′-AGGAAGGTGCAGCTTTTCAA-3′, and ACTB forward 5′-GCCGAGACCGCGTCC-3′, reverse 5′-ATCATCCATGGCGAACTGGT-3′. The reaction was conducted in Viia7 real time PCR system (Applied Biosystems, Carlsbad, CA), performing three technical replicas for each sample. The amplification reactions consisted of 2× SYBR Green PCR master mix (Applied Biosystems), water, and 0.5 μM each primer in a total volume of 20 μL. The cycles were as follows: 2 min at 50°C, 10 min at 95°C, and 40 cycles at 95°C for 15 s and 60°C for 1 min. Melting curve was determined at 15 s for 95°C, 1 min at 58°C and 15 s at 95°C.
Results
Caprine primary mammary cell culture.
The primary cell culture was a heterogeneous population of epithelial and fibroblast-like cell types. When grown at low density on a thin layer of basement membrane matrix, typical cobblestone morphology of epithelial cells was observed (Fig. 1a, b ). Densely packed islands of smaller tear drop-shaped cells and cells randomly spreading around these islands that morphologically resembled fibroblasts (Fig. 1a ) were observed. The sub-cultured pgMEC proliferated without changes in morphology or growth pattern for more than ten passages. At first, cells dissociated from the tissue exhibited slow growth rate when seeded into culture dishes. After reaching confluence (in approximately 10–14 d) and passaging, the sub-cultured cells grew on average twice as fast as primary cells before first passage. Dome-like structures appeared as a result of cell to cell contact induced differentiation (Fig. 1c ) when cells were grown for extended period of time at high density. Additionally, cells formed sphere-like aggregates on top of the monolayer (Fig. 1d ). When cells were grown to an over confluent state, forming domes, the formation of lipid droplets (Fig. 2) and expression of beta-casein (Fig. 7) were noticed.
Morphology of caprine primary cells observed after 8 d in culture (a) with close up of island of densely packed epithelial cells (b). Dome-like structures (c) and aggregates (d) in 15 d old post-confluent culture grown on a thin layer of basement membrane matrix. Scale bars are 50, 100, 200, and 200 μm, respectively.
Expression of cytoskeletal proteins.
A monolayer of pgMEC was stained with cell-type-specific primary antibodies followed by secondary antibodies. Myoepithelial cells stained positively for CK 14 (green staining in Fig. 3a ), CK 5 (green staining in Fig. 3e ) and for basal marker CD49f (Fig. 3b ), whereas luminal cells stained positively for CK 18 (red staining in Fig. 3a, d ), ER (green nuclear staining in Fig. 3d ), and CSN2 (Fig. 3f ). Cells also stained positively for intermediate filament protein vimentin (Fig. 3c ), which is frequently expressed by cells of mesenchymal origin. A minor part of cells was marked with antibody against SMA (Fig. 3e ), exhibiting sheets of filaments in the cell cytoplasm. The domes, which arose under over confluent conditions, were mainly composed of luminal cells, marked by cytokeratin 18 (Fig. 4, red). A minor fraction of dome forming cells were CK14-positive myoepithelial cells (Fig. 4, green). Domes were surrounded by a thick wall of cells which were not marked by epithelial markers, indicating their stromal origin.
Primary mammary culture cultured for 5 d on a thin layer of basement membrane matrix. Bright field and fluorescence images of cells incubated with antibodies against cytokeratin 14 (a; green) and cytokeratin 18 (a and d; red), CD49f (b), vimentin (c), estrogen receptor (d; green), and smooth muscle actin and cytokeratin 5 (e; red and green). Synthesis of beta-casein of primary mammary cells in fourth passage cultured for 11 d on a thin layer of basement membrane matrix under lactogenic conditions (f). Nuclei have been counterstained with DAPI (blue). Scale bars are 100 μm.
3D organization in culture.
The morphological differentiation of mammary epithelial cells was evaluated when the cells were grown in suspension using 3D culture methods. Under non-adherent culture conditions mammospheres formed as irregularly shaped floating masses at day 6, having size of 53.02 ± 17.13 μm (n = 33) (Fig. 5a ). In medium supplemented with basement membrane matrix, aggregates arose, which were mostly round in shape (Fig. 5b ); some of them connected through duct-like structures or were organized as a large cohort of single cells. These mammospheres were larger than mammospheres formed in medium without added basement membrane matrix, reaching average size of 102.74 ± 33.38 μm (n = 10) at day 7.
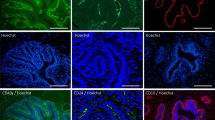
When mammospheres were immunofluorescently stained in suspension, the expression of cytokeratin 14 was observed (Fig. 6a, b ). The stained sections of paraffin embedded 3D structures clearly showed presence of lumen (Fig. 6c, d ) and several layers of myoepithelial cells. Positive staining for luminal marker cytokeratin 18 was noticed in smaller structures, mainly in mammospheres with fewer layers of CK14-positive myoepithelial cells (Fig. 6d ), whereas larger mammospheres consisted mainly of myoepithelial cells (Fig. 6c ).
Expression of cytokeratins 14 (green) and 18 (red) in mammospheres formed under non-adherent conditions with cells from goat mammary gland. Mammospheres in images a and b were stained in suspension and in images c and d were embedded in paraffin, and 5 μm sections were stained. Nuclei have been counterstained with DAPI (blue). Scale bar = 100 μm (A–C) and 20 μm (D).
mRNA marker expression.
Transcriptome profile was generated from primary goat mammary samples comprising of approximately 15 million reads. In the draft of approximately 20,000 different bovine RefSeq identifiers (i.e., genes or predicted genes), we searched for markers, previously described in different species, which would indicate presence of specific mammary cell lineages (Table 1). The frequency of reads coding for the different markers could be used to characterize mammary cell culture and infer presence of certain cell types. The highest expression was observed for markers typical for myoepithelial cells, i.e., CK 5, 17, and 14. Highly expressed luminal markers were CK6A, EpCAM, CK18, and CK19. High amount of mRNA for basal marker integrin alpha 6 (CD49f) was detected in the pgMEC. Mesenchyme specific markers SMA, vimentin, catenin-beta 1, SPARC, fibronectin 1, NOV, TGFBR2, ADAM, and matrix metalloproteinases (MMP) were also present, but in lesser amounts. Additionally, proliferation marker Ki67 was detected. mRNA for components of extracellular matrix (ECM), mainly collagens and laminins, were also expressed in the primary culture.
Expression of CSN2 in pgMECs.
CSN2 products were detected at cycle-threshold (Ct) value of 21.81 ± 0.07 and of housekeeping ACTB at Ct value of 15.66 ± 0.10 (Fig. 7). RT-qPCR confirmed that, under certain conditions, the cells were able to synthesize CSN2 mRNA.
Discussion
Our study describes characterization of the caprine primary culture with the emphasis on morphology, differentiation potential, and lineage marker expression. Growth of primary mammary cell cultures from lactating mammary gland on plastic usually results in loss of tissue specific functions (Huynh et al. 1991). Because the growth of MEC on pre-formed extracellular matrices was shown to result in morphological differentiation as well as synthesis of milk (Rose et al. 2002) and additionally enables cytological polarization in vitro (Barcelloshoff et al. 1989), our primary culture was grown on a thin layer of basement membrane matrix. Dissociated goat mammary gland cells grew in a monolayer. Epithelial cells were typically organized as densely packed islands composed of tear drop-shaped cells. Many outlying cells grew individually around the blister-like structures of epithelial islands. During the first passages, large multinucleated cells were frequently noticed. Spontaneous dome formation was observed in post confluent cultures under lactogenic conditions, which is in a way reminiscent of 3D organization of cells in the mammary tissue. It has been previously shown that formation of dome-like structures was connected with fluid under the epithelial cells (Pickett et al. 1975). Functional and structural changes that take place in dome-forming cells might correspond to cellular changes occurring in vivo when tubules and alveoli are developed in the mammary gland at pregnancy (Zucchi and Dulbecco 2002). The domes were composed of mainly luminal cells, indicated by cytokeratin 18 marker. Formation of acini-like aggregates, morphologically similar to those described in mammary epithelial cell lines (German and Barash 2002; Rose et al. 2002), was observed when growing cells for prolonged time on basement membrane matrix. It seems that formation of such structures is necessary for luminal cells to become fully competent. Namely, CSN2 expression and lipid droplets were detected only in dome-forming confluent cultures.
In this paper, we have examined the expression of cytokeratins by pgMEC, using antibodies that are monospecific for a single cytokeratin. Antibodies directed to other markers of myoepithelial, luminal, and stromal cells were also used to define the cultured cells. In addition, RNA sequencing was employed to assess expression of markers, which were selected based on literature data mainly performed in mice and humans. In previous studies using primary cell cultures of goat mammary glands mainly CK18 and pan-cytokeratin were used as markers of epithelial cells (Lerondelle et al. 1999; Milhau et al. 2005; Zheng et al. 2010; Liu et al. 2012; Sorg et al. 2012). In our study, the lineage marker expression indicates that two major cell types were predominant in caprine primary culture, the myoepithelial cells, which stained positively for CK14, CK5, and CD49f and luminal cells, which were CK18 positive. Similarly, in rodents and humans, development of the mammary cell phenotypes has been studied using immunocytochemistry, which identified epithelial and myoepithelial cells (Smalley et al. 1999). It has been previously described for mouse mammary epithelial cells that when grown on plastic, the expression of CK14 and CK18 varied (Smalley et al. 1998). However, when growing the same cells on basement membrane, the cells maintained their differentiated identity of myoepithelial cells, whereas retention of luminal markers by luminal cells depended on homotypic cell–cell contacts and interactions (Smalley et al. 1999). When growing the pgMEC on a basement membrane matrix at lower cell densities, specific types of cells formed isolated islands, whereas at the post confluent stage the luminal and myoepithelial cells grew over each other, but mostly remained in groups of specific lineages even before forming domes.
RNAseq data confirmed that previously described mammary markers are highly expressed also in goat mammary cells. For example, myoepithelial markers CK 5, 17, and 14 were the most expressed. Amongst transcripts previously associated with myoepithelial cells was also integrin alpha 6, also named CD49f, which is a common human and mouse mammary gland stem cell marker, highly expressed by basally located cells (Koukoulis et al. 1991; Stingl et al. 2001; Shackleton et al. 2006; Stingl et al. 2006). MEC use integrins to adhere to the basement membrane and secure their position in the mammary environment. High expression was also observed for luminal markers. In addition to CK18, highly expressed luminal markers were CK6A, EpCAM, and CK19.
The third type of observed cells were fibroblast-like, spindle shaped cells, growing individually around islands of epithelial cells in a random pattern. Some of these cells stained positively for SMA, exhibiting sheets of filaments in the cell cytoplasm. We have previously shown that SMA marks goat mammary myoepithelial cells in vivo (Prpar et al. 2012); but in pgMEC, it might mark a minor fraction of remaining fibroblasts because marked cells exhibit typical spindle-shaped morphology of fibroblasts and do not stain for cytokeratin markers. Similar observation was made for human samples where it has been noticed that myoepithelial cells in vitro often do not express SMA (Stingl et al. 2005). Expression of intermediate filament vimentin, frequently expressed by cells of mesenchymal origin, and also described as a marker of myoepithelial cells in vitro (Zavizion et al. 1996; Pantschenko et al. 2000a), was recognized in pgMEC. Although vimentin was previously described as marker of myoepithelial cells, recent observations indicate that vimentin expression could be attributed to the process of epithelial to mesenchymal transition, which is common during in vitro culture of mouse and human mammary epithelial cells (Stingl et al. 2006; Mani et al. 2008). Other stromal fibroblast-specific markers detected by RNAseq were catenin beta 1, SPARC, fibronectin 1, NOV, TGFBR2, ADAM and MMP proteases, and SFRP1, as also noticed by Casey et al. in mammary stromal fractions of prepartum cows (Casey et al. 2011).
Although the expression of CK18 was relatively low in comparison to CK5, CK17, and CK14, the immunofluorescent staining revealed that there were about 50% of pgMEC that expressed this protein. Similar observation was made for vimentin. It seems that absolute expression of CK5, CK17, and CK14 in myoepithelial cells is greater than that of CK18 in luminal cells. Therefore, the overall abundance of markers is not indicative of the actual proportion of individual cell lineages in the cell culture. Similarly, estrogen receptor was observed to be expressed in luminal cells by immunological staining but its transcripts were not detected by sequencing. In this study the goat sequences were mapped against the bovine transcriptome, therefore it is possible that some of the reads were discarded in the process of mapping, due to insufficient sequence similarities. Consequently, expression of some genes might have been overlooked.
Although the cells were provided with basement membrane matrix, composed of laminin, collagen IV and entactin, they also expressed mRNA coding for ECM proteins. Cells expressed transcription factors involved in cell fate specification, such as STAT5A and GATA3 (Kouros-Mehr et al. 2006; Asselin-Labat et al. 2007; Yamaji et al. 2009). Transcription of Ki67, which is present in all proliferating cells in the active parts of the cell cycle (Gerdes 1990) was also detected, indicating a fraction of proliferating cells. There was no mRNA transcripts detected for caseins, probably because the sequenced RNA originated from non-confluent pgMEC. Rosen and coworkers studied transcription of beta-casein and suggested that expression of CSN2 is induced synergistically by lactogenic hormones, local growth factors, and cell–cell and cell–substratum interactions (Rosen et al. 2009). In our case, the proper cell–cell and cell–substratum interactions were probably lacking in non-confluent cells because when primary culture was grown to an over confluent state expression of beta-casein mRNA was detected, using RT-qPCR. The same cells were also capable of producing milk fat because some lipid droplets were observed by oil red O staining.
To date, mammospheres were described in bovine species (Rauner and Barash 2012), but not in goat. It is pivotal to note that several structures were called mammospheres in literature in recent years, but herein we refer to those which arose under low-attachment conditions in suspension culture. When cells were seeded in B27 supplemented medium, the mammospheres were irregularly shaped, resembling non-spherical floating colonies as observed with bovine MEC (Rauner and Barash 2012). In the medium supplemented with basal membrane matrix pgMEC self-organized in big, lumen forming spherical structures. They were twice as big as structures in medium supplemented with B27, indicating the importance of basement membrane matrix for the formation of 3D structures. After mammosphere dissociation and replating of single cells, we also observed formation of secondary mammospheres, indicating that the cells were able to self-renew. We noted that larger mammospheres had several layers of myoepithelial cells and were sometimes lacking lumen, whereas smaller mammospheres were composed of myoepithelial and luminal cells. Smaller structures probably originated from stem/progenitor cell and the bigger structures arose due to aggregation.
It has been argued recently that mammospheres arise due to aggregation of cells and not from stem/progenitor cells. If the cells are used at proper dilution this can be omitted and the mammosphere model used as a good source of progenitor cells (Dong et al. 2013). Additionally, mammospheres are a good model for lactation studies because they were shown to express milk protein and genes responsible for fat synthesis (Riley et al. 2010).
Based on marker expression it is evident that the primary culture was composed of epithelial and stromal cells. The most expressed epithelial-specific markers in pgMEC were various cytokeratins and integrins, which coincide with data obtained on mammary lineages in mice (Kendrick et al. 2008). Our findings are in accordance with results on human mammary cells, showing that adhesion molecules, tight junction proteins and metalloproteases are highly expressed in the primary culture, which is consistent with expected higher levels of expression in differentiating conditions (Dontu et al. 2003). Additionally, the expression of ECM components, such as laminins and different types of collagens indicates that epithelial cells were able to produce ECM proteins in vitro.
Conclusions
To our knowledge, this is the first report of extensive goat mammary cell culture characterization that combines RNA expression patterns, immunostainings, mammosphere assay, and morphological analyses. In the present study we assessed expression of several markers on RNA and protein level and concluded that mainly cells of epithelial and stromal origin are present in the primary culture. We selected several markers that can be used for characterization of goat mammary cells and tested a number of antibodies, not primarily produced to be used in goat species, but can be successfully used for characterization of goat mammary cells.
References
Anand V, Dogra N, Singh S, Kumar SN, Jena MK, Malakar D, Dang AK, Mishra BP, Mukhopadhyay TK, Kaushik JK, Mohanty AK (2012) Establishment and characterization of a buffalo (Bubalus bubalis) mammary epithelial cell line. Plos One 7(7):e40469
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE (2007) Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9:201–209
Barcelloshoff MH, Aggeler J, Ram TG, Bissell MJ (1989) Functional-differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement-membrane. Development 105:223–235
Bonnefont CMD, Rainard P, Cunha P, Gilbert FB, Toufeer M, Aurel MR, Rupp R, Foucras G (2012) Genetic susceptibility to S. aureus mastitis in sheep: differential expression of mammary epithelial cells in response to live bacteria or supernatant. Physiol Genomics 44:403–416
Casey T, Dover H, Liesman J, DeVries L, Kiupel M, VandeHaar M, Plaut K (2011) Transcriptome analysis of epithelial and stromal contributions to mammogenesis in three week prepartum cows. Plos One 6
Dong QX, Wang DH, Bandyopadhyay A, Gao H, Gorena KM, Hildreth K, Rebel VI, Walter CA, Huang CJ, Sun LZ (2013) Mammospheres from murine mammary stem cell-enriched basal cells: clonal characteristics and repopulating potential. Stem Cell Res 10:396–404
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Gen Dev 17:1253–1270
Duchler M, Schmoll F, Pfneisl F, Brem G, Schellander K (1998) OMEC II: a new ovine mammary epithelial cell line. Biol Cell 90:199–205
Eirew P, Stingl J, Raouf A, Turashvili G, Aparicio S, Emerman JT, Eaves CJ (2008) A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med 14:1384–1389
Gerdes J (1990) Ki-67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluations in human malignancies. Semin Cancer Biol 1:199–206
German T, Barash I (2002) Characterization of an epithelial cell line from bovine mammary gland. In Vitro Cell Dev Biol Anim 38:282–292
Hennighausen L, Robinson GW (2001) Signaling pathways in mammary gland development. Dev Cell 1:467–475
Hu H, Wang JQ, Bu DP, Wei HY, Zhou LY, Li FD, Loor JJ (2009) In vitro culture and characterization of a mammary epithelial cell line from chinese holstein dairy cow. PlosOne 4
Hurley WL (1989) Mammary-gland function during involution. J Dairy Sci 72:1637–1646
Huynh HT, Robitaille G, Turner JD (1991) Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp Cell Res 197:191–199
Ilan N, Barash I, Gootwine E, Shani M (1998) Establishment and initial characterization of the ovine mammary epithelial cell line NISH. In Vitro Cell Dev Biol Anim 34:326–332
Jedrzejczak M, Szatkowska I (2013) Bovine mammary epithelial cell cultures for the study of mammary gland functions. In Vitro Cell Dev Biol Anim 2013
Johansson KE, Heldtander MU, Pettersson B (1998) Characterization of mycoplasmas by PCR and sequence analysis with universal 16S rDNA primers. Methods Mol Biol 104:145–165
Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, Smalley MJ (2008) Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9:591
Keys JE, Cifrian E, Guidry AJ, Farrell HM (1997) Bovine mammary explant versus primary cell cultures: effect of bovine somatotropin and insulin-like growth factor-I on DNA content and protein synthesis. In Vitro Cell Dev Biol Anim 33:206–211
Knight CH, Peaker M (1984) Mammary development and regression during lactation in goats in relation to milk secretion. Q J Exp Physiol 69:331–338
Koukoulis GK, Virtanen I, Korhonen M, Laitinen L, Quaranta V, Gould VE (1991) Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol 139:787–799
Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z (2006) GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127:1041–1055
Lerondelle C, Godet M, Mornex JF (1999) Infection of primary cultures of mammary epithelial cells by small ruminant lentiviruses. Vet Res 30:467–474
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760
Liu XQ, Yang H, Peng JY, Chen QJ, Cao BY (2012) Establishment and evaluation of a new method tissue re-shift for isolation of goat mammary epithelial cells. J Anim Vet Adv 11:2403–2408
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Milhau N, Renson P, Dreesen I, Greenland T, Bellaton C, Guiguen F, Mornex JF, Le Jan C (2005) Viral expression and leukocyte adhesion after in vitro infection of goat mammary gland cells with caprine arthritis-encephalitis virus. Vet Immunol Immunopathol 103:93–99
Pantschenko AG, Barber MR, Woodcock-Mitchell J, Bushmich SL, Yang TJ (2000a) Establishment and characterization of a caprine mammary myoepithelial cell line (CMMyoEC). In Vitro Cell Dev Biol Anim 36:351–356
Pantschenko AG, Woodcock-Mitchell J, Bushmich SL, Yang TJ (2000b) Establishment and characterization of a caprine mammary epithelial cell line (CMEC). In Vitro Cell Dev Biol Anim 36:26–37
Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A 89:9064–9068
Pickett PB, Pitelka DR, Hamamoto ST, Misfeldt DS (1975) Occluding junctions and cell behavior in primary cultures of normal and neoplastic mammary gland cells. J Cell Biol 66:316–332
Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK (2009) Cell contacts in the mouse mammary gland: i. Normal gland in postnatal development and the secretory cycle. 1973. J Mammary Gland Biol 14:295–316
Prpar S, Martignani E, Dovc P, Baratta M (2012) Identification of goat mammary stem/progenitor cells. Biol Reprod 86:117
Rainard P, Riollet C (2006) Innate immunity of the bovine mammary gland. Vet Res 37:369–400
Rauner G, Barash I (2012) Cell hierarchy and lineage commitment in the bovine mammary gland. Plos One 7
Riley LG, Gardiner-Garden M, Thomson PC, Wynn PC, Williamson P, Raadsma HW, Sheehy PA (2010) The influence of extracellular matrix and prolactin on global gene expression profiles of primary bovine mammary epithelial cells in vitro. Anim Genet 41:55–63
Rose MT, Aso H, Yonekura S, Komatsu T, Hagino A, Ozutsumi K, Obara Y (2002) In vitro differentiation of a cloned bovine mammary epithelial cell. J Dairy Res 69:345–355
Rosen JM, Kabotyanski EB, Rijnkels M, Freeman-Zadrowski C, Buser AC, Edwards DP (2009) Lactogenic hormonal induction of long distance interactions between beta-casein gene regulatory elements. J Biol Chem 284:22815–22824
Sanchez O, Montesino R, Toledo JR, Rodriguez E, Diaz D, Royle L, Rudd PM, Dwek RA, Gerwig GJ, Kamerling JP, Harvey DJ, Cremata JA (2007) The goat mammary glandular epithelial (GMGE) cell line promotes polyfucosylation and N, N '-diacetyllactosediaminylation of N-glycans linked to recombinant human erythropoietin. Arch Biochem Biophys 464:322–334
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE (2006) Generation of a functional mammary gland from a single stem cell. Nature 439:84–88
Shi H, Luo J, Zhu J, Li J, Sun Y, Lin X, Zhang L, Yao D, Shi H (2013) PPARɣ regulates genes involved in triacylglycerol synthesis and secretion in mammary gland epithelial cells of dairy goats. PPAR Res 2013:10
Smalley MJ, Titley J, O’Hare MJ (1998) Clonal characterization of mouse mammary luminal epithelial and myoepithelial cells separated by fluorescence-activated cell sorting. In Vitro Cell Dev Biol Anim 34:711–721
Smalley MJ, Titley J, Paterson H, Perusinghe N, Clarke C, O’Hare MJ (1999) Differentiation of separated mouse mammary luminal epithelial and myoepithelial cells cultured on EHS matrix analyzed by indirect immunofluorescence of cytoskeletal antigens. J Histochem Cytochem 47:1513–1524
Smith GH, Mehrel T, Roop DR (1990) Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ 1:161–170
Sorg D, Potzel A, Beck M, Meyer HHD, Viturro E, Kliem H (2012) Effects of cell culture techniques on gene expression and cholesterol efflux in primary bovine mammary epithelial cells derived from milk and tissue. In Vitro Cell Dev Anim 48:550–553
Stiening CM, Hoying JB, Abdallah MB, Hoying AM, Pandey R, Greer K, Collier RJ (2008) The effects of endocrine and mechanical stimulation on stage I lactogenesis in bovine mammary epithelial cells. J Dairy Sci 91:1053–1066
Stingl J (2009) Detection and analysis of mammary gland stem cells. J Pathol 217:229–241
Stingl J, Eaves CJ, Zandieh I, Emerman JT (2001) Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat 67:93–109
Stingl J, Raouf A, Emerman JT, Eaves CJ (2005) Epithelial progenitors in the normal human mammary gland. J Mammary Gland Biol Neoplasia 10:49–59
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439:993–997
Sun YL, Lin CS, Chou YC (2005) Gene transfection and expression in a primary culture of mammary epithelial cells isolated from lactating sows. Cell Biol Int 29:576–582
Sun YL, Lin CS, Chou YC (2006) Establishment and characterization of a spontaneously immortalized porcine mammary epithelial cell line. Cell Biol Int 30:970–976
Tong HL, Li QZ, Gao XJ, Yin DY (2012) Establishment and characterization of a lactating dairy goat mammary gland epithelial cell line. In Vitro Cell Dev Anim 48:149–155
Villadsen R, Fridriksdottir AJ, Rønnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, Bissell MJ, Petersen OW (2007) Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol 177:87-101
Yamaji D, Na RS, Feuermann Y, Pechhold S, Chen WP, Robinson GW, Hennighausen L (2009) Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Gen Dev 23:2382–2387
Yuan YG, Cheng Y, Guo L, Ding GL, Bai YJ, Miao MX, An LY, Zhao JH, Cao YJ (2009) Cloned kids derived from caprine mammary gland epithelial cells. Theriogenology 72:500–505
Zavizion B, van Duffelen M, Schaeffer W, Politis I (1996) Establishment and characterization of a bovine mammary epithelial cell line with unique properties. In Vitro Cell Dev Anim 32:138–148
Zheng YM, He XY, Zhang Y (2010) Characteristics and EGFP expression of goat mammary gland epithelial cells. Reproduction in domestic animals =. Zuchthygiene 45:e323–e331
Zucchi I, Dulbecco R (2002) Proteomic dissection of dome formation in a mammary cell line. J Mammary Gland Biol 7:373–384
Acknowledgments
This work was supported by funding from Slovenian Research Agency (ARRS; Research programme P4-0220 and Research project J4-3621), through the ARRS young researcher scheme and by a grant from Slovene Human Resources Development and Scholarship Fund for funding of cooperation of Slovene graduate students abroad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Prpar Mihevc, S., Ogorevc, J. & Dovc, P. Lineage-specific markers of goat mammary cells in primary culture. In Vitro Cell.Dev.Biol.-Animal 50, 926–936 (2014). https://doi.org/10.1007/s11626-014-9796-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-014-9796-4