Abstract
In spite of previous reports, the role of transforming growth factor-β1 (TGF-β1) on cardiomyocyte differentiation, especially in the present autologous serum (AS) in culture medium, is still unclear. So, the purpose of this study was to investigate the potential of rat bone marrow mesenchymal stem cells (rBMSCs) to proliferate and differentiate towards cardiomyocyte lineage with the use of AS. Most expansion protocols use a medium supplemented with fetal bovine serum (FBS) as nutritional supplement. FBS is an adverse additive to cells that are proliferated for therapeutic purposes in humans because the use of FBS carries the risk of transmitting viral and bacterial infections and proteins that may initiate xenogeneic immune responses. Therefore, bone marrow cells were cultured in a medium supplemented with 10% AS, 10% FBS, and serum free medium (SFM). Then, rBMSCs were cultured with TGF-β1 (10 ng/ml) for 2 wk. The number of viable cells in AS and FBS groups were measured with MTT assay. Beating areas frequency, up to fourth week after plating, were monitored and evaluated daily. The characteristics of cardiomyocytes were assessed by semi-quantitative reverse transcription polymerase chain reaction and western blot. MTT result indicated that rBMSCs in AS proliferated markedly faster than FBS and SFM. The number of beating areas significantly increased in AS compared to FBS medium. A noticeable increase in the cardiac genes expression was observed in AS. Moreover, western blot analysis confirmed that cardiac proteins were increased in the AS condition. In conclusion, the present study could be extended toward the safe culture of MSCs for the treatment of heart defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) is one of the major causes of death and disability in the developed world (Wei et al. 2009). Since mammalian cardiomyocytes rarely regenerate after birth, a necrosis in myocardium is repaired by fibrous scar (Liu et al. 2003). Although drugs are usually used for the treatment of chronic heart failure, for heart failure for patients with end-stage disease, cardiac transplantation is necessary. New therapeutic strategies are needed to improve the prognosis and quality of life for patients who survived an acute myocardial infarction (AMI) (Wei et al. 2009). Cell therapy has become a new therapeutic choice for patients with ischemic heart disease. Cardiac cell therapy aims to restore myocardial tissue and to get better neovascularization (Beeres et al. 2005). A hopeful therapy to return the cardiac function is the local transplantation of stem/progenitor cells. It is now accepted that adherent cells isolated from bone marrow and proliferated in vitro represent a source of mesenchymal stem cells (MSCs) that can convert to connective tissue cell types. MSCs present a good source of cells for MI therapy (Ohnishi et al. 2007), as they could have been gotten by routine bone marrow aspiration easily, they exhibit low immunogenicity and the ability to proliferate and differentiate. Several studies have been developed to differentiate stem cells into cardiomyocytes. Co-culturing MSCs with cardiomyocytes converted MSCs into cardiomyocytes (Fukuhara et al. 2003). MSCs were treated with 5-azacytidine, which were induced to express cardiomyocyte phenotype characteristics (Burlacu et al. 2008). Transforming growth factor-β1 (TGF-β1) is a multifunctional cytokine involved in the differentiation, survival, and growth of a variety of cells (Li et al. 2005a, b). TGF-β1 stimulated cardiogenic differentiation of MSCs in vitro (Gwak et al. 2009). TGF-β1 and TGF-β2 stimulated cardiogenic differentiation of MSCs (Li et al. 2005a, b) and embryonic stem cells in vitro (Singla and Sun 2005), respectively. Some clinical studies have shown safety and possibility of BMSCs transfer in patients with heart failure, and it was shown that clinical parameters improved after cell therapy (Tse et al. 2003; Wollert et al. 2004). Some studies have shown that MSCs differentiate to express cardiomyocyte-specific markers after their local delivery into the injured heart (Kudo et al. 2003). In vitro studies have shown that MSCs can secrete a range of angiogenic, antiapoptotic, and mitogenic factors, such as hepatocyte growth factor, vascular endothelial growth factor (VEGF), and insulin-like growth factor 1 (Kinnaird et al. 2004). Main hurdles for clinical use of MSCs are related to using fetal bovine serum (FBS) for their culture. Most expansion protocols use a medium supplemented with FBS. Serum provides vital nutrients, growth factors, and attachment factors for cells. FBS, however, is an undesired source of xenogeneic antigens that are internalized by MSCs (Kocaoemer et al. 2007). It consists of possible viral or bacterial infections, prions, and immune or local inflammatory reactions due to contamination with bovine proteins, which cannot be eliminated even by several washings after proliferation, and this may lead to antibody formation or rejection of the transplanted MSCs (Stute et al. 2004). Anaphylactic reactions to FBS in patients treated with cultured cells have been previously reported (Macy et al. 1989), even leading to arrhythmias after cellular cardioplasty (Chachques et al. 2004a, b). Increasing the safety of clinical treatments with MSCs requires utilizing autologous human serum. Therefore, MSCs culture require appropriate alternatives for the use of animal serums as a supplement, such as serum free medium (SFM) especially for embryonic stem cells (Li et al. 2005a, b; Passier et al. 2005; Chen et al. 2007), autologous serum (AS) (Yamamoto et al. 2003; Chachques et al. 2004a, b), plasma (Sun et al. 2008), and platelet-rich plasma (PRP) (Kocaoemer et al. 2007).
In the present study, we expanded rat bone marrow MSCs (rBMSCs) with rat serum, FBS, and SFM and used TGF-β1 for cardiogenic induction. We present the results of the experiments comparing expansion, morphology, cardiomyocyte differentiation potential, and cardiac gene expression of rBMSCs expanded in different serum preparations.
Materials and Methods
Autologous serum preparation.
Five hundred-microliter peripheral blood from each sample twice weekly was collected and then was allowed to coagulate at 4°C for 2 h and centrifuged at 2,500 rpm for 10 min. Then, the serum was collected, and the yield was about 150–200 μl serum every time.
Isolation and culture of rBMSCs.
Under general anesthesia (intraperitoneal administration of chloral hydrate, 300 mg/kg), about 100 μl bone marrow was aspirated from the tibia with a syringe containing 1 ml Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Big Cabin, OK) with 40 U/ml heparin. Then, it was centrifuged at 1,200 rpm for 5 min. The bone marrow pellet was resuspended with DMEM, and it was divided into three groups, first group containing 10% FBS (Gibco and Biosera, France), second group containing 10% AS, and third group in SFM (StemPro MSC SFM, Gibco), and they were cultured in 3-cm2 Petri dish at 37°C in humid air with 5% CO2 incubator for 48 h before the first medium change. The mesenchymal population was isolated on the base of its adherence ability (Hahn et al. 2008). At 80–90% confluence, the cells were trypsinized and subcultured. Second passage MSCs were used in all experiments.
Flow cytometric analysis.
After the 0.25% trypsin-EDTA treatment, the cells cultured in AS and FBS were resuspended at a density of 105 cells/200 μl PBS, and the incubations were performed on ice. Cells were incubated with the monoclonal antibodies CD34 (Abcam, Cambridge, MA, ab81289) and CD90-flurescin isothiocyanate (FITC; AbD Serotec, Raleigh, NC) conjugated (at concentrations indicated by the manufacturer) for 30 min, washed in PBS–FBS, and then incubated with the FITC-conjugated secondary antibodies, for 20 min. After washing, cells were analyzed in flow cytometry (BD, FACSCalibur).
MTT assay.
MTT assay for viability of MSCs was previously described (Eslaminejad et al. 2009). Briefly, third passage AS, and FBS-supplemented and SFM cultures were transferred into 96-well plates containing a 5:1 ratio of medium and MTT solution (5 mg/ml in PBS) and incubated for 2 h at 37°C. After removing the culture media, 0.5 ml of extraction solution [dimethyl sulfoxide (DMSO)] was added. The absorbance of the supernatant was read with a microplate reader at 540 nm. Cell number was determined through a standard curve calibrated using a known number of cells counted in a Coulter counter.
In vitro differentiation potential.
For osteogenic differentiation, the 80% subconfluent culture of MSCs from second passage (AS and FBS) was used. Induction medium containing DMEM supplemented with 50 mg/ml ascorbic 2-phosphate (Sigma, St. Louis, MO, A8960), 10 nM dexamethasone (Sigma, d4902), and 10 mM β-glycerol phosphate (Sigma, G9422) was added to cultures. After 3 wk, cells were fixed in methanol for 10 min and stained with Alizarin Red solution for 2 min to reveal the deposition of mineralized matrix.
For adipogenic differentiation, DMEM containing 100 nM dexamethasone and 50 mg/ml indomethacin (Sigma) was used. After 3 wk, cells were fixed with 4% formalin at room temperature, washed with 70% ethanol, and stained with Oil red solution in 99% isopropanol for 15 min (Eslaminejad et al. 2009).
Cardiomyogenic differentiation.
Myocardial differentiation of MSCs was previously described (Gwak et al. 2009). Briefly, to induce cardiogenic differentiation in all groups, rBMSCs were cultured with a 10-ng/ml TGF-β1 supplement (R&D Systems, Minneapolis, MN) for 2 wk. rBMSCs cultured without TGF-β1 served as a control group. Differentiation medium applied in our study consisted of Dulbecco’s modified Eagle’s medium (DMEM, high glucose, with sodium pyruvate formulation; l-glutamine; Gibco) supplemented with 1% penicillin–streptomycin (Gibco), 10% FBS (Gibco), and 10% AS and SFM. Each group was observed daily, and the percent of beating areas in different groups was determined.
Semi-quantitative RT-PCR.
In reverse transcription polymerase chain reaction (RT-PCR) test, total RNA was extracted from native neonatal rat cardiomyocytes (NRC, as a positive control), differentiated rBMSCs in AS, FBS, and SFM, and untreated cells (as a negative control) at second week after treatment using RNX plus™ according to the manufacturer’s recommendations (Cinnagen, Tehran, Iran). cDNA synthesis was performed using primers, oligo-dT, and reverse transcriptase (k1632; Fermentas, Hanover, MD). cDNA was amplified by PCR using 200 μM dNTPs, 2.5 μl cDNA, 1X PCR buffer (AMS), 1 unit/25 μl reaction Taq DNA polymerase (Fermentas), and 0.5 μM of each primer pair. The endogenous “house-keeping” gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was also quantified to normalize differences in the added RNA and efficiency of RT. PCR primers were designed for cardiac troponin T and GATA4 as follows: cardiac troponin T (For: AAG AGT GAG AAG AGA CAG AC, Rev: CGT TGA TTT CGT ATT TCT G) GATA4 (For: AAA TTA AGG GGA CCA CAC, Rev: GAG AGA CAA CGT AAA TAA), and GAPDH (For: ATA GAC AAG AAG GTG ATG GT, Rev: GCA TCA GAA GGT AGA AAG). PCR was carried out for 35 cycles of denaturing (94°C, 30 s), annealing (58°C for cardiac troponin T, 62°C for GATA4, 30 s), and extension (72°C, 60 s) with a final extension at 72°C for 5 min. The PCR products were visualized by electrophoresis on 1.7% (w/v) agarose gels with ethidium bromide staining and analyzed with a gel documentation system (Gel Doc 1000, Bio-Rad Laboratories, Hercules, CA). Relative genes expression was normalized to GAPDH expression.
Western blot analysis.
To evaluate rBMSCs cardiomyogenic differentiation, samples from native neonatal rat cardiomyocyte (NRC, as a positive control) and cultured rBMSCs treated with or without TGF-β1 (as a negative control) in AS, FBS-supplemented medium, and SFM were analyzed with western blot analysis at the second week. Samples were washed two times with cold PBS and lysed by adding a buffer containing 52 mM Tris HCL, pH 6.8, glycerol 10%, 150 mM NaCl, 1 mM EDTA, and 1% Triton X100. Proteins were electrophoretically separated on 10% SDS polyacrylamide gels and transferred to polyvinyl difluoride (PVDF, Bio-Rad, 162-0177). For protein detection, membranes were incubated with primary antibodies against cardiac troponin T and GAPDH (Abcam, Cambridge, UK) for 2 h at room temperature, then washed and incubated with secondary antibodies conjugated to horseradish peroxidase (HRP, Sigma) for 1 h at room temperature. Proteins were visualized using Immobilon™ Western blotting detection reagents (Millipore, Billerica, MA).
Statistical analysis.
Values are reported as mean ± SD. Statistical significance was evaluated with Student’s t test and ANOVA. A significant difference was defined as p < 0.05.
Results
Characterization of cultured BMSCs.
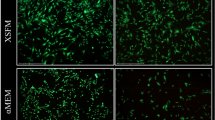
The culture of rBMSCs in FBS-supplemented medium yielded variably high numbers of contaminating hematopoietic cells. In contrast, AS-supplemented cultures and SFM resulted in few numbers of hematopoietic cells (Fig. 1). rBMSCs cultured in the presence of either FBS, AS, or SFM exhibited the characteristic MSCs-like spindle-shape; however, fine differences in morphology were detected. MSCs cultured in AS-containing medium and SFM showed a slender shape compared to the wider MSCs cultured in the presence of FBS (Fig. 1).
Phenotype of rBMSCs.
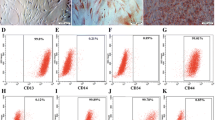
rBMSCs were isolated from rat bone marrow on the basis of the adherent properties of these cells and characterized by flow cytometry for common stem cell surface markers in AS and FBS medium. After second passage, many BMSCs in AS and FBS media expressed CD90, which is a mesenchymal cell-specific surface marker (AS, 99.54%; FBS, 99.77%; Fig. 2A, C , respectively). In contrast, the majority of the cells were negative for CD34, a hematopoietic or hemangioblastic surface marker (AS, 2.79%; FBS, 3.93%; Fig. 2B, D , respectively). This result suggests that majority of ADSCs exhibit MSCs properties.
Phenotypes of rBMSCs isolated from rat bone marrow by flow cytometry. (A, B) Cells cultured in AS; (C, D) cells cultured in FBS. Expressions of cell surface antigens are shown with black and isotype controls by purple. CD90, a mesenchymal stem cells marker and CD34, a hematopoietic progenitor marker.
Multilineage differentiation potential.
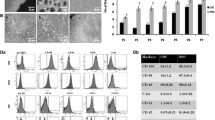
rBMSCs at second passage in AS and FBS were investigated for their potential to differentiate into adipogenic and osteogenic lineages. After osteogenic induction, BMSCs began to mineralize their matrix then cultures were stained positively with Alizarin Red; also, they differentiated to adipocytes then their lipid droplets were stained with Oil red staining (Fig. 3).
Viability of MSCs.
The confluent passage 2 cultures supplemented with AS show that it had more viable cells than those supplemented with FBS and SFM (Fig. 4).
Myocardial differentiation.
After treatment with TGF-β1, the morphological differentiation of BMSCs into cardiomyocytes cells changed gradually. Cells connected with adjacent cells and formed spherical structures within 2–3 wk.
Serum affected the number of beating areas. They were also observed from second week up to fourth week. Statistical analysis of the beating areas percent at different serums showed significant increase between AS group compared with SFM and FBS group. On average, 31.7 ± 2.1 beating areas per plate were observed in 10% AS, whereas 2.3 ± 0.6 beating areas were observed in 10% FBS and 18.8 ± 1.6 in SFM. Cells in control group did not show any beating area up to 4 wk after plating (Fig. 5).
Semi-quantitative RT-PCR.
To determine whether the increase in the number of beating areas resulted in a comparable increase in the expression of cardiac genes, RT-PCR analysis was performed on BMSCs in AS, SFM, and FBS groups. We evaluated the effects of serum treatment on differentiation of rBMSCs into cardiomyocyte. After 2 wk treatment with TGF-β1, RT-PCR results showed that expression of GATA4 and cardiac troponin T was observed in three experimental groups. Semi-quantitative analysis revealed that in AS group, cardiac genes were higher compared with SFM and FBS during differentiation (cardiac troponin T, 305.83 ± 2.8, 201.43 ± 1.7, and 90.814 ± 1.9; GATA4, 315.37 ± 1.8, 230.568 ± 1.2, and 98.546 ± 1, respectively), and control group (without TGF-β1) did not show cardiac genes expression. In control (not treated) group, at 4 wk after plating, only the expression of GAPDH was detectable (P < 0.05, Fig. 6).
Effect of serum on the expression of cardiac genes rBMSCs-derived cardiomyocytes in AS, FBS, and SFM (A), relative expression GATA4 and cardiac troponin T (*P < 0.05, AS vs. SFM and FBS; **P < 0.05, SFM vs. FBS) genes using GAPDH mRNA levels as an internal control (B). NRCs neonatal rat cardiomyocytes.
Western blot.
Increased expression of cardiac proteins in AS-supplemented medium was confirmed by western blot analysis. In FBS medium, cardiac troponin T is at the detection limit of the assay, but in SFM, it had a better band than FBS, whereas in AS group, there was clear band for cardiac troponin T. The reliability of the analysis was confirmed with cultured neonatal rat cardiomyocytes under the same conditions (Fig. 7).
Discussion
In the present study, we demonstrated that rBMSCs cultured in induction medium containing AS and TGF-β1 exhibit higher proliferation rates and capacity to differentiate into cardiomyocyte than rBMSCs cultured with medium supplemented with 10% FBS and SFM in vitro. The differentiated cells expressed cardiac troponin T and GATA4 (Figs. 6 and 7). Previous studies reported that MSCs differentiation into cardiomyocytes can be induced by co-culturing with cardiomyocytes (Fukuhara et al. 2003), or 5-azacytidine treatment (Burlacu et al. 2008). The co-culture method results in a low cardiogenic differentiation rate, and 5-azacytidine has a potentially unsafe nonspecific demethylating activity (Liu et al. 2003), suggesting that these process are not likely clinically applicable. TGF-β1 is known to play an important role in embryonic heart development (Akhurst et al. 1990). TGF-β1 has been shown to induce cardiomyocytes differentiation from mouse embryonic stem cells (Singla and Sun 2005). C-kit-positive MSCs treated with TGF-β1 increased expression of cardiac markers, such as troponin T, connexin-43, troponin I, NKx-2.5, and GATA-4 (Li et al. 2005a, b). TGF-β can also induce the differentiation of functional smooth muscle cells from a neural crest stem cells line by the activation of Smad2 and Smad3 signaling pathways (Chen and Lechleider 2004). Our results show that TGF-β1 promotes BMSCs cardiogenic differentiation in vitro.
Cell-based myocardial regenerative therapy is one promising way. In this method, usually, expansion of MSCs is performed by cell culture with medium supplemented with FBS (Wollert et al. 2004; Gwak et al. 2009; Wei et al. 2009). For clinical application of stem cells expanded with FBS, some problems may occur. These include possible viral, bacterial, and prion transmission from cows (Nimura et al. 2008). In one experiment, osteogenesis imperfecta antibodies against bovine serum proteins developed in a patient after treatment with hMSCs cultured by FBS; finally, this patient did not exhibit successful cellular transplantation (Horwitz et al. 2002). To overcome these problems, the use of AS is strongly recommended for the isolation and proliferation of MSCs. Several studies have compared the effects of FBS and AS on the expansion of MSCs (Nimura et al. 2008; Bogdanova et al. 2010). Yamamoto and his collaborators investigated proliferation and differentiation of MSCs in AS (Yamamoto et al. 2003). Also, it was indicated in our previous experiment that AS enhances osteogenic differentiation and cell viability in MSCs (Eslaminejad et al. 2009). In 2008, Pountos et al. found AS could have enhanced osteogenic differentiation (Pountos et al. 2008). Directly using cells cultured with FBS into ischemic myocardium appears to present a substrate for electrical instability, leading to malignant arrhythmia. Chachques et al. in 2004 cultured autologous myoblasts in medium supplemented with AS and transplanted these cells into infarcted LV in 20 patients (Chachques et al. 2004a, b). In the present study, we examined whether AS could increase bone marrow MSCs cardiogenic differentiation compared to FBS and SFM.
MSCs showed the same characteristics as those previously described for MSCs culture. The cells showed a higher proliferative rate; using flow cytometry analysis, we recognized that isolated MSCs, when expanded in vitro, maintained their phenotypic markers such as CD90 but not hematopoietic lineage marker CD34 (Fig. 2) and their differentiation capacity to osteogenic and adipogenic lineages (Fig. 3). It has been already demonstrated that increasing cardiomyocyte differentiation from human embryonic stem cells (ESCs) reduced FBS and in serum-free cultures (Passier et al. 2005; Taha and Valojerdi 2008). However, the results from these studies suggested that FBS in early stage of ESCs differentiation fallowing SF medium is necessary to maximize differentiation efficiency.
In order to assess whether rBMSCs viability could have been influenced by serum, MTT assay was performed. The number of viable cells in AS-supplemented was much higher than in FBS-supplemented and SFM in second passage cultures. These studies indicate that AS supports better extension of rBMSCs than FBS. The beating areas frequency rate of the rBMSCs-derived cardiomyocytes in the group with AS was better than the group with FBS. The increase in the number of beating areas in AS suggested a greater efficiency in cardiogenic differentiation. We evaluated the effects of TGF-β1 treatment on induction of rBMSCs into cardiomyocytes in AS, FBS, and SFM. After 2 wk treatment with TGF-β1 in AS, FBS, and SFM group, semi-quantitative RT-PCR showed that expression of GATA4 and cardiac troponin T was higher in rBMSCs cultured with AS-supplemented medium than SFM and FBS-supplemented. These results indicate that AS has a higher ability to support cardiac differentiation of rBMSCs. Results of western blot analysis also confirmed increased expression of cardiac proteins such as cardiac troponin T in AS-containing medium.
In the present study, we investigated the bovine serum-induced changes of one batch to other batches. So, we tried to use different batches or even different brands of FBS for cell culturing. Ultimately, our results showed that no significant differences were observed between groups.
Passier et al. (2005) reported mRNA and protein expression of cardiac markers were increased in low percentage of FBS especially SFM. Taha and Valojerdi (2008) also investigated effect of bone morphogenetic protein-4 on cardiac differentiation from mouse embryonic stem cells (ESCs) in serum-free and low-serum media. They found that in the complete lack of serum, neither control nor BMP-4 groups resulted in cardiomyocytes differentiation. Addition of FBS to hanging drop stage resulted in the form of beating area in some BMP-4-treated EBs. In the best designed model, only hanging drop and the first 24 h of plating stage were carried out in the presence of FBS. Noticeably, using AS, we could evade infection of cultured stem cells from bovine diseases and adverse immune reaction.
In conclusion, the present study could be extended toward the safe culture of MSCs for the treatment of heart defects. Pre-differentiating MSCs toward cardiomyocytes using AS prior to transplantation could be useful in enhancing the therapeutic efficiency for MI. It is required to examine whether BMSCs proliferated in AS have the ability to produce heart-like tissue in vivo.
References
Akhurst R. J.; Lehnert S. A.; Faissner A.; Duffie E. TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development 108: 645–56; 1990.
Beeres S. L.; Atsma D. E.; van der Laarse A.; Pijnappels D. A.; van Tuyn J.; Fibbe W. E.; de Vries A. A.; Ypey D. L.; van der Wall E. E.; Schalij M. J. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J. Am. Coll. Cardiol. 46: 1943–52; 2005.
Bogdanova A.; Bērziņš U.; Brūvere R.; Eivazova G.; Kozlovska T. Adipose-derived stem cells cultured in autologous serum maintain the characteristics of mesenchymal stem cells. Proc. Latv. Acad. Sci., Sect. B, Nat. Exact Appl. Sci. 64: 106–13; 2010.
Burlacu A.; Rosca A. M.; Maniu H.; Titorencu I.; Dragan E.; Jinga V. Promoting effect of 5-azacytidine on the myogenic differentiation of bone marrow stromal cells. Eur. J. Cell Biol. 87: 173–84; 2008.
Chachques J. C.; Herreros J.; Trainini J.; Juffe A.; Rendal E.; Prosper F. Autologous human serum for cell culture avoids the implantation of cardioverter-defibrillators in cellular cardiomyoplasty. Int. J. Cardiol. 95: 29–33; 2004a.
Chachques J. C.; Herreros J.; Trainini J.; Juffe A.; Rendal E.; Prosper F.; Genovese J. Life-threatening arrhythmias after cellular cardioplasty. Autologous human serum for cell culture avoids the implantation of cardioverter-defibrillators in cellular cardiomyoplasty. Int. J. Cardiol. 95: S29–S33; 2004b.
Chen H. F.; Kuo H. C.; Chien C. L.; Shun C. T.; Yao Y. L.; Ip P. L.; Chuang C. Y.; Wang C. C.; Yang Y. S.; Ho H. N. Derivation, characterization and differentiation of human embryonic stem cells: comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum. Reprod. 22: 567–77; 2007.
Chen S.; Lechleider R. J. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ. Res. 94: 1195–202; 2004.
Eslaminejad M. B.; Rouhi L.; Arabnajafi M.; Baharvand H. Rat marrow-derived mesenchymal stem cells developed in a medium supplemented with the autologous versus bovine serum. Cell Biol. Int. 33: 607–16; 2009.
Fukuhara S.; Tomita S.; Yamashiro S. Direct cell-cell interaction of cardiomyocytes is key for bone marrow stromal cells to go into cardiac lineage in vitro. J. Thorac. Cardiovasc. Surg. 125: 1470–80; 2003.
Gwak S. J.; Bhang S. H.; Yang H. S.; Kim S. S.; Lee D. H.; Lee S. H. In vitro cardiomyogenic differentiation of adipose-derived stromal cells using transforming growth factor-beta1. Cell Biochem. Funct. 27: 148–54; 2009.
Hahn J. Y.; Cho H. J.; Kang H. J.; Kim T. S.; Kim M. H.; Chung J. H.; Bae J. W.; Oh B. H.; Park Y. B.; Kim H. S. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J. Am. Coll. Cardiol. 51: 933–43; 2008.
Horwitz E. M.; Gordon P. L.; Koo W. K.; Marx J. C.; Neel M. D.; McNall R. Y. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc. Natl. Acad. Sci. U. S. A. 99: 8932–7; 2002.
Kinnaird T.; Stabile E.; Burnett M. S. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 94: 678–85; 2004.
Kocaoemer A.; Kern S.; Klüter H.; Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 25: 1270–8; 2007.
Kudo M.; Wang Y.; Wani M. A.; Xu M.; Ayub A.; Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J. Mol. Cell. Cardiol. 35: 1113–9; 2003.
Li T. S.; Hayashi M.; Ito H.; Furutani A.; Murata T.; Matsuzaki M.; Hamano K. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation 111: 2438–45; 2005a.
Li Y.; Powell S.; Brunette E.; Lebkowski J.; Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol. Bioeng. 91: 688–98; 2005b.
Liu Y.; Song J.; Liu W.; Wan Y.; Chen X.; Hu C. Growth and differentiation of rat bone marrow stromal cells: does 5-azacytidine trigger their cardiomyogenic differentiation? Cardiovasc. Res. 58: 460–8; 2003.
Macy E.; Bulpitt K.; Champlin R. E.; Saxon A. Anaphylaxis to infusion of autologous bone marrow: an apparent reaction to self, mediated by IgE antibody to bovine serum albumin. J. Allergy Clin. Immunol. 83: 871–5; 1989.
Nimura A.; Muneta T.; Koga H.; Mochizuki T.; Suzuki K.; Makino H.; Umezawa A.; Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 58: 501–10; 2008.
Ohnishi S.; Ohgushi H.; Kitamura S.; Nagaya N. Mesenchymal stem cells for the treatment of heart failure. Int. J. Hematol. 86: 17–21; 2007.
Passier R.; Oostwaard D. W.; Snapper J.; Kloots J.; Hassink R. J.; Kuijk E. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells. 23: 772–80; 2005.
Pountos I.; Georgouli T.; Giannoudis P. V. The effect of autologous serum obtained after fracture on the proliferation and osteogenic differentiation of mesenchymal stem cells. Cell. Mol. Biol. 54: 33–9; 2008.
Singla D. K.; Sun B. Transforming growth factor-b2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochem. Biophys. Res. Commun. 332: 135–41; 2005.
Stute N.; Holtz K.; Bubenheim M.; Lange C.; Blake F.; Zander A. R. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp. Hematol. 32: 1212–25; 2004.
Sun X.; Gan Y.; Tang T.; Zhang X.; Dai K. In vitro proliferation and differentiation of human mesenchymal stem cells cultured in autologous plasma derived from bone marrow. Tissue Eng. Part A. 14: 391–400; 2008.
Taha M. F.; Valojerdi M. R. Effect of bone morphogenetic protein-4 on cardiac differentiation from mouse embryonic stem cells in serum-free and low-serum media. Int. J. Cardiol. 127: 78–87; 2008.
Tse H. F.; Kwong Y. L.; Chan J. K.; Lo G.; Ho C. L.; Lau C. P. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 361: 47–9; 2003.
Wei H. M.; Wong P.; Hsu L. F.; Shim W. Human bone marrow-derived adult stem cells for post-myocardial infarction cardiac repair: current status and future directions. Singap. Med. J. 50: 935–42; 2009.
Wollert K. C.; Meyer G. P.; Lotz J.; Ringes-Lichtenberg S.; Lippolt P.; Breidenbach C.; Fichtner S.; Korte T.; Hornig B.; Messinger D.; Arseniev L.; Hertenstein B.; Ganser A.; Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364: 141–8; 2004.
Yamamoto N.; Isobe M.; Negishi A.; Yoshimasu H.; Shimokawa H.; Ohya K. Effects of autologous serum on osteoblastic differentiation in human bone marrow cells. J. Med. Dent. Sci. 50: 63–9; 2003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Rouhi, L., Kajbafzadeh, A.M., Modaresi, M. et al. Autologous serum enhances cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells in the presence of transforming growth factor-β1 (TGF-β1). In Vitro Cell.Dev.Biol.-Animal 49, 287–294 (2013). https://doi.org/10.1007/s11626-013-9597-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-013-9597-1