Abstract
Recent findings have demonstrated that amniotic fluid cells are an interesting and potential source of mesenchymal stem cells (MSCs). In this study, we isolated MSCs from canine amniotic fluid and then characterized their multilineage differentiation ability. Canine amniotic fluid stem (cAFS) cells at passage 5 had a fibroblast-like morphology instead of forming colonies and were positive for pluripotent stem cell markers such as OCT4, NANOG, and SOX2. Flow cytometry analysis showed the expression of MSC surface markers CD44, CD29, and CD90 on the cAFS cells. In addition, these cells were cultured under conditions favorable for adipogenic, chondrogenic, and osteogenic induction. The results of this experiment confirmed the mesenchymal nature of cAFS cells and their multipotent potential. Interestingly, although the cells exhibited a fibroblast-like morphology after hepatogenic induction, reverse transcription-polymerase chain reaction revealed that the expression of several hepatic genes, such as albumin, tyrosine aminotransferase, and alpha-1 antiproteinase, increased following maturation and differentiation. These findings indicated that cAFS cells have functional properties similar to those of hepatocytes. Taken together, the results of our study demonstrated that cAFS cells with mesenchymal characteristics can be successfully isolated from canine amniotic fluid and possess functional properties characteristic of hepatocytes. The findings of our work suggest that cAFS cells have the potential to be a resource for cell-based therapies in a canine model of hepatic disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous reports have suggested that amniotic fluid cells (AFCs) are a new potential source of mesenchymal stem cells, especially since AFCs are not associated with the ethical issues surrounding embryonic stem cell research (Sessarego et al. 2008; Cananzi et al. 2009). Amniotic fluid is considered an important diagnostic tool for evaluating the fetal status during pregnancy due to its contact with the fetus during development. Several recent reports have found that stem cells derived from human AFCs express pluripotent markers such as OCT4, NANOG, and SOX2 (You et al. 2009; Yadav et al. 2010; Decembrini et al. 2011). Further research has also demonstrated the multipotent potential of amniotic fluid stem (AFS) cells by showing that they undergo successful differentiation into multiple lineages including adipocytes, osteocytes, chondrocytes, and neuronal cells (Kim et al. 2007). Some of these differentiated cells are being tested for possible use in cell-based therapy (You et al. 2009). However, the safety and efficacy of AFS cells must first be tested in preclinical animal models.
Dogs have been gaining attention from biomedical researchers as a new bridge laboratory animal model for the study of human diseases (Neff and Rine 2006; Schneider et al. 2007, 2008; Naggara et al. 2010). The dog has been considered an alternative animal model for evaluating the mechanisms of new therapeutic drugs or for preclinical studies (Ladiges et al. 1990; Storb et al. 1998) because these animals have physiological characteristics similar to those of humans. Interestingly, the time-course of liver disease development in dogs and humans is similar, and a high degree of similarity between dog and human liver diseases has been reported in clinical, molecular, and pathological studies (Spee et al. 2006, 2007). Since stem cells can differentiate into hepatocytes, therapies using these cells could be a potential alternative treatment for terminal liver diseases. However, an appropriate source of human mesenchymal stem cells for producing hepatocytes has not been identified (Atala 2006).
Therefore, we performed the present study to isolate canine amniotic fluid stem (cAFS) cells, which are also mesenchymal stem cells (MSCs). We also evaluated the pluripotency, multipotency, and hepatogenic differentiation potential of these cells. Based on our findings, we suggest possible strategies for developing cell-based therapies in a canine model of hepatic disease.
Materials and Methods
Isolation and expansion of cAFS cells.
The study protocol was approved by the Research Ethics Committee and the Institutional Animal Care and Use Committee of Chungnam National University (Republic of Korea). Canine amniotic fluid samples were collected from pregnant beagles (n = 3) at the full-term stage of gestation by Cesarean section using an 18-gauge needle. The EUB-405 PLUS ultrasound unit (Hitachi, Tokyo, Japan) with convex 5 MHz and/or linear 7.5 MHz probes was used for taking imaging of fetus. The cAFS cells were immediately isolated from amniotic fluid and cell debris by centrifugation at 3,000 rpm for 30 min. The pelleted cells were then resuspended in 5 mL of phosphate-buffered saline (PBS; Gibco, Carlsbad, CA) supplemented with 1% penicillin/streptomycin (Gibco). After being washed twice, the cAFS cells were then grown in low-glucose Dulbecco’s modified Eagle’s medium (L-DMEM; Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Gibco), 1% penicillin/streptomycin, 5 ng basic fibroblast growth factor (bFGF; Sigma-Aldrich), and 10 ng epidermal growth factor (EGF; Sigma-Aldrich) in a 25-cm2 flask at 39°C with 5% CO2 and 5% O2. After 16 h, the unattached cells were removed by changing medium. The attached cAFS cells were allowed to grow to 70–80% confluence (5–7 d) before being routinely trypsinized with 0.25% trypsin–EDTA (Sigma-Aldrich) and subcultured at a dilution of 1:3 (or plated at a density of 5,000 cells/cm2). The remaining cells were transferred to cryopreservation media (10% dimethylsulfoxide, 90% FBS), frozen at −80°C in an isopropanol-jacketed closed container, and stored in liquid nitrogen the next day. The population doubling time of cAFS cells was calculated from passage 2 to passage 12 using the algorithm provided at http://www.doubling-time.com (Widera et al. 2009).
Total RNA isolation and reverse transcription-PCR.
Total RNA was extracted from adipogenically, chondrogenically, and osteogenically differentiated cAFS cells, and canine bone marrow stem cells (cBMs) (as a control) using an RNA extraction kit (Macherey–Nagel; Duren, Germany), according to the manufacturer’s instructions. RNA samples (1 μg total RNA) were combined with an oligo dT primer to synthesize cDNA using iScript reverse-transcriptase (Bio-Rad, Hercules, CA). cDNA was amplified by polymerase chain reaction (PCR) using appropriate primers for several pluripotent markers and expression of genes specific for adipogenic (lipoprotein lipase (LPL) and leptin), osteogenic (osteocalcin (Osteo) and runt-related transcription factor 2 (RUNX2)), and chondrogenic (aggrecan (AGG) and cartilage oligomeric matrix protein (COMP)) differentiation (Table 1). PCR was carried out with a 3-min denaturation at 94°C followed by 30–35 cycles at 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s, and a final extension for 5 min at 72°C. As the positive control, β2-microglobulin was amplified with 25 cycles of reverse transcription-polymerase chain reaction (RT-PCR) and a primer annealing temperature of 60°C. The amplified DNA fragments were separated by 2% agarose gel electrophoresis with a known standard (100-bp ladder; Elpis Biotech., Daejeon, Republic of Korea), stained with ethidium bromide, and visualized under ultraviolet light. Digital images were captured with ImageQuant (GE Healthcare), and the RT-PCR results were evaluated using ImageJ software (National Institute of Health, Bethesda, MD). Band intensities were measured by densitometry. Quantification of relative gene expression was performed using that of β2-microglobulin as a control. Identities of the target genes amplified by RT-PCR were confirmed by DNA sequencing (SolGent Co., Ltd., Daejeon, Republic of Korea).
Flow cytometric analysis for cAFS cell identification.
Cell surface proteins on the cAFS cells were analyzed by flow cytometry. Cells from passage 5 were washed with L-DMEM and detached from the culture disc with 0.25% trypsin–EDTA. The cells were pelleted, resuspended in PBS containing 2% FBS at a concentration of 1 × 105 cells/mL, and labeled with monoclonal antibodies against the following factors: CD29 (Abcam, Cambridge, MA), CD34 (Acris; Herford, Germany), CD44 (Serotec; Kidlington, UK), and CD90 (DB Biosciences-Pharmingen, Franklin Lakes, NJ). After being washed three times with PBS containing 2% FBS, the cells were further incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA). The stained cells were washed three times with PBS containing 2% FBS and fixed with 4% paraformaldehyde before they were analyzed by flow cytometry using a FACScan instrument operating with CELLQuest software (Becton Dickinson, Franklin Lakes, NJ). Flow cytometer settings were established using unstained cells. The cells were gated by forward scatter to eliminate debris. To reduce possible autofluorescence, we removed the signal from unstained cells in the measurement channel. A minimum of 10,000 events was counted for each analysis.
Immunocytochemistry.
The AFS cells were plated on a four-well dish (Nunc; Roskilde, Denmark), fixed with 4% paraformaldehyde in PBS at room temperature for 20 min and rinsed with PBS for 5 min. The cells were then permeabilized and blocked with 0.1% Triton X-100 (Sigma-Aldrich), 1% bovine serum albumin (BSA; Gibco), and 10% normal donkey serum (Invitrogen) in PBS for 45 min at room temperature. After blocking, the cells were incubated overnight at 4°C in a humidified chamber with antibodies specific for SOX2 SSEA-1, SSEA-4, alkaline phosphatase (Santa Cruz, Santa Cruz, CA), OCT4, and NANOG (Abcam) diluted in PBS containing 1% BSA. Next, cells were washed three times for 15 min with 0.1% Tween-20 in PBS. The cells then incubated with appropriate secondary antibodies diluted in PBS containing 1% BSA for 1 h at room temperature. The following antibodies were used: SOX2, Rhodamine Red-conjugated donkey anti-goat secondary antibody; SSEA-1 and alkaline phosphatase, Rhodamine Red-conjugated donkey anti mouse IgG secondary antibody; SSEA-4, Rhodamine Red-conjugated donkey anti-mouse IgM secondary antibody; and OCT4 and NANOG, Alexa Fluor 488 rabbit anti-mouse IgG secondary antibody (Invitrogen). Finally, the cells were counterstained with 1 ng/mL 6-diamino-2-phenylindole (DAPI; Sigma-Aldrich) for 30 min at 25°C. Images were captured with a confocal laser scanning microscope (LSM5 Live Configuration Variotwo VRGB, Germany).
Measurement of the multilineage differentiation potential.
The capacity of cAFS cells to differentiate into adipogenic, chondrogenic, and osteogenic lineages was analyzed as described by Zuk et al. (2001) at passage 5 in order to evaluate their MSC properties. Non-induced control samples were maintained in proliferation media during all differentiation studies. A total of 20,000 cAFS cells/well were seeded in six-well plates and cultured as a monolayer until confluence was reached before adipogenic, chondrogenic, and osteogenic differentiation was induced. After 3 d, the cells were treated with different induction media made with L-DMEM supplemented with different components listed in Table 2. All media were changed every third day for 21 d.
Adipogenic differentiation was confirmed on day 21 by intracellular accumulation of lipid-rich vacuoles stained with Oil Red O (Sigma-Aldrich). Cells were fixed with formaldehyde solution (10% [v/v] in PBS; Samchun Chemical, Seoul, Republic of Korea), washed with 3% (v/v) isopropanol (Amresco, Solon, OH), and stained with a working solution of 0.5% (w/v in 60% isopropanol) Oil Red O for 20 min. Chondrogenic and osteogenic differentiation were confirmed by the presence of calcium mineralization and deposition, respectively, observed with Alizarin Red S (Sigma-Aldrich) staining. Cells were washed twice with distilled water (DW) and fixed in ice-cold 70% ethanol for 1 h. After being carefully washed three times with DW, cells were stained with 40 mM Alizarin Red S for 10 min and then washed twice with DW at room temperature. And then, the stained cells were analyzed by light microscopy.
Hepatogenic differentiation.
Hepatogenic differentiation was induced using a previously described method (Zuk et al. 2001) with modifications. After reaching 85% confluence, cells were pre-cultured in L-DMEM supplemented with 20 ng/mL EGF and 10 ng/mL bFGF (conditioning step) to stop cell proliferation prior to the induction of differentiation into a hepatic phenotype. We then performed a two-step differentiation protocol. Cells were cultured for 2 wk in an induction medium consisting of L-DMEM supplemented with 20 ng/mL hepatocyte growth factor (HGF; Sigma-Aldrich) and 5 ng/mL bFGF. This was followed by incubation in maturation medium consisting of L-DMEM supplemented with 20 ng/mL oncostatin M (Sigma-Aldrich), 1 μmol/L dexamethasone, and 5 mmol/L nicotinamide (Sigma-Aldrich) to achieve maturation in up to 4 wk. All culture media were changed twice a week, and hepatic differentiation was assessed at different time points by RT-PCR specific for liver-associated genes using appropriate primers (Table 1) and β2-microglobulin as a positive control.
Statistical analysis.
All data were analyzed using a one-way ANOVA. All calculations were carried out using the GraphPad Prism 2.0 software package (GraphPad Software, Inc., San Diego, CA). All experiments were independently performed at least three times.
Results
cAFS cell culturing.
In order to establish a method for isolating and culturing cAFS cells, we determined whether the culturing of these cells was affected by gestation stage. We also analyzed the number and size of viable cAFS cells that were obtained. Although the concentration of cAFS cells decreased during the full-term stage of gestation, we obtained a sufficient number of cells. Therefore, cAFS cells collected during the full-term stage of gestation were used in this study. As shown in Fig. 1A, B, and C , morphological differences between passages 0, 3, and 5 were observed. Cells in primary culture and in passage 3 appeared to form colonies. However, cells at passage 5 developed a fibroblast-like morphology instead of forming colonies. A difference in doubling time was observed between early (2–6) and late (7–12) passages. The cumulative population doubling level was calculated and plotted in a graph (Fig. 1D ). Cells were cultured and maintained until passage 12. After the passage 6, cAFS cell proliferation decreased rapidly. These results indicated that cAFS cells undergo morphologic changes over time, and proliferation capacity also varies with passage.
Changes in morphology and proliferation of canine amniotic fluid stem (cAFS) cells according to passage. Morphological differences were observed between passages 0 (A), 3 (B), and 5 (C). The cumulative population doubling level was calculated and is displayed graphically (D). Scale bar = 20 μm. Data in the bar graph represent the means ± SEM of three independent measurements. * P < 0.01, compared with passage 6.
The expression of stemness markers in cAFS cells.
We determined whether the cAFS cells had the potential of pluripotent adult stem cells by immunocytochemistry and RT-PCR. At passage 5, the cAFS cells were immunostained for pluripotent stem cell markers OCT4, NANOG, and SOX2 (Fig. 2A–C ). RT-PCR was performed to measure the expression of stem cell markers (Fig. 2D ). Immunocytochemistry and RT-PCR results revealed that cAFS cells have a differentiation potential similar to that of pluripotent adult stem cells. In addition, we analyzed cell surface markers by flow to evaluate the multipotency of cAFS cells as a potential source of MSCs. At passage 5, the majority of cAFS cells expressed CD29 (β1 integrin), CD44, and CD90 (Thy1) adhesion molecules (Fig. 2E, G, and H ) but did not express the hematopoietic lineage marker CD34 (Fig. 2F ). Immunocytochemical staining also confirmed that the cAFS cells were positive for the mesenchymal stem cell markers CD44, CD29, and CD90 but were negative for CD34 (data not shown). These results demonstrated that cAFS cells have the same potential as pluripotent and multipotent adult stem cells.
Expression of stemness and mesenchymal stem cell markers in cAFS cells. After passage 5, cAFS cells were immunostained for the pluripotent stem cell markers OCT4 (A), NANOG (B), and SOX2 (C), and observed at ×400 magnification (scale bar = 30 μm). Nuclei were visualized by DAPI staining. Expression of OCT4, NANOG, and SOX2 was confirmed by RT-PCR (D). Canine bone marrow stem cells (cBMs) were used as a control. The housekeeping gene β2-microglobulin (β-MG) was used for normalization. Expression of cell surface makers CD29 (E), CD34 (F), CD 44 (G), and CD90 (H) on cAFS cells was measured by flow cytometry.
Multilineage differentiation potential.
cAFS cells were examined under conditions favorable for adipogenic, chondrogenic, and osteogenic induction. Fat vesicles were observed within 21 d of adipogenic differentiation induction. Lipid-laden vesicles increased in size as shown by Oil Red O staining (Fig. 3A and D ). Seven to 14 d after inducing chondrogenic differentiation, the cAFS cells showed positive Alizarin Red S staining (Fig. 3B and E ). In the osteogenic differentiation medium, the cAFS cells developed an osteogenic phenotype after 21 d. After differentiation was confirmed by a transition from a spindle to cuboidal shape, these cells were positive for Alizarin Red S staining (Fig. 3C and F ).
Differentiation potential of cAFS cells. Adipogenic (A, D), chondrogenic (B, E), and osteogenic (C, F) differentiation of the cAFS cells. (A, B, C): unstained group; (D): Oil Red O staining; (E, F): Alizarin Red S staining (×400 magnification). The expression of genes specific for adipocytes (LPL and leptin), osteoblasts (RUNX2 and osteocalcin), and chondrocytes (COMP and aggrecan) was evaluated by RT-PCR in cAFS cells and cBMs (G). M: 100-bp DNA ladder, cAFS: canine amniotic fluid stem cell, cBM: canine bone marrow stem cell.
Expression of adipocyte-specific genes such as LPL and leptin were detected by RT-PCR (Fig. 3G ). The expression levels of these two genes in the cAFS cells were similar to those in the cBMs that had differentiated into adipocytes (Table 3). The expression of chondrocyte-specific genes, such as COMP and aggrecan, were also detected by RT-PCR (Fig. 3G ). COMP gene expression was detected only in cBMs, but similar levels of aggercan gene expression were found in the cAFS cells and cBMs (Table 3). The differentiated cAFS cells also expressed osteoblast-specific genes such as RUNX2 and osteocalcin (Fig. 3G ). RUNX2 gene expression was detected only in cAFS cells whereas osteocalcin gene expression in cAFS cells was higher compared with that in the cBMs (Table 3). These results confirmed the mesenchymal nature of cAFS cells.
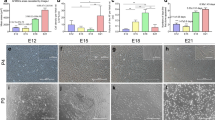
RT-PCR detection of hepatic gene expression in cAFS cells differentiated into hepatocyte-like cells.
Morphological changes of the cAFS cells were assessed during different stages of hepatogenic induction. Although the cells exhibited a fibroblast-like morphology after differentiation, we did not observe typical hepatocyte morphology, such as small, round, or epithelioid. In order to determine whether the differentiated cAFS cells possessed the functional characteristics of hepatocytes, expression of several hepatic genes was measured by RT-PCR 28 d after differentiation. cBMs were used as a control. RT-PCR results showed that the expression levels of albumin (ALB), tyrosine aminotransferase (TAT), and alpha-1 antiproteinase (α1-AT) increased after maturation and differentiation (Fig. 4). ALB and TAT gene expression was induced in the cAFS cells, but this was not detected in the cBM cells (Fig. 4). Early hepatocyte markers such as AFP (expressed in fetal hepatocytes) and TTR (pre-albumin) were not detected during either the induction of differentiation or maturation (data not shown). Results of this experiment indicated that cAFS cells have functional properties similar to those of hepatocytes.
RT-PCR analysis of hepatic gene expression in cAFS cells differentiated into hepatocyte-like cells. RT-PCR was performed to evaluate the expression of markers specific for hepatogenic differentiation [alpha-fetoprotein (AFP), albumin (ALB), tyrosine aminotransferase (TAT), alpha-1 antiproteinase (α1-AT), and transthyretin (TTR; pre-albumin)]. Total RNA was extracted from cAFS cells and cBMs 28 d after differentiation. UD: undifferentiated, D: differentiated, M: 100-bp DNA ladder. β2-microglobulin (β-MG) was used as a control.
Discussion
Isolation and characterization of MSCs derived from various tissues and sources are critical for stem cell therapy, regenerative medicine research, and tissue engineering (Miki et al. 2005; De Coppi et al. 2007a, b; Perin et al. 2007). Recently, an increasing number of reports have been published regarding the characterization of AFCs and suggest that these cells are a new potential source of MSCs (Cananzi et al. 2009). Accordingly, the study of MSCs from amniotic fluid has become a new focus in stem cell research (Zhang and Chen 2008; Park et al. 2011). However, the potential use of AFCs in medical applications has yet to be explored. The dog has historically been a useful model for studying mechanisms and testing new treatments for several human pathologic conditions including prostate cancer, cardiovascular diseases, bone regeneration, diabetes, leukemia, spinal cord injury, and solid organ transplantation (Miyamoto et al. 1996; Andrawiss et al. 1999; Fleeman and Rand 2001; Junghanss et al. 2003; Cui et al. 2007; Lim et al. 2007; Perin et al. 2008). Consequently, canines may also be very useful for studying the use of AFCs for clinical applications.
In the present investigation, we demonstrated that it is possible to isolate MSCs derived from canine amniotic fluid. We subsequently characterized the multilineage differentiation ability of these cells. We also determined whether the culturing of cAFS cells was affected by the stage of gestation (early [20–30 d] versus full-term [50–60 d]). After isolating and culturing cAFS cells collected during both stages, we determined the number and size of viable cells. Although the concentrations of cAFS cells decreased during the full-term stage of gestation, we still obtained a sufficient number of cells (data not shown). Therefore, cAFS cells isolated from amniotic fluid during full-term gestation were used in this study. Doubling time of the cAFS cells increased gradually with the number of passages, but our results demonstrated that cAFS cells isolated during the full-term stage could be cultured and expanded in vitro for up to 12 passages (Fig. 1D ). The morphology of cAFS cells was fibroblast-like after passage 5 (Fig. 1C ). This finding was consistent with data from previous reports that indicated this type of morphology is developed by MSCs derived from various tissues (Bossolasco et al. 2006; Patki et al. 2010; Vieira et al. 2010).
Stem cell pluripotency is defined as the potential to differentiate into any of the three germ layers: endoderm, mesoderm, and ectoderm. OCT4, NANOG, and SOX2 are pluripotency markers and are usually found in embryonic stem cells. According to the results of our RT-PCR and immunocytochemistry assays (Fig. 2A–D ), cAFS cells expressed all three of these pluripotency-specific markers, indicating the stemness of these cells. In addition, expression of NANOG in cAFS cells was higher than that in cBMs (control cells). NANOG is required to maintain the undifferentiated state and for the self-renewal of stem cells. As shown in Fig. 2D , NANOG expression in cBMs was weak. In a human study, NANOG was shown to be involved in the proliferation and colony formation of MSCs (Liu et al. 2009). Moreover, forced expression of NANOG in human bone marrow-derived MSCs sustains their expansion and differentiation capabilities (Go et al. 2008). Thus, the importance of NANOG remains unclear, and comprehensive investigations are required to elucidate the stemness markers of MSCs. In addition, cAFS cells expressed high levels of the mesenchymal cell markers CD44, CD29, and CD90 but were negative for the hematopoietic lineage marker CD34 (Fig. 2E–H ). These findings are similar to those reported for multipotent and pluripotent stem cells derived from human AFCs (Tsai et al. 2004; Chen et al. 2009; Zucconi et al. 2010).
In the current study, cAFS cells differentiated into adipogenic, osteogenic, and chondrogenic lineage cells. Cheong et al. (2005) found that cord-lining mesenchymal cells from explants of human umbilical cord amniotic membrane develop to adipocyte-like cells and observed the accumulation of lipid droplets with Oil Red O staining. Consistent with our data, lipid-laden vesicles were visualized using Oil Red O in cAFS cells cultured under conditions that promoted adipogenic differentiation (Fig. 3A and D ). Additionally, RT-PCR also showed that adipocyte-related genes, such as LPL and leptin, were abundantly expressed in the cells (Fig. 3G ).
Under osteogenic conditions, intact human amniotic membranes are able to differentiate into an osteogenic lineage. Calcium contents as well as and RUNX2 and osteocalcin (bone morphogenetic protein) expression are also increased (Lindenmair et al. 2010). In the present study, osteogenic-differentiated cAFS cells were positive for Alizarin Red S staining, thereby confirming the presence of calcium mineralization and deposition. These cells also expressed osteoblast-specific genes such as RUNX2 and osteocalcin. Compared with cBMs, cAFS cells expressed higher levels of these genes. Our results suggest that the osteogenic differentiation potential of cAFS cells is greater than that of cBM cells.
It has been reported that COMP and aggrecan are expressed during chondrogenic differentiation of human amniotic fluid-derived stem cells (Kolambkar et al. 2007; Kruger et al. 2012). In the present study, chondrogenic-differentiated cAFS cells were negative for the expression of COMP but positive for aggrecan gene expression (Fig. 3G ). Nevertheless, the cells were positive for Alizarin Red S staining (Fig. 3F ). These findings imply that the aggrecan gene plays a more important role in chondrocyte differentiation of cAFS cells than the COMP gene. Taken together, these results demonstrated for the first time that cAFS cells have a capacity for multilineage differentiation. However, it remains unclear whether these cells have greater therapeutic potential than adult MSCs.
ES and induced pluripotent stem (iPS) cells readily form teratomas when transplanted into immunodeficient mice (Takahashi et al. 2007). To test pluripotency in vivo, we transplanted cAFS (passages 4–5, 2 × 106 or 1 × 107/0.5 mL) and mouse embryonic stem cells (1 × 106/0.5 mL) subcutaneously into severe combined immunodeficiency mice (Taconic), respectively. However, although mouse embryonic stem cells as control were observed tumor formation, we could not find that within 1 mo after transplanting cAFS cells (data not shown). Since teratoma formation can be a serious complication of stem cell therapy, cAFS cells could have a therapeutic advantage over ES cells or iPS cells in the context of tissue engineering or the treatment of diseases that require organ transplantation.
Liver transplantation is a last resort for patients with end-stage liver disease. However, this procedure is limited by a lack of suitable organ donors, tissue rejection, and the high cost of surgery. Once the technical hurdles associated with in vitro hepatocyte differentiation of adult stem cells are overcome, many more patients may benefit from stem cell therapy than the current number of patients who receive liver transplants. Therefore, many investigators have been searching for various methods to induce the differentiation of adult stem cells into hepatocytes. In the current study, hepatocyte differentiation was induced by major factors such as HGF, OSM, nicotinamide, and dexamethasone. HGF, which is an endocrine or paracrine factor, is essential for liver development. OSM can promote the maturation of hepatocytes, which is confirmed by intracellular glycogen accumulation and morphological changes that result in differentiated hepatocytes expressing hepatic differentiation markers (Kamiya et al. 1999). Additionally, nicotinamide and dexamethasone are important factors in the hepatogenic development pathway. Chivu et al. (2009) demonstrated that dexamethasone promotes the development of hepatogenic morphology through the suppression of cell division. Unexpectedly, cAFS cells that underwent hepatic differentiation in the present study did not show typical hepatocyte morphological changes. However, we did observe the expression of genes critical for hepatocyte differentiation. After being induced to hepatocytic differentiation, cAFS cells were strongly positive for TAT, α1-AT, GS, and ALB, all markers of mature hepatocytes (Fig. 4). The expression of some hepatocyte-specific genes, including ALB and TAT, was greater in cAFS cells compared with cBM cells. After 14 d of induction and 28 d of differentiation (data not shown), expression of early hepatocyte markers (such as AFP and TTR) was not detected. These results indicated that nicotinamide, dexamethasone, HGF, and OSM could induce hepatogenesis in cAFS cells, which is consistent with data from other studies (Takashima et al. 2004; Hong et al. 2005; De Kock et al. 2009).
Taken together, these results suggest that cAFS cells have the capacity for multilineage differentiation. Following differentiation, these cells possessed functional properties similar to those of hepatocytes. Our findings suggest that cAFS cells have the potential to be a resource for cell-based therapies in canine models of hepatic disease.
References
Andrawiss M.; Opolon P.; Benihoud K.; Devauchelle P.; Di Falco N.; Villette J. M.; Kremer E.; Saulnier P.; Berthon P.; Perricaudet M.; Cussenot O. Adenovirus-mediated gene transfer in dog prostate: a preclinical study of a relevant model system for gene therapy of human prostatic cancer. Prostate Cancer Prostatic Dis. 2: 25–35; 1999.
Atala A. Recent developments in tissue engineering and regenerative medicine. Curr. Opin. Pediatr. 18: 167–171; 2006.
Bossolasco P.; Montemurro T.; Cova L.; Zangrossi S.; Calzarossa C.; Buiatiotis S.; Soligo D.; Bosari S.; Silani V.; Deliliers G. L.; Rebulla P.; Lazzari L. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 16: 329–336; 2006.
Cananzi M.; Atala A.; De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod. Biomed. Online 18(Suppl 1): 17–27; 2009.
Chen C. P.; Liu S. H.; Huang J. P.; Aplin J. D.; Wu Y. H.; Chen P. C.; Hu C. S.; Ko C. C.; Lee M. Y.; Chen C. Y. Engraftment potential of human placenta-derived mesenchymal stem cells after in utero transplantation in rats. Hum. Reprod. 24: 154–165; 2009.
Cheong H. H.; Masilamani J.; Phan T. T.; Chan S. Y. Cord lining progenitor cells: potential in vitro adipogenesis model. Int. J. Obes. 34: 1625–1633; 2005.
Chivu M.; Dima S. O.; Stancu C. I.; Dobrea C.; Uscatescu V.; Necula L. G.; Bleotu C.; Tanase C.; Albulescu R.; Ardeleanu C.; Popescu I. In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl. Res. 154: 122–132; 2009.
Cui L.; Liu B.; Liu G.; Zhang W.; Cen L.; Sun J.; Yin S.; Liu W.; Cao Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 28: 5477–5486; 2007.
Decembrini S.; Cananzi M.; Gualdoni S.; Battersby A.; Allen N.; Pearson R. A.; Ali R. R.; De Coppi P.; Sowden J. C. Comparative analysis of the retinal potential of embryonic stem cells and amniotic fluid-derived stem cells. Stem Cells Dev. 20: 851–863; 2011.
De Coppi P.; Bartsch Jr. G.; Siddiqui M. M.; Xu T.; Santos C. C.; Perin L.; Mostoslavsky G.; Serre A. C.; Snyder E. Y.; Yoo J. J.; Furth M. E.; Soker S.; Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25: 100–106; 2007a.
De Coppi P.; Callegari A.; Chiavegato A.; Gasparotto L.; Piccoli M.; Taiani J.; Pozzobon M.; Boldrin L.; Okabe M.; Cozzi E.; Atala A.; Gamba P.; Sartore S. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J. Urol. 177: 369–376; 2007b.
De Kock J.; Vanhaecke T.; Biernaskie J.; Rogiers V.; Snykers S. Characterization and hepatic differentiation of skin-derived precursors from adult foreskin by sequential exposure to hepatogenic cytokines and growth factors reflecting liver development. Toxicol. In Vitro 23: 1522–1527; 2009.
Fleeman L. M.; Rand J. S. Management of canine diabetes. Vet. Clin. N. Am Small Anim. Pract. 31: 855–880; 2001. vi.
Go M. J.; Takenaka C.; Ohgushi H. Forced expression of Sox2 or Nanog in human bone marrow derived mesenchymal stem cells maintains their expansion and differentiation capabilities. Exp. Cell Res. 314: 1147–1154; 2008.
Hong S. H.; Gang E. J.; Jeong J. A.; Ahn C.; Hwang S. H.; Yang I. H.; Park H. K.; Han H.; Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem. Biophys. Res. Commun. 330: 1153–1161; 2005.
Junghanss C.; Takatu A.; Little M. T.; Maciej Zaucha J.; Zellmer E.; Yunusov M.; Sale G.; Georges G. E.; Storb R. Adoptive immunotherapy against kidney targets in dog-leukocyte antigen-identical mixed hematopoietic canine chimeras. Transplantation 75: 268–274; 2003.
Kamiya A.; Kinoshita T.; Ito Y.; Matsui T.; Morikawa Y.; Senba E.; Nakashima K.; Taga T.; Yoshida K.; Kishimoto T.; Miyajima A. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 18: 2127–2136; 1999.
Kim J.; Lee Y.; Kim H.; Hwang K. J.; Kwon H. C.; Kim S. K.; Cho D. J.; Kang S. G.; You J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 40: 75–90; 2007.
Kolambkar Y. M.; Peister A.; Soker S.; Atala A.; Guldberg R. E. Chondrogenic differentiation of amniotic fluid-derived stem cells. J. Mol. Histol. 38: 405–413; 2007.
Kruger J. P.; Endres M.; Neumann K.; Stuhlmuller B.; Morawietz L.; Haupl T.; Kaps C. Chondrogenic differentiation of human subchondral progenitor cells is affected by synovial fluid from donors with osteoarthritis or rheumatoid arthritis. J. Orthop. Surg. Res. 7: 10; 2012.
Ladiges W. C.; Storb R.; Thomas E. D. Canine models of bone marrow transplantation. Lab. Anim. Sci. 40: 11–15; 1990.
Lim J. H.; Byeon Y. E.; Ryu H. H.; Jeong Y. H.; Lee Y. W.; Kim W. H.; Kang K. S.; Kweon O. K. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J. Vet. Sci. 8: 275–282; 2007.
Lindenmair A.; Wolbank S.; Stadler G.; Meinl A.; Peterbauer-Scherb A.; Eibl J.; Polin H.; Gabriel C.; van Griensven M.; Redl H. Osteogenic differentiation of intact human amniotic membrane. Biomaterials 31: 8659–8665; 2010.
Liu T. M.; Wu Y. N.; Guo X. M.; Hui J. H.; Lee E. H.; Lim B. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 18: 1013–1022; 2009.
Miki T.; Lehmann T.; Cai H.; Stolz D. B.; Strom S. C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 23: 1549–1559; 2005.
Miyamoto T.; Hachimura H.; Amimoto A. A case of megakaryoblastic leukemia in a dog. J. Vet. Med. Sci. 58: 177–179; 1996.
Naggara O.; Darsaut T. E.; Salazkin I.; Soulez G.; Guilbert F.; Roy D.; Weill A.; Gevry G.; Raymond J. A new canine carotid artery bifurcation aneurysm model for the evaluation of neurovascular devices. AJNR Am. J. Neuroradiol. 31: 967–971; 2010.
Neff M. W.; Rine J. A fetching model organism. Cell 124: 229–231; 2006.
Park S. B.; Seo M. S.; Kang J. G.; Chae J. S.; Kang K. S. Isolation and characterization of equine amniotic fluid-derived multipotent stem cells. Cytotherapy 13: 341–349; 2011.
Patki S.; Kadam S.; Chandra V.; Bhonde R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum. Cell 23: 35–40; 2010.
Perin E. C.; Silva G. V.; Assad J. A.; Vela D.; Buja L. M.; Sousa A. L.; Litovsky S.; Lin J.; Vaughn W. K.; Coulter S.; Fernandes M. R.; Willerson J. T. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J. Mol. Cell. Cardiol. 44: 486–495; 2008.
Perin L.; Giuliani S.; Jin D.; Sedrakyan S.; Carraro G.; Habibian R.; Warburton D.; Atala A.; De Filippo R. E. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 40: 936–948; 2007.
Schneider M. R.; Adler H.; Braun J.; Kienzle B.; Wolf E.; Kolb H. J. Canine embryo-derived stem cells—toward clinically relevant animal models for evaluating efficacy and safety of cell therapies. Stem Cells 25: 1850–1851; 2007.
Schneider M. R.; Wolf E.; Braun J.; Kolb H. J.; Adler H. Canine embryo-derived stem cells and models for human diseases. Hum. Mol. Genet. 17: R42–R47; 2008.
Sessarego N.; Parodi A.; Podesta M.; Benvenuto F.; Mogni M.; Raviolo V.; Lituania M.; Kunkl A.; Ferlazzo G.; Bricarelli F. D.; Uccelli A.; Frassoni F. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica 93: 339–346; 2008.
Spee B.; Arends B.; van den Ingh T. S.; Brinkhof B.; Nederbragt H.; Ijzer J.; Roskams T.; Penning L. C.; Rothuizen J. Transforming growth factor beta-1 signalling in canine hepatic diseases: new models for human fibrotic liver pathologies. Liver Int. 26: 716–725; 2006.
Spee B.; Arends B.; van den Ingh T. S.; Roskams T.; Rothuizen J.; Penning L. C. Major HGF-mediated regenerative pathways are similarly affected in human and canine cirrhosis. Comp. Hepatol. 6: 8; 2007.
Storb R.; Yu C.; Deeg H. J.; Georges G.; Kiem H. P.; McSweeney P. A.; Nash R. A.; Sandmaier B. M.; Sullivan K. M.; Wagner J. L.; Walters M. C. Current and future preparative regimens for bone marrow transplantation in thalassemia. Ann. N. Y. Acad. Sci. 850: 276–287; 1998.
Takahashi K.; Tanabe K.; Ohnuki M.; Narita M.; Ichisaka T.; Tomoda K.; Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872; 2007.
Takashima S.; Ise H.; Zhao P.; Akaike T.; Nikaido T. Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell. Struct. Funct. 29: 73–84; 2004.
Tsai M. S.; Lee J. L.; Chang Y. J.; Hwang S. M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 19: 1450–1456; 2004.
Vieira N. M.; Brandalise V.; Zucconi E.; Secco M.; Strauss B. E.; Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 19: 279–289; 2010.
Widera D.; Zander C.; Heidbreder M.; Kasperek Y.; Noll T.; Seitz O.; Saldamli B.; Sudhoff H.; Sader R.; Kaltschmidt C.; Kaltschmidt B. Adult palatum as a novel source of neural crest-related stem cells. Stem Cells 27: 1899–1910; 2009.
Yadav P.; Mann A.; Singh V.; Yashveer S.; Sharma R.; Singh I., 2010. Expression of pluripotency genes in buffalo (Bubalus bubalis) amniotic fluid cells. Reprod Domest Anim 46:705-711
You Q.; Tong X.; Guan Y.; Zhang D.; Huang M.; Zhang Y.; Zheng J. The biological characteristics of human third trimester amniotic fluid stem cells. J Int Med Res 37: 105–112; 2009.
Zhang S.; Chen F. Characterization of amniotic fluid-derived stem cells. J. Clin. Rehabil. Tissue Eng. Res. 12: 3152–3155; 2008.
Zucconi E.; Vieira N. M.; Bueno D. F.; Secco M.; Jazedje T.; Ambrosio C. E.; Passos-Bueno M. R.; Miglino M. A.; Zatz M. Mesenchymal stem cells derived from canine umbilical cord vein—a novel source for cell therapy studies. Stem Cells Dev. 19: 395–402; 2010.
Zuk P. A.; Zhu M.; Mizuno H.; Huang J.; Futrell J. W.; Katz A. J.; Benhaim P.; Lorenz H. P.; Hedrick M. H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7: 211–228; 2001.
Acknowledgment
This study was supported by a grant (no. 110056-3) from the Korea Institute of Planning and Evaluation for Technology of Food, Agriculture, Forestry and Fisheries.
Author disclosure statement
None of the authors have any competing financial interests to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Choi, SA., Choi, HS., Kim, K.J. et al. Isolation of canine mesenchymal stem cells from amniotic fluid and differentiation into hepatocyte-like cells. In Vitro Cell.Dev.Biol.-Animal 49, 42–51 (2013). https://doi.org/10.1007/s11626-012-9569-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-012-9569-x