Abstract
Humic acids are known for their overall positive health and productivity effects in animal feeding trials and, controversially, as an aetiological factor of cancer. We tried to assess the in vitro effect of humic acids from a selected source in Slovakia when used at recommended prophylactic dosage. We investigated antioxidant properties, enzymatic and non-enzymatic antioxidant defence system in liver mitochondria and cultured cancer cell lines in vitro. We observed a significant decrease in superoxide dismutase activity after humic acids treatment irrespective of dissolving in dimethyl sulphoxide or direct addition to mitochondria suspension in a respiration medium. Activities of other antioxidant enzymes measured, such as glutathione peroxidase and glutathione reductase, showed no significant differences from the control as well as the reduced glutathione content. Percentage of inhibition by humic acids of superoxide radical indicated lower efficacy compared with that of hydroxyl radical. Survival of six different cancer cells lines indicated that only the acute T lymphoblastic leukaemia cell line was sensitive to the tested humic acids. Despite relatively low solubility in aqueous solutions, humic acids from the selected source participated in redox regulation. By recapturing the radicals, humic acids reloaded the antioxidant defensive mechanism. Results from in vitro study conducted with humic acids from the natural source showed potential of these substances as promising immunity enhancing agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humic acids (HAs) are high molecular polymeric aromatic substances having complicated structure of polyaromatic and heterocyclic chemicals with multiple carboxylic acid side chains and showing significant physicochemical properties. They are naturally occurring decomposed organic constituents of soil and lignite produced from decayed plant matter by soil bacteria (Yoruk et al. 2004). Additionally, humic acids are described as organic molecules that can be soluble and chemically active at neutral or alkaline pH (Masini 1994; Choppin and Labonne-Wall 1997). Humates are salts of humic acid in which the cation/anion exchange sites of humic acid are Ca2+, Na+, Al3+ and Fe2+ rather than hydrogen (Choppin and Labonne-Wall 1997). Since long ago, humic acids have been used for growth stimulation in the field of plant production (Chen et al. 2000). Humic acids are active substances used in prophylaxis and as therapeutical drugs in veterinary practice in Europe (EMEA 1999). They are administered as antidiarrhoeal, analgesic, immunostimulatory and antimicrobial agents and as substances used in replacement therapy to enhance feed conversion ratio and treat malnutrition and diarrhoea (Klocking et al. 2002; Joone et al. 2003; Schepetkin et al. 2003; Joone and Rensburg 2004; Kucukersan et al. 2005; Rath et al. 2005). Although many experimental studies have shown HAs to be largely nontoxic and nonteratogenic (EMEA 1999; Yasar et al. 2002), they have been implicated as the cause of Kashin–Beck disease characterized by arthrosis of joints and growth plate in children and adolescents in certain endemic areas of East Asia (Zhai et al. 1990; Liang et al. 1999).
Overall positive effects of humates on health and even productivity of animals are known from animal feeding trials (Yoruk et al. 2004; Kucukersan et al. 2005; Demeterová et al. 2009). The mechanism of HA action either in vivo or in vitro is not clear. It was observed that interaction between HAs and endothelial cells may increase directly their Ca2+ permeability, increase NO/peroxynitrite production and could lead to apoptotic cell death (Hseu et al. 2002a, b). Via oxidative generation and reduction in the activities of antioxidant enzymes in human erythrocytes, HAs induced echinocyte transformation (Hseu et al. 2000) and oxidative DNA damage in human peripheral blood lymphocytes (Hseu et al. 2008). HAs stimulate rat liver mitochondria respiration (Visser 1987).
Some similarities of their molecular structure with coenzyme Q indicated that they may interfere with the mitochondrial redox system. Mitochondria have been recognized as an effective source of superoxide anions and hydrogen peroxides even under normal conditions. Our aim was to assess the antioxidant properties and effect of HAs on enzymatic and non-enzymatic antioxidant activities in mitochondria from the organ with major metabolic functions to estimate the potential toxic influence of HAs as well as the effect on cultured cancer cell lines in vitro at recommended prophylactic dosage.
Materials and Methods
All chemicals used were of analytical grade if not specified otherwise. Solutions were freshly prepared using redistilled water. HAs were purchased as Humac Natur (Humac Ltd., Košice, Slovakia). The original amount of HAs was 10 mg/10 ml (10% DMSO or phosphate buffered saline (PBS) pH 7.4). However, solubility of humic acids is low; therefore, the HA solution was sonicated two times for 10 min. After filtration, the filter papers were dried to the constant weight, weighed and after abstraction, the remaining amount of HAs was determined in the solution to be 6.4 mg/10 ml (three times) in DMSO and 3.2 mg/ml in PBS.
Male Wistar rats (Velaz, Prague, Czech Republic) weighing 200–250 g were used. Adhering to the procedures approved by the University of Košice Animal Care and Use Committee, the animals were sacrificed by cervical displacement and decapitation.
Liver mitochondria were isolated from male Wistar rats using the method by Johnson and Lardy (1967). Mitochondrial protein content was determined by Bradford (1976) protein assay.
The activities of glutathione reductase (GR; E.C. 1.6.4.2) were determined according to Carlberg and Mannervik (1985), glutathione peroxidase (GPx; E.C. 1.11.1.9) by the method of Flohe and Gunzler (1984) and superoxide dismutase (SOD; E.C. 1.15.1.1) by kit user manual (Fluka, Tokyo, Japan). Reduced glutathione (GSH) content in mitochondria treated with HAs was measured by the modified method of Floreani et al. (1997) using Ellman’s reagent (R 2 = 0.991).
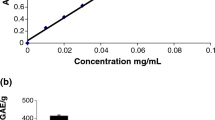
The antioxidant properties of HAs were evaluated by Beauchamp and Fridovich (1971). By UV illumination under aerobic conditions, riboflavin is reduced by l-methionine and the reduced form reacts with oxygen forming a peroxide derivative which, after decomposition, provides superoxide radical anion. The ions are captured by nitrotetrazolium blue (NTB). This compound changes colour upon the reduction. The original yellow colour turns blue. The transformation can be followed by spectrophotometry, measuring the absorbance at 450 and 560 nm. When an antioxidant is present, it captures the superoxide radical ion; consequently, the photoreduction of NTB is inhibited (the solution is decolourized). The reaction mixture used contained 300 μl of 0.2 mmol l−1 riboflavin, 30 μl of 5 mmol l−1 NTB (Merck, Darmstadt, Germany), 8.7 ml of 50 mmol l−1 PBS (KH2PO4 and K2HPO4) pH 7.4 containing 0.1 mmol l−1 EDTA, all from ITES (ITES, Vranov nad Topľou, Slovakia), and 13 mmol l−1 l-methionine (Merck), and the humic acids were added at a concentration of 6.4 mg/10 ml (DMSO or PBS). Riboflavin was added last and the reaction was initiated by placing vessels under an Hg lamp. The illumination time was 10 and 20 min. The role of EDTA is to remove the disturbing metal ions. From the absorbance (450 and 560 nm), we calculated percentage of inhibition by the formula: % inhibition = [K (time) − A x (time)/K (time)] × 100, where K is the absorbance of the composition without antioxidant and A x is absorbance at the same wavelength of the composition with antioxidant at the time of use.
2-Deoxy-d-ribose (9 mmol l−1; Sigma, Roedermark, Germany) was dissolved in 30 mmol l−1 PBS pH 7.4 containing 40 mmol l−1 NaCl and 30 μmol l−1 ammonium iron (II) sulphate hexahydrate [NH4]2[Fe][SO4]2·6H2O and 50 μmol l−1 H2O2 was added to the mixture. The mixture was incubated at 37°C for 10 min. The 2-deoxyribose degradation was monitored by determination of the thiobarbituric acid (TBA; Merck) reactive substances. The TBA reactivity was developed by mixing 1.0 ml aliquot of the incubation product with 0.5 ml thiobarbituric acid reagent (1%, w/v), TBA dissolved in 50 mmol l−1 NaOH solution and 0.5 ml 5.6% (w/v) trichloroacetic acid (Microchem, Bratislava, Slovakia) followed by heating at 100°C for 8 min. When the mixture was cooled, the developed chromogen was determined by reading the absorbance at 532 nm against an appropriate blank solution.
A-549 (lung carcinoma), MDA-MB-231 and MCF-7 (mammary gland adenocarcinoma), HeLa (cervical carcinoma) and Jurkat cells (acute T lymphoblastic leukaemia) all kindly provided by Dr. M. Hajdúch (Olomouc, Czech Republic), and CEM (acute T lymphoblastic leukaemia; DSMZ, Braunschweig, Germany), all mycoplasma free, were cultivated in RPMI 1640 cultivation medium (Invitrogen, Paisley, UK) with Glutamax-I supplemented or D-MEM medium with Glutamax-I and glucose supplemented in with 10% foetal calf serum, penicillin (100 IU ml−1) and streptomycin (100 μg ml−1; all from Invitrogen), in 5% CO2 atmosphere in humidified air at 37°C. Cell viability, estimated by trypan blue exclusion, was greater than 95% before each experiment.
3-(Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma. Cycle TEST™ PLUS DNA Reagent Kit, annexin V-FITC and propidium iodide (Becton Dickinson, Franklin Lakes, NJ) were used. The cytotoxic effect of the tested compounds was studied by colorimetric microculture assay with the MTT end-point. The amount of MTT reduced to formazan was proportional to the number of viable cells. Briefly, 8 × 104 cells were plated per well in 96-well polystyrene microplates (Sarstedt, Germany) in the culturing medium containing the tested chemicals at final concentrations 10−4–10−6 mmol l−1. After 72 h incubation, 10 μl of MTT (5 mg ml−1) was added to each well. After an additional 4 h, during which insoluble formazan was produced, 100 μl of 10% sodium dodecyl sulphate was added to each well and another 12 h were allowed for the formazan to be dissolved. The absorbance was measured at 540 nm using an automated MRX microplate reader (Dynatech, Willenhall, UK). The absorbance of control wells was taken as 100%, and the results were expressed as percent of the control.
Results are presented as mean ± SD of at least three independent experiments. Statistical significance was determined by ANOVA t test.
Results
We observed a significant decrease in SOD activity after the HAs treatment irrespective of dissolving in DMSO or direct addition to mitochondria suspension in respiratory medium (PBS). Likewise, activities of other measured antioxidant enzymes as GPx, removing the peroxides, showed a non-significant decrease in comparison with the control (Table 1). Thus, superoxide anions and peroxides generated and derived from electrophilic compounds in mitochondria, as potential mitochondria response to treatment, did not induce variations in enzymes. The levels of cellular dynamic indicator of oxidative stress, GSH, showed no significant changes in comparison with the control. The last examined mitochondrial intrinsic enzyme involved in the control of oxidative stress was GR. GR regenerated GSH from the oxidised form to maintain glutathione redox state in the optimum ratio, and the enzyme activities measured did not differ from the control in both treatment groups (Table 1).
Based on the data measured, counteracting several published studies, we estimated the antioxidant properties of HAs against the radicals reacting with the mentioned enzymes. The percentage of inhibition by HAs dissolved in DMSO (A) and PBS (B) of the superoxide radical is shown in Fig. 1. The percentage of inhibition by HAs dissolved in DMSO (A) and PBS (B) of the hydroxyl radical is shown in Fig. 2.
Survival of different cancer cells (Jurkat T, CEM, MCF-7, MDA, HeLa, A 549) exposed to 72 h incubation with the tested compound is shown in Table 2. Our data indicate that only the CEM cell line was sensitive to the tested HAs. An average of 58% surviving cells showed 42% effectiveness in comparison with the control (±12.88 SEM). No cytotoxic effect was observed on other cell lines used in the experiment. The tested compounds showed cytotoxic activity with IC50 = 0.6033 ng.
Discussion
HAs have been used in animal husbandry and agriculture trials in an effort to improve economy and ecology of the production. On the other hand, HAs have been suggested to be an etiological factor of cancer, with HAs induced oxidative damage as a potential causative mechanism of carcinogenesis (Hseu et al. 2008). HAs are the most widespread natural polymers derived from biological, chemical and microbial decomposition of organic matter. According to the summary by Yang et al. (2004), HAs represent a group of natural high molecular weight macromolecules composed of aromatic rings forming a very complex structure in the presence of phenolic, hydroxyl, phenolic hydroxyl, ketonyl, quinone, semiquinone, carboxyl, carbonyl and alkoxyl groups. The HAs often form complexes with a mixture of metallic elements. Because of the above-mentioned data, one cannot expect that two separate natural sources of HAs can contain identical molecules. Thus, HAs from one source may exert positive influence while the effect of those from another source can be a negative one. Our study was carried out using HAs from a selected natural source in concentrations corresponding to recommended veterinary prophylactic doses.
In an organism, there are cells that in their peculiar “professional” roles are exposed to chemical (i.e. hepatocytes) or oxidative (i.e. monocytes) damage in order to maintain the homeostasis of the organism (Coppola and Ghibelli 2000). The mitochondrion is the main metabolic site of generation of reactive oxygen species (ROS). The reactive oxygen species of primary biological importance include superoxide (O2 −), hydroxyl radicals (OH·) and hydrogen peroxide (H2O2). Cell damage occurs when there is an increased generation of reactive oxygen species or a relative shortage of antioxidant molecules (Medina and Moreno-Otero 2005). To study the effect of HAs showing positive effects on organism when used in animal feeding trials‚ the liver mitochondria were selected. The treatment dose corresponded to the approved prophylactic dosage. The solubility of HAs is generally low and only certain defined components are dissolved depending on pH. The in vitro experiment was carried out in environments routinely used for maintaining viability of mitochondria and cell lines. The undissolved portion of HAs in both media corresponded to the range declared by the producer for the content of HAs and free HAs in dry matter.
Due to their chemical nature, HAs strongly interact with both inorganic and organic agents and thus might participate in redox regulation (Woo et al. 2002). HAs have been shown to generate ROS, such as the superoxide anion, and deplete glutathione and several antioxidant enzymes (Cheng et al. 2003). However, results from our study do not indicate effect on antioxidant enzymes or redox potential reduction (Table 1).
GPx is an antioxidant enzyme that scavenges various peroxides. Three isoenzymes, cellular GPx, extracellular GPx and phospholipid hydroperoxide GPx are known to convert peroxides by an electron donor from nonprotein sulfhydryl, GSH. Cellular GPx can react with hydrogen peroxide and organic peroxides but not with lipid hydroperoxide. Also catalase is found in many types of cells and scavenges hydrogen peroxide as its sole substrate. The content of catalase is lower than the content of GPx in the majority of cells, except for hepatocytes, but the K m value of catalase for hydrogen peroxide is higher than that of GPx (Asahi et al. 1995). Considering our findings and the properties of enzymes, we could state that the GPx activities are not indicative of enhanced peroxide degradation in comparison with the control.
Within mitochondria, exposed thiols are present at a high concentration (Schafer and Buettner 2001). Because the concentration of GSH is much higher than that of the other two systems (NADPH/NADP+ and Trx(SH)2/TrxSS), it is often considered the principal redox buffer of the cell (Aon et al. 2007). The regeneration of GSH from GSSG trapped inside the mitochondria requires GR, which harnesses the more negative reduction potential of NADPH in the process. NADPH in turn will be regenerated by the transhydrogenase, which in animal mitochondria couples hydride transfer between NADH and NADP to the proton motive force (Rydstrom 2006). GR continually recycles oxidised thiol back to its reduced form, since the potentiality of GSH regeneration within the mitochondrial matrix is proportionately responsible for constant reactive oxygen species degradation. In this respect, insufficiency in GR enzyme activities leading to mitochondria GSH depletion was not observed.
Another point to be considered is that mitochondrial GSH is not synthesized in the matrix but is imported from cytoplasmic compartment (Schafer and Buettner 2001). Thus, variation in the mitochondria matrix GSH ratio could be balanced by the import. However, in the case of mitochondria, the supply by GSH, GR activities in both treatment groups did not reflect any increase in response to GSH/GSSG ratio change.
Several studies have indicated that HA could induce oxidative injury (Jezierski et al. 2000) mediated by superoxide anion production (Liang et al. 1999) and apoptosis induction (Hseu et al. 2000; Hseu et al. 2002a, b; Yang et al. 2004; Hseu et al. 2008). Measurement of antioxidant properties of the selected natural HAs in both media showed that HAs displayed low antioxidant properties against superoxide radical, reaching maximally 20% of inhibition irrespective of the solvent used and the time of illumination (see Fig. 2). Regarding the unknown structure of HAs, one can only assume that their benzene rings lack enough phenolic groups to give antioxidant properties or these are not in favourable positions (Rice-Evans et al. 1996) or are blocked, e.g. by methyl groups.
Much better antioxidant properties of HAs against hydroxyl radicals are shown in Fig. 2. Fifty percent of inhibition was recorded at approximately 0.35 μg ml−1 for HA dissolved in DMSO and 0.2 μg ml−1 for HA dissolved in PBS. That relatively high activity is probably related to the free positions for substitution by hydroxy group in benzene rings of HA.
So, it can be concluded from our experiments that HA in spite of relatively low solubility in aqueous solutions and DMSO shows high antioxidant activity against hydroxyl radicals, while superoxide radicals, the electron in excess, respectively, is accepted by HA only very little. So the mechanism of electron transfer is not preferred by HA (Foti 2007).
High flow through mitochondria electron transport chain leads to increased use of coenzyme Q as an electron acceptor. Formed semiquinones after electronic acceptance readily reduce forming superoxide radicals. Dismutation of superoxide leads to hydrogen peroxide formation. The presence of numerous structures in HAs resembling those of quinones predetermines them to transferring the accepted electrons within a HA molecule without involvement of mitochondrial enzymes that otherwise reduce the developing peroxides. Decrease in other measured antioxidant enzymes GPx and GR point out that electrophilic properties of HA markedly balance mitochondria redox status. In vitro mitochondria exposed to HA showed assumed capacity of defensive enzymes in metabolically induced oxygen radical formation by the donor–acceptor charge transfer mechanism.
Cancer is a leading cause of death worldwide. The possibility that intake of natural substances might reduce risk of cancer has attracted attention as eventual chemopreventive or chemotherapeutic agent. Many clinically successful anticancer drugs are themselves either natural products or have been developed from naturally occurring lead compounds (Pouget et al. 2001). These compounds had the opposite effect, i.e. enhanced human lung cancer cell progression (Lee et al. 1999). In our study, cultivation of a number of cancer cell lines showed no oxidative stress injury (Table 2) resulting in induction of cell death, although the viability of CEM cells was decreased.
Conclusions
The results obtained indicated involvement of HAs from the selected source in redox regulation. They did not show HA-supported generation of ROS. On the contrary, they indicated recapture of superoxide with lower efficiency than hydroxyl radicals. In agreement with this, levels of glutathione, glutathione related and other antioxidant enzymes suggested moderate disabling of these protective mechanisms. As HA are naturally occurring, decomposed organic constituents, largely depending on the source used, they might constitute a promising group in search for an agent in terms of immunity enhancement.
References
Aon M. A.; Cortassa S.; Maack Ch; O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J. Biol. Chem. 282(30): 21889–21900; 2007.
Asahi M.; Fujii J.; Suzuki K.; Seo H. G.; Kuzuya T.; Hori M.; Tada M.; Fujii S.; Taniguchi N. Inactivation of glutathione peroxidase by nitric oxide. J. Biol. Chem. 270(36): 21035–21039; 1995.
Beauchamp C.; Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44: 276–287; 1971.
Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248; 1976.
Carlberg I.; Mannervik B. Glutathione reductase. Methods Enzymol. 113: 484–485; 1985.
Chen Y.; Katan J.; Gamliel A.; Aviad T.; Schnitzer M. Involvement of soluble organic matter in increased plant growth in solarized soils. Biol. Fertil. Soils 32: 28–34; 2000.
Cheng M. L.; Ho H. Y.; Huang Y. W.; Lu F. J.; Chiu D. T. Y. Humic acid induces oxidative DNA damage, growth retardation, and apoptosis in human primary fibroblasts. Exp. Biol. Med. 228: 413–423; 2003.
Choppin G. R.; Labonne-Wall N. Comparison of two models for metal-humic interactions. J. Radioanal. Nucl. Chem. 221: 67–71; 1997.
Coppola S.; Ghibelli L. GSH extrusion and the mitochondrial pathway of apoptotic signalling. Biochem. Soc. Trans. 28(2): 56–61; 2000.
Demeterová M.; Mariščáková R.; Pistl J.; Naď P.; Šamudovská A. The effect of the probiotic strain Enterococcus faecium DSM 7134 in combination with natural humic substances on performance and health of broiler chickens. Berl. Münch. Tierärztl. Wochenschr. 122(9/10): 370–377; 2009.
EMEA. Humic acids and their sodium salts, summary report. Committee for Veterinary Medicinal Products. European Agency for the Evaluation of Medicinal Products Available via http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014416.pdf; 1999.
Flohe L.; Gunzler W. A. Assay of glutathione peroxidase. Methods Enzymol. 105: 114–121; 1984.
Floreani M.; Petrone M.; Debetto P.; Palatini P. A comparison between different methods for the determination of reduced and oxidized glutathione in mammalian tissues. Free Radic. Res. 26: 449–455; 1997.
Foti M. C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 59: 1673–1685; 2007.
Hseu Y. C.; Chen S. C.; Chen Y. L.; Chen J. Y.; Lee M. L.; Lu F. J.; Wu F. Y.; Lai J. S.; Yang H. L. Humic acid induced genotoxicity in human peripheral blood lymphocytes using comet and sister chromatid exchange assay. J. Hazard. Mater. 153: 784–791; 2008.
Hseu Y. C.; Huang H. W.; Wang S. Y.; Chen H. Y.; Lu F. J.; Gau R. J.; Yang H. L. Humic acid induces apoptosis in human endothelial cells. Toxicol. Appl. Pharmacol. 182: 34–43; 2002a.
Hseu Y. C.; Lu F. J.; Engelking L. R.; Chen C. L.; Chen Y. H.; Yang H. L. Humic acid-induced echinocyte transformation in human erythrocytes: characterization of morphological changes and determination of the mechanism underlying damage. J. Toxicol. Environ. Health A 60: 215–230; 2000.
Hseu Y. C.; Wang S. Y.; Chen H. Y.; Lu F. J.; Gau R. J.; Chang W. C.; Liu T. Z.; Yang H. L. Humic acid induces the generation of nitric oxide in human umbilical vein endothelial cells: stimulation of nitric oxide synthase during cell injury. Free Radic. Biol. Med. 32: 619–629; 2002b.
Jezierski A.; Czechowski F.; Jerzykiewicz M.; Chen Y.; Drozd J. Electron paramagnetic resonance (EPR) studies on stable and transient radicals in b\humic acids from compost, soil, peat and brown coal. Spectrochim. Acta A Mol. Biomol. Spectrosc. 56: 379–385; 2000.
Johnson D.; Lardy H. Isolation of liver or kidney mitochondria. Methods Enzymol. 10: 94–96; 1967.
Joone G. K.; Dekker J.; van Rensburg C. E. Investigation of the immunostimulatory properties of oxihumate. Z. Naturforsch. 58: 263–267; 2003.
Joone G. K.; Rensburg C. E. An in vitro investigation of the anti-inflammatory properties of potassium humate. Inflammation 28: 169–174; 2004.
Klocking R.; Helbig B.; Schotz G.; Schacke M.; Wutzler P. Anti-HSV-1 activity of synthetic humic acid-like polymers derived from p-diphenolic starting compounds. Antivir. Chem. Chemother. 13: 241–249; 2002.
Kucukersan S.; Kucukersan K.; Colpan I.; Goncuoglu E.; Reisli Z.; Yesilbag D. The effect of humic acid on egg production and egg traits of laying hen. Vet. Med. 50: 406–410; 2005.
Lee C. K.; Klopp R. G.; Weindruch R.; Prolla T. A. Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390–1393; 1999.
Liang H. J.; Tsai C. L.; Chen P. Q.; Lu F. J. Oxidative injury induced by synthetic humic acid polymer and monomer in cultured rabbit articular chondrocytes. Life Sci. 65: 1163–1173; 1999.
Masini J. C. The use of linear potentiometric titration curve in the determination of alkalinity and acid-based properties of diluted solutions of humic substances. Talanta 41: 1383–1389; 1994.
Medina J.; Moreno-Otero R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs 65: 2445–2461; 2005.
Pouget Ch; Lauthier F.; Simon A.; Fagnere C.; Basly J.-F.; Delage Ch; Chulia A.-J. Flavonoids: structural requirements for antiproliferative activity on breast cancer cells. Bioorg. Med. Chem. Lett. 11(24): 3095–3097; 2001.
Rath N. C.; Richards M. P.; Huff W. E.; Huff G. R.; Balog J. M. Changes in the tibial growth plates of chickens with thiram-induced dyschondroplasia. J. Comp. Pathol. 133: 41–52; 2005.
Rice-Evans C. A.; Miller N. J.; Paganga G. Structure–antioxidant activity relationships of flavanoids and phenolic acids. Free Radic. Biol. Med. 20(7): 933–956; 1996.
Rydstrom J. Mitochondrial transhydrogenase—a key enzyme in insulin secretion and, potentially, diabetes. Trends Biochem. Sci. 31: 355–358; 2006.
Schafer F. Q.; Buettner G. R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30: 1191–1212; 2001.
Schepetkin I. A.; Khlebnikov A. I.; Ah S. Y.; Woo S. B.; Jeong C. S.; Klubachuk O. N.; Kwon B. S. Characterization and biological activities of humic substances from mumie. J. Agr. Food Chem. 51: 5245–5254; 2003.
Visser S. A. Effect of humic substances on mitochondrial respiration and oxidative phosphorylation. Sci. Total Environ. 62: 347–354; 1987.
Woo S. H.; Park I. C.; Park M. J.; Lee H. C.; Lee S. J.; Chun Y. J.; Lee S. I.; Rhee C. H. Arsenic trioxide induces apoptosis through a reactive oxygen species-dependent pathway and loss of mitochondrial membrane potential in HeLa cells. Int. J. Oncol. 21: 57–63; 2002.
Yang H. L.; Hseu Y Ch; Hseu Y. T.; Lu F. J.; Lin E.; Lai J. S. Humic acid induces apoptosis in human premyelocytic leukemia HL-60 cells. Life Sci. 75: 1817–1831; 2004.
Yasar S.; Gokcimen A.; Altunas I.; Yonden Z.; Petekkaya E. Performance and ideal histomorphology of rats treated with humic acid preparations. J. Anim. Physiol. Anim. Nutr. 86: 257–264; 2002.
Yoruk M. A.; Gul M.; Hayirli A.; Macit M. The effects of supplementation of humate and probiotic on egg production and quality parameters during the late laying period in hens. Poultry Sci. 83: 84–88; 2004.
Zhai S. S.; Kimbrough R. D.; Meng B.; Han J. Y.; LeVois M.; Hou X.; Yi X. N. Kashin–Beck disease: a cross-sectional study in seven villages in the People’s Republic of China. J. Toxicol. Environ. Health 30: 239–259; 1990.
Acknowledgement
Financial support of the Slovak Grant Agency for Science VEGA 1/0799/09 is appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Vašková, J., Veliká, B., Pilátová, M. et al. Effects of humic acids in vitro. In Vitro Cell.Dev.Biol.-Animal 47, 376–382 (2011). https://doi.org/10.1007/s11626-011-9405-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-011-9405-8