Abstract
In botryllid ascidians, astogeny is executed through blastogenesis, a weekly, highly synchronized phenomenon of growth and death cycles, each constitutes four major developmental stages (A–D), operating simultaneously on three coexisting asexually derived generations, including primary and secondary palleal buds. This study documents the de novo expression of Piwi transcript and protein in extirpated blastogenic stage “D” buds isolated from Botryllus schlosseri colonies that are maintained in vitro, days after the disappearance of corresponding intact zooids in control colonies. Under in vitro conditions, floating buds attach to substrates and develop monolayers of epithelial sheets that live for long periods (compared to intact colonial buds) prior to their deterioration. Here, we further demonstrate that various cell types within floating blastogenic stage “D” buds are labeled as Piwi +, as do other cells that are dispersed over the epithelial sheets (that are Piwi −, representing highly differentiated state), all revealing a surprising new flag for stemness in these tissue fragments that developed exclusively under in vitro conditions. No single permanent cell-line is currently available from colonial tunicates or from other marine invertebrates, since cells stop dividing in vitro within 24–72 h after their isolation and start cellular quiescence. The development of epithelial sheets from isolated Botryllus palleal buds and the recorded molecular stemness flag of various cells, remaining for long periods under in vitro conditions, may pave the way for establishing cell cultures from Botryllus epithelial cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colony formation in botryllid ascidians is carried out by blastogenesis, a weekly, highly synchronized phenomenon of growth and death cycles, occurring simultaneously in three coexisting asexually derived generations, zooids and primary and secondary palleal buds (Berrill 1941a,b; Lauzon et al. 2002; Manni et al. 2007). Each blastogenic cycle is composed of three major developmental stages (stages A–C; sensu the staging method by Mukai and Watanabe 1976) followed by a degenerative stage D (also called takeover), in which a whole-organism apoptotic event coupled with fast maturation of the primary buds culminates in the reconstitution of the colony. During blastogenic stages “A” to “C”, up to three to four new palleal buds develop from the body wall of each parental zooid within a colony. Stage “A” primary buds (each may reach 0.3 mm) develop secondary bud rudiments that emerge from the atrial epithelium. The secondary buds, together with the primary buds, progress through blastogenic stages “B” and “C” (primary buds reach sizes of up to 1 mm), whereby the secondary buds form a double-layered vesicles that host a rapid organogenesis process.
Botryllus schlosseri a shallow water encrusting marine species, has emerged as an accessible model system for studying various scientific disciplines (including stem cells biology, immunology, development senescence, and regeneration). It is a worldwide-distributed species, with colonies that are composed of up to several hundreds of modular units of adult zooids (each 1–2 mm long). All zooids and buds are embedded within a semi-translucent gelatinous matrix, the tunic, and are connected via ramified vasculature system.
Botryllus schlosseri has also been studied for the in vitro fate of developing primary palleal buds, extirpated from mature colonies at four blastogenic stages (Rinkevich and Rabinowitz 1997; Rabinowitz and Rinkevich 2003, 2004; Rabinowitz et al. 2009). Results revealed that abrogating in vivo homeostasis at the colonial-level resulted in in vitro major changes. Following a few days of transient period, extirpated buds at stages “B” to “D” (but not stage “A”) developed under suitable in vitro conditions new structures that were not found when following normal in vivo blastogenesis. Initially, extirpated buds adhered to the substrates, developed spheres (up to 1 mm diameter) and then, they developed epithelial monolayers on substrates that deteriorated after about 7–10 d, all culminating in about fivefold in vitro increase in life expectancy, as compared to in vivo developing zooids. Another biological feature change was the necrotic mode of death of epithelial monolayers in vitro (Rabinowitz and Rinkevich 2004) in contrast to the apoptotic mode of death at the in vivo takeover phase (Lauzon et al. 2002). In contrast to the in vivo outcomes, results also revealed the unexpected regenerative power of blastogenic stage “D” zooids (at the takeover phase) under in vitro conditions. Extirpated stage “D” buds developed almost three times more epithelial monolayers than developing stages “B” and “C” buds, with a higher order of magnitude in monolayers-to-spheres ratio (Rabinowitz and Rinkevich 2004). In addition, the vast majority of blastogenic stage “D” buds developed epithelial monolayers directly, without forming first in vitro spheres. Working on actin, PL10, P-MEK, MAP-kinase, and cadherin expressions, we (Rabinowitz et al. 2009) documented that extirpated buds and monolayers were very active on the molecular/biochemical levels, exhibiting fast changes in the protein levels on a daily basis. This study also revealed the importance of fibroblast growth factor (FGF) administration for the formation of epithelial monolayers.
Piwi belongs to the evolutionarily conserved PIWI/Argonaute superfamily of RNA interference effector proteins, which are essential for both self-renewal and maintenance of germline and somatic stem cells in diverse multicellular organisms (Reddien et al. 2005; Carmell et al. 2007; Rinkevich et al. 2010), including lower eukaryotes and plants. Working on the expression of Piwi in botryllid ascidians, Rinkevich et al. (2010) revealed that whole body regeneration in the tunicate Botrylloides leachi develops through activation, mobilization, and expansion of “dormant” cells, presumably stem cells, which normally line the internal vasculature epithelium of blood vessels. Following a mechanical assault, these cells express Piwi de novo, change morphology, and invade niches of the vasculature lumen, where they proliferate and differentiate, regenerating to a functional organism. In contrast, in normally developed Botryllus schlosseri colonies, colonies at blastogenic stages A–C showed no Piwi transcript or protein expressions.
As new cellular and morphological structures are associated with extirpated buds under in vitro conditions, this study tries to elucidate the possible de novo appearance of stemness signatures in the Botryllus model assay. This was performed by tracing Piwi expressions in mechanically extirpated palleal buds.
Materials and Methods
Bud isolation.
Botryllus schlosseri colonies were reared on glass slides under standard conditions (Rinkevich and Shapira 1998). Laboratory-bred colonies at sizes of up to 200 zooids were first starved for 24 h by incubation in filtered (0.2 μm) seawater (FSW) containing 50 μM/ml gentamycin (Biological Industries, Kibbutz Beit-HaEmek, Israel). Blastogenic stage “D” buds (sensu Mukai and Watanabe 1976), at an average size of 300 μm, were extirpated as described (Rabinowitz and Rinkevich 2003, 2004). In brief, the colonies were removed from the sterile FSW incubation solution, washed for 30 s in 70% ethanol, and immersed in FSW with 1% mixed antibiotic solution containing 104 U/ml penicillin, 10 mg/ml streptomycin, and 25 μg/ml amphotericin-B (PSA). Primary buds at blastogenic stage “D” were excised under stereomicroscope, in sterile conditions, using a pair of 1 ml disposable syringes (Artsana S.P.A. Grandate, Italy) equipped with 28-G size needles. By means of the needle tip, the tunic was removed and buds were dissected out, transferred to a sterile 100-μm-pore-size nylon cell strainer (Falcon, Becton Dickenson Labware, NJ) immersed in FSW–PSA solution. Extensive rinsing of buds with FSW (200–300 ml) through the filter was than performed.
Culture conditions.
Groups of up to five buds were transferred into a single 35 mM Petri dish (Cell Star, Greiner-Bio-One, Frickenhausen, Germany). Excess of FSW was removed and substituted with 2 ml of tissue culture medium essentially based on L-15 synthetic medium iso-osmotic to seawater, prepared as previously described (Rinkevich and Rabinowitz 1993; Rabinowitz and Rinkevich 2003). Medium was supplemented with 4 mM l-glutamine, 20 mMol/l HEPES, 3% heat inactivated fetal calf serum, 1% PSA, and 1 ng/ml recombinant human FGF. Medium and medium supplements were purchased from Biological Industries (Kibbutz Beit-HaEmek, Israel). Cultures were incubated at 20°C in a humidified incubator. They were grown either after developing epithelial monolayer or at earlier culture stages when still in sphere forms. Then, they were harvested, fixed, and processed for immunohistochemistry and Western analyses.
Immunohistochemistry of monolayers and buds.
Epithelial cells attached to plastic substrate as a monolayer were fixed with Bouin fixative (70 ml picric acid, 25 ml of 37% formaldehyde, and 5 ml glacial acetic acid, all purchased from Sigma, St. Louis, MO) followed by 70% ethanol. Combined methodologies for tissue permeabilization using proteinase K and antigen retrieval by means of heat treatment (90°C, 20 min) were performed on monolayers. Blocking buffer, composed of Tris-buffered saline (TBS) plus 0.05% Tween-20 (TBS-T) and 1% of bovine serum albumin (BSA, MP Biomedicals, LLC, Irvine, CA), was added for overnight incubation in order to block nonspecific signals. Primary rabbit anti-Bl-Piwi antibodies (Rinkevich et al. 2010) at a concentration of 1:500 dilutions in TBS–BSA solution (4°C) was added to the monolayers (n = 3). We used goat anti-rabbit alkaline phosphatase conjugates (Jackson Immunoresearch Laboratories Inc., West Grove, PA) as the secondary antibody, at a concentration of 1:10,000 dilutions in TBS-T containing 1% BSA. Incubations were performed for 90 min at room temperature, followed by washes with TBS-T 5 × 10 min. Monolayers were then incubated with NBT/BCIP diluted in developing buffer consisting of 100 mM NaCl, 50 mM MgCl2, and 100 mM Tris at a pH = 9.5. The stained tissues were protected from light by foil paper, and as soon as color developed, the substrate was removed, washed with tap water, and mounted with Hydromount™ medium (National Diagnostics, Atlanta, GA). For easy viewing through the microscope, (Olympus microscope, BX-FLA BX50, Olympus Optical Co., Hamburg, Germany), the plastic rim of the dish was removed by using heated sharp scalpel knife. Photomicrographs were captured using Color View camera (Soft Imaging System, Munster, Germany).
For histological sections, groups of five to seven isolated buds from different plates, at various in vitro culture periods (0 h, 24 h, 5 d, and 8 d after isolation), were harvested and fixed with Bouin fixative, for 30 min. Serial paraplast sections of 5 μm were obtained using a rotary microtome. Sections were attached to microscope slides (SuperFrost Plus, Menzel-Glaser, Braunschweig, Germany) and processed for immunostaining as previously described (Rosner et al. 2006) with modifications as described (Rabinowitz et al. 2009). In brief, sections were rehydrated and washed with TBS, microwaved for 30 min in citrate buffer (pH = 6.0) for antigen retrieval, and blocked in TBS plus 1% BSA. Finally, they were immunostained for Bl-Piwi protein, with the same polyclonal primary antibody and at the same concentrations as for whole-mount monolayers.
Immunoblotting.
Western blotting analyses were performed on isolated buds under in vitro conditions prior to the expected development of monolayers (at ~9 d) as was previously described (Rabinowitz et al. 2009). In brief, groups of up to 10 buds were either harvested immediately or left under culture conditions for 1, 5, and 8 d. Buds were washed in FSW and lysed in 35 μl lysis buffer solution containing 50 mM Tris buffer (pH = 6.8), 2% sodium dodecyl sulfate (SDS), and 250 mM β-mercaptoethanol. Extracts were collected and stored at −20°C. When all samples of an experiment were collected, they were boiled at 95°C, cooled on ice, and centrifuged at 14,000 rpm for 10 min. Supernatants were collected, and 1 μl sample of each protein extract was added onto Whatman GB002 gel blotting paper (Schleicher & Schuell, Dassel, Germany). BSA of five known standard concentrations was added in order to draw a standard curve. Protein concentrations were determined using Tina’s software (package version 2.07, Raytest, Straubenhardt, Germany). Samples of 5 μg total protein extract, each, were prepared to a final volume of 20 μl sample buffer containing bromophenol blue and loaded onto 8% SDS polyacrylamide gel, for electrophoretic separation according to their molecular weight. Subsequently, the proteins were transferred electrophoretically onto nitrocellulose membrane. Then, membranes were initially stained with membrane stain PROACT™ (Ameresco, Ohio, USA) in order to confirm equal protein concentrations in all lanes. The stain was removed by washing, and membranes were subjected to the indicated primary antibodies, separately. Blocking of nonspecific binding sites was carried out with 1% BSA dissolved in TBS containing 0.05% Tween®-20 (Sigma, TBS-T), incubated overnight at 4°C. Membranes were probed first to the primary specific antibody diluted with TBS-T and 1% BSA for overnight at 4°C. Secondary antibody, goat anti-rabbit horseradish peroxidase conjugate (Sigma) was diluted 1:12,000 in TBS-T and BSA and added to membrane for 1.5–2.0-h incubation. Visualization of antibody binding was done using SuperSignal West Pico Chemiluminescent Substrate kit (Pierce, Rockford, IL) according to manufacturer’s instructions. Membranes were exposed to a Kodak maximum resolution imaging film (Kodak BioMax MR Film, Kodak, Stuttgart, Germany). The membranes were then stripped off and re-probed with anti BS-PL10 (used at 1:2,000 dilutions) as previously described (Rosner et al. 2006) and with rabbit anti-actin (I-19; sc-1616) antibodies (1:5,000 dilution).
Results
We expiated 350–400 blastogenic stage “D” buds. As previously documented (Rabinowitz et al. 2009), most of blastogenic stage “D” surgically detached buds remained floating and retained a vigorous heart beatings under in vitro conditions for several d, without developing into mature zooids. This is in contrast to the corresponding blastogenic stage “D” buds that were left untouched in the control colonies. In vivo primary buds at this stage that usually “metamorphose” into functional zooids within a few hours survived for another week. Certain tissues (blood cells, epithelial tissues) in extirpated buds that did not develop the epithelial sheets survived under in vitro conditions for up to 5 mo. Heart beatings of these transplants gradually ceased during the first wk of explant culture, and all freely floating buds (71% of total explants) remained spherical, and in time, swelled up and filled with loose cells originated from dissociated visceral tissues and circulating blood cells. As described (Rabinowitz et al. 2009), about 90% of isolated buds attached to the substrate by day 9 developed epithelial monolayer, following the FGF treatment that was administered for 24 h 1 d post-bud extirpation.
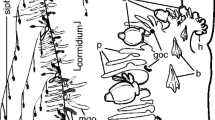
The epithelial cells of the monolayers were not stained by the Bl-Piwi polyclonal antibody. However, very few (about 65 cells per monolayer at a size of 1.5 mm in diameter) 8-μm-size cells, lying atop the epithelial sheets, were intensely stained by Bl-Piwi (Fig. 1a – d ). The protein was located in the cytoplasm but not in the nucleus of the cell (Fig. 1c ). The proportion of stained cells to unstained epithelial cells was 1:34. Bl-Piwi + cells exhibited large round nucleus of 4–5 μm (Fig. 1b, c ) and spread on epithelial sheets with radially extruded slender cytoplasmic projections, the filopodia (Fig. 1b ).
Expression of Piwi protein in Botryllus schlosseri monolayers. (a–c) Three epithelial monolayers (originated from 9 to 14-d-old cultures) were immunolabeled with anti-Bl-Piwi polyclonal antibody. At the background, the epithelial sheets were not stained as opposed to the strong cytoplasmic stain appearing in individual cells, lying on top of the monolayers. These cells are irregular in shape, spreading on epithelial sheets with slender cytoplasmic projections, the filopodia (arrows in b). (a, ×200; b, ×400; c, ×1,000). (d) 4′,6-Diamidino-2-phenylindole (DAPI)-stained cell nuclei in the monolayer (blue dots) on which the silhouettes of the lying cells are seen (arrows). DAPI was added following labeling with anti-Bl-Piwi antibodies and was observed by fluorescence microscope equipped with an excitation filter of 360–370-nm wavelengths and a barrier filter of 420 nm (×200). Bars: a = 100 μm, b–d = 10 μm.
Histological sections made on intact and detached blastogenic stage “D” buds (Fig. 2) revealed no Bl-Piwi + expression in intact buds at the time of extirpation that followed for the next few days. Weak Bl-Piwi + expression started on day 5 of culture with substantial increase in expression at 8 d when cells in the entire extirpated bud were positively labeled (Fig. 2). Histological sections from floating blastogenic stage “D” buds, harvested on the eight day of culture (Fig. 3) and immunostained with anti-Bl-Piwi polyclonal antibodies, revealed Bl-Piwi + protein staining appearing de novo in various cell types. Bl-Piwi + cells include loose cell clumps (Fig. 3a ), cells of the external thickened epithelial envelop of the dissected bud (Fig. 3b ) and individual cells in close contact with the epithelial layer (Fig. 3c ).
Bl-Piwi immunohistochemistry on 5-μm-thick histological sections originated from in vitro cultured blastogenic stage “D” buds and harvested at different time points (up to 8 d) following extirpation. The “in situ” microphotograph represents the control situation of undetached bud with nonspecific staining of the tunic matrix.
Immunostaining with anti-Bl-Piwi polyclonal antibodies of histological sections (5 μm) made on floating blastogenic stage “D” buds harvested on the eight day of culture. The expression of this protein is present de novo in various cell types. (a) A loose cell clump adjacent to the external epithelial envelope with varying Bl-Piwi + levels. (b) The external thickened epithelial envelop is stained strongly at this stage. (c) Single Bl-Piwi + round-shaped cells in close contact with a thin and weakly stained epithelium layer. All photomicrographs are of same magnifications (×1,000). Bars = 20 μm.
Western blot analyses of total protein extracts from pools of 10 isolated buds, collected at various culture time points (0, 1, 5, and 8 d; all taken prior to monolayer development), were performed on Bl-Piwi as well as on actin and Pl-10 protein levels. In time, actin and Bl-PIWI showed increased levels of protein expression (Fig. 4). While levels of the actin protein were high from the start and almost doubled by day 8, the Bl-Piwi bands that appeared very weak at first became positively visible on day 5 and highly increased by day 8. Pl-10 protein levels remained stable and showed constant expression along the eight culture days (Fig. 4).
Discussion
In this study, we have documented the de novo expression of Piwi transcript and protein in extirpated blastogenic stage “D” buds, days after the disappearance of corresponding zooids that were left intact in control colonies. Various cell types within floating blastogenic stage “D” buds were labeled as Piwi + in addition to other cells that, after the establishment of the epithelial monolayers, were dispersed over these epithelial sheets (that were Piwi −, representing highly differentiated state), revealing a surprising new flag for stemness in these tissue fragments, developed exclusively under in vitro conditions. In regular zooids, intense Piwi staining was recorded in the macrophage-like cells around the endostyle (presumably the stem cells niche; Voskoboynik et al. 2008); in test cells, tunic cells, and cells of the digestive system, mainly in the stomach (Rosner et al. 2009), a complete different profile from the stemness signature presented in vitro. These results add to earlier outcomes (Rabinowitz et al. 2009) showing that extirpated buds and monolayers were very active on the molecular/biochemical levels, revealing various cells and cellular organelles stains and rapid changes in the protein levels on a daily basis, for example, cells situated in the center of the monolayers stained differently for some proteins than peripheral cells (Rabinowitz et al. 2009).
Irrespective of their origin, stem cells have to respond in similar ways to regulate self-renewal and differentiation lineages, and therefore, it is likely that different stem cells will be harnessed for stemness by ubiquitous mechanisms and omnipresent orthologue genes. One avenue is Piwi orthologues. The Piwi belongs to a class of such universal genes, originally identified as encoding regulatory protein responsible for maintaining incomplete differentiation in stem cells and maintaining the stability of cell division rates in germline cells (Reddien et al. 2005; Carmell et al. 2007; O’Donnell and Boeke 2007). Not all stemness markers (i.e., Pl-10, Vasa, Nanos, Piwi, and Oct4) depict real stem cell situations and mark stem cell lineage exclusivities. One example is the DDX3 proteins (termed also Pl-10) that are less representing solely real stemness capacities, as being involved in various developmental pathways, highly expressed in adult undifferentiated soma and germ cells and in some adult and embryo’s differentiating tissues (Rosner and Rinkevich 2007). We found that this was reflected by the Pl-10 protein expression levels in blot analyses as well.
As in earlier studies (Rosner et al. 2009; Rinkevich et al. 2010), this study revealed that Piwi − cells are repeatedly appearing in the colony, independently of its sexual state, emerging also in cell lineages unrelated to the somatic cell line, a results that may have considerable importance for the developing of permanent cell lines from these organisms. Despite extensive research efforts, there is yet no single permanent cell line available from colonial tunicates or from other marine invertebrates because isolated cells stop dividing in vitro within 24–72 h after their isolation, starting cellular quiescence (Mothersill and Austin 2000; Rinkevich 2005). The development of epithelial sheets from isolated Botryllus palleal buds (Rinkevich and Rabinowitz 1997; Rabinowitz and Rinkevich 2003, 2004; Rabinowitz et al. 2009), the fact that free-floating stage “D” buds are highly active, exhibiting differential expressions of various proteins along incubation (actin, Pl-10, FGF-R, P-MEK, MAP-kinase, and Cadherin; Rabinowitz et al. 2009; this study) and the recorded molecular stemness flag of various cells for prolonged periods under in vitro conditions (this study) may pave the way for the establishment of cell cultures from Botryllus epithelial cells. When cultured “correctly”, these Piwi + cells can remain in their primitive stage and proliferate without differentiating into cells lineages, harnessing the stem cell’s inherent ability to create self-replicate progenies, developing into immortal cell lines.
References
Berrill N. J. The development of bud in Botryllus. Biol. Bull 80: 169–184; 1941a.
Berrill N. J. Size and morphogenesis in the bud of Botryllus. Biol. Bull. 80: 185–193; 1941b.
Carmell M. A.; Girard A.; van de Kant H. J.; Bourc’his D.; Bestor T. H.; de Rooij D. G.; Hannon G. J. et al. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell. 12: 503–514; 2007.
Lauzon R. J.; Ishizuka K. J.; Weissman I. L. Cyclical generation and degeneration of organs in a colonial urochordate involves crosstalk between old and new: a model for development and regeneration. Dev. Biol. 249: 333–348; 2002.
Manni L.; Zaniolo G.; Cima F.; Burighel P.; Ballarin L. Botryllus schlosseri: a model ascidian for the study of asexual reproduction. Dev. Dyn. 236: 335–352; 2007.
Mothersill C.; Austin B. Aquatic invertebrate cell culture. Springer, Berlin; 2000.
Mukai H.; Watanabe H. Studies on the formation of germ cells in a compound ascidian Botryllus primiginus Oka. J. Morphol. 148: 337–362; 1976.
O’Donnell K. A.; Boeke J. D. Mighty piwis defend the germline against genome intruders. Cell. 129: 37–44; 2007.
Rabinowitz C.; Alphasi G.; Rinkevich B. Further portrayal of epithelial monolayers, emergent de novo from extirpated ascidians’ palleal buds. In Vitro Cell. Dev. Bio. Anim. 45: 334–342; 2009.
Rabinowitz C.; Rinkevich B. Epithelial cell cultures from Botryllus schlosseri palleal buds: accomplishments and challenges. Methods Cell. Sci. 25: 137–148; 2003.
Rabinowitz C.; Rinkevich B. In vitro delayed senescence of extirpated buds from zooids of the colonial tunicate, Botryllus schlosseri. J. Exp. Biol. 207: 1523–1532; 2004.
Reddien P. W.; Oviedo N. J.; Jennings J. R.; Jenkin J. C.; Alvarado A. S. SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science. 310: 1327–1330; 2005.
Rinkevich B. Marine invertebrate cell cultures: new millennium trends. Mar. Biotechnol. 7: 429–439; 2005.
Rinkevich B.; Rabinowitz C. In vitro culture of blood cells from the colonial protochordate Botryllus schlosseri. In Vitro Cell. Dev. Biol. Anim. 29A: 79–85; 1993.
Rinkevich B.; Rabinowitz C. Initiation of epithelial cell cultures from palleal buds of Botryllus schlosseri, a colonial tunicate. In Vitro Cell. Dev. Biol. Anim. 33: 422–424; 1997.
Rinkevich Y.; Rosner A.; Rabinowitz R.; Lapidot Z.; Moiseeva E.; Rinkevich B. Piwi positive cells that line the vasculature epithelium, underlie whole body regeneration in a basal chordate. Dev. Biol. 345: 94–104; 2010.
Rinkevich B.; Shapira M. An improved diet for inland broodstock and the establishment of an inbred line from Botryllus schlosseri, a colonial sea squirt (Ascidiacea) Aquat. Living Resour. 11: 163–171; 1998.
Rosner A.; Moiseeva E.; Rinkevich Y.; Lapidot Z.; Rinkevich B. Vasa and the germ line lineage in colonial urochordate. Dev. Biol. 331: 113–128; 2009.
Rosner A.; Paz G.; Rinkevich B. Divergent roles of the DEAD-Box protein BS-PL10, the urochordate homologue of human DDX3 and DDX3Y proteins, in colony astogeny and ontogeny. Dev. Dyn. 235: 1508–1521; 2006.
Rosner A.; Rinkevich B. The DDX3 subfamily of the DEAD Box helicases: divergent roles as unveiled by studying different organisms and in vitro assays. Curr. Med. Chem. 14: 2517–2525; 2007.
Voskoboynik A.; Soen Y.; Rinkevich Y. Identification of the endostyle as a stem cell niche in a colonial chordate. Cell Stem Cell 3: 456–464; 2008.
Acknowledgements
This study was supported by grants from the United States–Israel Bi-National Science Foundation (2003-010) and the Israel Academy of Science (550-06). We thank G. Paz for the figure preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Rabinowitz, C., Rinkevich, B. De novo emerged stemness signatures in epithelial monolayers developed from extirpated palleal buds. In Vitro Cell.Dev.Biol.-Animal 47, 26–31 (2011). https://doi.org/10.1007/s11626-010-9357-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-010-9357-4