Abstract
The Wharton’s Jelly (WJ) of the umbilical cord (UC) is an excellent source of mesenchymal stem cells (MSCs) with a range of potential therapeutic applications. The present study was conducted to demonstrate the efficiency of the protocols used by Biogenea-Cellgenea Ltd. for isolation and expansion of WJ MSCs from donors across Greece. Umbilical cord samples were collected from 599 females following childbirth and processed for WJ MSC isolation. Stem cells were expanded using DMEM-based media and cell counts and overall viability figures derived using Trypan blue exclusion. To investigate the application of isolation and expansion protocols on samples received 1, 2, 3, 4 and 5 d after their collection, ten fresh samples were processed at these time intervals and evaluated. The cellular yield of most WJ samples was 1.1–5.0 × 106 cells at 21–30 d after processing. As culture time increased, cell counts decreased. Statistical analysis of mean cell counts showed a significant reduction after 21 d. Finally, we demonstrate for the first time that it is possible to obtain satisfactory cell numbers from samples processed 1, 2, 3, 4 and even 5 d after collection. We have derived favourable data on the protocols used at Biogenea-Cellgenea Ltd. to isolate and culture MSCs from the WJ. Protocol choice is crucial when handling large numbers of samples on a daily basis and should be made to ensure the best possible outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human umbilical cord is considered an excellent source of stem cells, which may be therapeutically beneficial through autologous transplantation. Although human umbilical cord blood haematopoietic stem cells (HUSCs) were the first to be thoroughly characterised and stored in stem cell banks, another type of stem cells has recently been isolated from the Wharton’s Jelly of the human umbilical cord (McElreavey et al. 1991). These are mesenchymal in origin, highly proliferative, multipotent and pleomorphic yet fibroblast-like cells, located in the mucoid connective tissue known as the Wharton’s Jelly (McElreavey et al. 1991; Ma et al. 2005). The Wharton’s Jelly surrounds the two arteries and single vein of the umbilical cord, thus providing support during its development and maintenance (Romanov et al. 2003; Bakhshi et al. 2008). Since the initial identification of human umbilical cord mesenchymal stem cells (HUMSCs), a limited number of studies have been conducted on areas including HUMSC isolation and expansion.
In this study, we assess the effectiveness of the protocols used by the HUMSC bank Biogenea-Cellgenea Ltd. for the isolation and expansion of WJ HUMSCs obtained from umbilical cord samples shipped following labor from all over Greece.
Materials and Methods
Isolation and culture of WJ HUMSCs.
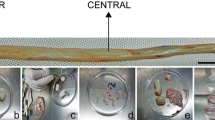
To enable the inclusion of samples in the present study, signed informed consent forms were obtained from all donors. Five hundred and ninety-nine umbilical cord samples 3–5 cm in length were used to isolate WJ HUMSCs. Samples were processed within a maximum period of 48 h since their collection, depending on the shipping period. To isolate stem cells, umbilical cord samples were rinsed in 75% ethanol (Sigma, Poole, UK) for 30 s and cut open in parallel to umbilical cord vessels, so as to expose them fully. The gelatinous tissue surrounding the vessels was excised and minced into very fine pieces of 0.5–1 mm2, which were plated on a sterile 100 × 20 mm petri dish (Corning, Ewloe, UK) and left for 5–10 min at room temperature, to facilitate tissue attachment. The minced tissue was carefully covered with 5 ml of growth medium comprised of low-glucose DMEM supplemented with 10% foetal bovine serum, 2 mM l-glutamine, penicillin (100 U/ml) and streptomycin (100 μg/ml) solution, 25 μg/ml Fungizone, 5 ng/ml basic fibroblast growth factor and 5 ng/ml epidermal growth factor (all from Invitrogen, Paisley, UK). WJ samples were incubated at 37°C in a humidified CO2 incubator for 5–10 d, before visible colonies of WJ HUMSCs were observed. Cells were then transferred to 75-cm2 flasks (Corning) at a density of 5 × 103/cm2 and cultured up to 70–80% confluency before data collection and cryopreservation. Cryopreservation was performed when cultures reached 70–80% confluency.
An additional experiment was carried out to determine the feasibility of obtaining viable HUMSC cultures from samples received by Biogenea-Cellgenea Ltd., Thessaloniki, Greece laboratories within a period of 1, 2, 3, 4 and 5 d of their collection. In this experiment, ten fresh umbilical cord samples were processed 1, 2, 3, 4 and 5 d after their collection and their viability and cell number measured.
WJ HUMSC number and viability count using Trypan blue exclusion.
Cells suspended in Ca2+- and Mg2+-free DPBS (Invitrogen) were diluted 1:2 with 0.25% (w/v) Trypan blue in PBS (Invitrogen) and incubated for 1–2 min at room temperature. Cells were loaded onto an improved Neubauer haemocytometer and live (clear) and dead (blue) cells were counted in the four corner squares. This test was used to establish percent viability and total cell count (TCC).
Flow cytometry.
WJ HUMSCs were assayed for the expression of CD45, CD44 and CD90 surface antigens through flow cytometry. Cell samples were rinsed in DPBS through two centrifugation steps at 1,500 rpm for 5 min at room temperature. Samples were used at a concentration of 1 × 106/ml and 1 ml volumes to stain with either anti-human CD45-FITC (Cytognos, Salamanca, Spain) or anti-human CD44-FITC (Beckman-Coulter, Krefeld, Germany) or anti-human CD90-PE (Beckman-Coulter) for 15 min at room temperature. Flow cytometric analysis was conducted using a Partec CyFlow® ML flow cytometer (Partec, Carate Brianza, Italy).
Results and Discussion
Most WJ samples were cryopreserved 21–30 d after their initial processing (Fig. 1a ). Fewer samples were stored after 10–20 d, probably due to the period required for HUMSCs to migrate from the plated tissue pieces to the plate surface and initiate colony formation. A minimum period of 10 d was required for the first colonies of WJ HUMSCs to appear and an additional 5-d period to gain 70–80% confluency prior to transfer to 75 cm2 flasks. The number of samples stored diminished after 31 d possibly due to sample donor age, which may result in a significant loss of stem cell proliferative capacity caused by telomere shortening. Another factor may be the prolonged culture period (Banfi et al. 2000; Baxter et al. 2004; Kan et al. 2005).
Population data—the distribution of WJ HUMSC samples with regard to TCC and culture period. Frequency distribution of processed WJ HUMSC samples with regard to TCC and isolation-culture duration prior to cryopreservation (a). Graphical representation of the mean TCC values vs isolation-culture duration prior to cryopreservation (b). The maximum interval between WJ sample collection and processing was 48 h, depending on the shipping period. 10–20 d: n = 133; 21–30 d: n = 359; 31–40 d: n = 69; 41–50 d: n = 29; 51–60 d: 9; total: n = 599. *p < 0.001, as derived from a combined one-way ANOVA and Tukey’s post-test analysis at p < 0.05.
The mean TCC of most samples was between 1.1 and 5.0 × 106 cells, with marked frequency decrease below the 1.1–2.0 × 106 region and above the 4.1–5.0 × 106 region. The highest mean TCC lay in the 10.1–11.0 × 106 region, thus indicating the ability of WJ HUMSCs to proliferate rapidly within a limited period of time, specifically four to five times in a period of 3–5 d, as previously reported (Kim et al. 2004). Donor age could explain that the samples with the highest mean TCC contained rapidly dividing cells.
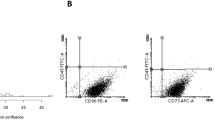
The overall viability of WJ HUMSC samples was >95%, as measured by both Trypan blue exclusion and flow cytometric analysis using 7-amino-actinomycin D (data not shown). Hence, our protocols for the isolation and expansion of WJ HUMSCs were validated since they produced viable stem cell populations. In addition, flow cytometry was carried out using monoclonal anti-human CD44 and CD90, since there is not a single marker for MSCs. Samples were >95% positive for either of these markers (Fig. 2a , b) and negative for the HUSC marker CD45 (Fig. 2a , b). These findings confirmed that WJ donor samples indeed contained WJ HUMSCs and are in agreement with the data presented by Wang et al. (2004).
Flow cytometry of UC samples. WJ culture samples were stained using a direct immunofluorescence protocol with anti-CD45-FITC (a and b), anti-CD44-FITC (a) and anti-CD90-PE (b) and analysed by flow cytometry to determine the percentage of HUMSCs. HUMSCs express CD44 and CD90, but not CD45. Mean percentage ± SEM data (%) was as follows: \({\text{CD}}45 + = 0.27 \pm 0.02\); \({\text{CD}}44 + = 97.35 \pm 3.02\); \({\text{CD}}90 + = 95.88 \pm 4.17\); n = 599.
The mean TCC values at various time intervals after plating are demonstrated in Fig. 1b . Mean TCC decreased with culture time, being highest after 10–20 d, and significantly lower after 21 d. Possible causes of this decline include donor age and shipping duration. Despite these potentially compromising factors, viable WJ HUMSC populations were obtained.
Figure 3 presents the outcome of processing ten fresh WJ samples after 1, 2, 3, 4 and 5 d since their collection. This independent experiment indicated that it was possible to derive viable WJ HUMSC populations 1, 2, 3, 4 and even 5 d after sample collection. Lack of WJ exposure due to its location in the umbilical cord could aid its protection and maintenance. This may be a valuable observation when considering the effects of long shipping times on successful WJ HUMSC isolation. Even though cells can be derived 4 and 5 d following the sample collection, they appear morphologically different (Fig. 3d , e) from those processed after 1, 2 and 3 d, a likely indicator of differentiation.
Mean TCC values ± SEM from the multiple processing experiments as obtained from Trypan blue exclusion were: 1 d: 2.50 ± 0.20 × 106; 2 d: 1.30 ± 0.18 × 106; 3 d: 1.20 ± 0.14 × 106; 4 d: 0.84 ± 0.07 × 106; 5 d: 0.74 ± 0.10 × 106. When analysed with one-way ANOVA and Tukey’s post-test at p < 0.05, the data revealed significant differences between 1 d and each of 2, 3, 4 and 5 d with p < 0.001. Statistical analysis therefore fully supports microscopical observations of the decrease in cell number after 2–5 d.
Most isolation protocols for WJ HUMSCs employ the use of several enzymes and long incubation times (Pereira et al. 2008; Seshareddy et al. 2008; La Rocca et al. 2009). Although the effectiveness of these protocols has been established, their application may be complicated when dealing with a large number of incoming samples simultaneously, as is usually the case with a stem cell bank. We therefore propose a simplified, reliable and less labour-intensive protocol, which leads to pure and viable cultures of WJ HUMSCs. Additionally, we have been able to confirm with our own experiments the protocol proposed by Friedman et al. (2007) to cryopreserve minced WJ tissue in autologous plasma and subsequently use it to derive viable WJ HUMSC cultures. Although this technique is considerably less complicated than ours, it may be disadvantageous when immediate product release is requested, since a period of at least 20 d was required to obtain a satisfactory cell number of 1–2 × 106 (data not shown).
Conclusively, this study presents population data obtained from UC tissue donors across Greece to assess the efficiency of isolation and culture protocols applied to a large number of samples. In addition, we were able to demonstrate the possibility of obtaining viable WJ HUMSC cultures when processing is performed within up to 5 d after collection. To the best of the authors’ knowledge, other relevant studies have used fresh samples. Considering the potential future clinical uses of WJ HUMSCs, the value of UC tissue becomes more apparent and its appropriate and effective handling mandatory.
References
Bakhshi T.; Zabriskie R. C.; Bodie S.; Kidd S.; Ramin S.; Paganessi L. A.; Gregory S. A.; Funq H. C.; Christopherson K. W. 2nd Mesenchymal stem cells from the Wharton’s jelly of umbilical cord segments provide stromal support for the maintenance of cord blood hematopoietic stem cells during long-term ex vivo culture. Transfusion 48: 2638–2644; 2008.
Banfi A.; Muraglia A.; Dozin B.; Mastrogiacomo M.; Cancedda R.; Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp. Hematol. 28: 707–715; 2000.
Baxter M. A.; Wynn R. F.; Jowitt S. N.; Wraith J. E.; Fairbairn L. J.; Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22: 675–682; 2004.
Friedman R.; Betancur M.; Boissel L.; Tuncer H.; Cetrulo C.; Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol. Blood Marrow Transplant 13: 1477–1486; 2007.
Kan I.; Melamed E.; Offen D. Integral therapeutic potential of bone marrow mesenchymal stem cells. Curr. Drug Targets 6: 31–41; 2005.
Kim J. W.; Kim S. Y.; Park S. Y.; Kim Y. M.; Kim J. M.; Lee M. H.; Ryu H. M. Mesenchymal progenitor cells in the human umbilical cord. Ann. Hematol. 83: 733–738; 2004.
La Rocca G.; Anzalone R.; Corrao S.; Magno F.; Loria T.; Lo Iacono M.; Di Stefano A.; Giannuzzi P.; Marasà L.; Cappello F.; Zummo G.; Farina F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem. Cell Biol. 131: 267–282; 2009.
Ma L.; Feng X. Y.; Cui B. L.; Law F.; Jiang X. W.; Yang L. Y.; Xie Q. D.; Huang T. H. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin. Med. J. 118: 1987–1993; 2005.
McElreavey K. D.; Irvine A. I.; Ennis K. T.; McLean W. H. Isolation, culture and characterization of fibroblast-like cells from the Wharton’s jelly portion of human umbilical cord. Biochem. Soc. Trans. 19: 29S; 1991.
Pereira W. C.; Khushnooma I.; Madkaikar M.; Ghosh K. Reproducible methodology for the isolation of mesenchymal stem cells from human umbilical cord and its potential for cardiomyocyte generation. J. Tissue Eng. Regen. Med. 2: 394–399; 2008.
Romanov Y. A.; Svintsitskaya V. A.; Smirnov V. N. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 21: 105–110; 2003.
Seshareddy K.; Troyer D.; Weiss M. L. Method to isolate mesenchymal-like cells from Wharton’s Jelly of umbilical cord. Methods Cell Biol. 86: 101–119; 2008.
Wang H. S.; Hung S. C.; Peng S. T.; Huang C. C.; Wei H. M.; Guo Y. J.; Fu Y. S.; Lai M. C.; Chen C. C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 22: 1330–1337; 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Petsa, A., Gargani, S., Felesakis, A. et al. Effectiveness of protocol for the isolation of Wharton’s Jelly stem cells in large-scale applications. In Vitro Cell.Dev.Biol.-Animal 45, 573–576 (2009). https://doi.org/10.1007/s11626-009-9227-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-009-9227-0