Abstract
Today there is a concern about the use of animal source proteins and peptides in cell culture applications due to potential contamination by adventitious infectious pathogens. Recombinant production of these proteins using a plant host provides a safe and cost effective alternative. In this paper, we tested the effect of rice-derived recombinant human lactoferrin (rhLF) on mammalian cell growth. The purified rhLF was partially (about 50%) iron-saturated (pis-rhLF). Chemical modification of pis-rhLF generated apo-rhLF (<10% iron saturation) or holo-rhLF (>90% iron saturation). All three forms of rhLF (pis, apo, holo) promoted growth of intestinal cells (HT-29) measured as [3H]-thymidine incorporation or viable cell count, but holo-rhLF was most effective. Holo-rhLF was further tested on hybridoma, osteoblast, and human embryonic kidney cells. Results showed that holo-rhLF promoted cell growth and reduced cell doubling time. The concentration of holo-rhLF in media was critical in promoting cell growth and each cell line had different concentration dependence with the most effective range from 5 to 200 mg/L. The effect of rhLF on antibody production was determined using a hybridoma cell line. Significantly, more antibodies were produced by cells grown with holo-rhLF than cells grown without holo-rhLF. We also compared the effect of holo-rhLF to that of human transferrin, a component commonly used in cell culture media as an iron source. Holo-rhLF was as effective as human transferrin in promoting cell growth and antibody production. Considering all the data obtained, we conclude that rhLF from rice is effective in promoting mammalian cell growth and increasing cell productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human lactoferrin (hLF) is an 80-kD glycoprotein expressed in milk, tears, and other fluids of the human body. Lactoferrin (LF) consists of two lobes, each binding one ferric iron molecule. It has been shown that LF is an antimicrobial protein playing a key role in the first line of defense against pathogenic invasion. LF has also demonstrated antiinflammatory and immuno-modulation effects (see Lonnerdal and Iyer 1995 for a review).
Studies have suggested that LF might function as a growth factor. LF enhances, in a dose-dependent manner, DNA synthesis and cell growth of neonatal rat hepatocytes (Kohno et al. 1993), primary rat and human osteoblast cells (Cornish et al. 2004) and human endometrial stroma cells (Yanaihara et al. 2000). Furthermore, LF has a synergistic effect with other growth factors such as epidermal growth factor (Hagiwara et al. 1995) and insulin (Kohno et al. 1993), as well as an effect on delaying apoptosis (Cornish et al. 2004; Baumrucker et al. 2005). For industrial application, LF has a stimulatory effect on hybridoma cell growth and production of immunoglobulin M (Yamada et al. 1990). However, the data from the literature are not consistent. Hashizume et al. demonstrated that LF promoted the growth of human B cells and T cells but had no effect on the growth of mouse B cells (Hashizume et al. 1983). Nichols et al. showed that [3H]-thymidine incorporation into DNA of crypt enterocytes (small intestinal cells of rats) was enhanced by approximately 35% in the presence of hLF (Nichols et al. 1987; Nichols et al. 1989) and Hagiwara et al. showed a positive effect of LF on the proliferation of rat intestinal epithelial cells (Hagiwara et al. 1995). However, Amouric et al. did not see the same effect of hLF on human colon adenocarcinoma cell line (HT29) (Amouric et al. 1984). Azuma et al. showed that hLF stimulated growth of fibroblast cells from mouse embryo (Azuma et al. 1989), but Nichols et al. (Nichols et al. 1987) did not observe the same effect. While one study showed that LF increased the cell cycle and delayed apoptosis of bovine epithelial cells (Baumrucker et al. 2005), another study showed that LF inhibited the growth of bovine mammary epithelial cells (Hurley et al. 1994). Therefore, the positive effect of lactoferrin as a general growth factor is not agreed on in the scientific community (Lonnerdal and Iyer 1995).
It is not clear why different results were obtained. Variation in media and differences in cell lines could contribute to the inconsistencies in the results. It is also possible that variations in the LF preparations contributed to the inconsistencies. These variations could be from different sources of LF or different methods of LF purification (see Wakabayashi et al. 2006 for a review of LF preparations). Thus, a consistent source of LF will help resolve some of the issues.
Recombinant human lactoferrin (rhLF) provides a foundation for producing a consistent product. We have expressed rhLF in rice at a high level (0.5% flour weight or 25% of total soluble protein) (Nandi et al. 2002). Biochemical and biophysical analyses indicate that rhLF is similar to native human lactoferrin (nhLF). The rhLF has the same isoelectric point, iron binding capacity, pH stability, thermal stability, and antimicrobial activity as nhLF. Furthermore, an efficient procedure to purify rhLF from rice has been developed (Nandi et al. 2005), which provides the basis for consistent large-scale manufacturing of rhLF for various applications.
In this report, we describe the use of rhLF from rice as a cell culture component to enhance the growth of intestinal cells, hybridoma cells, human embryonic kidney cells and osteoblasts. In some experiments, holo-transferrin (holo-TF) was used for comparison since holo-TF is commonly used in cell culture media such as serum-free medium for hybridoma cell growth in the cell culture industry (Kovar and Franek 1985). TF and LF are similar in molecular weight, structure and iron-binding (Testa 2002); thus, we expect that holo-rhLF will have similar effect on cell growth as holo-TF.
Materials and Methods
Preparation of rhLF for use in cell culture. A detailed description of rhLF expression and purification from rice has previously been described (Nandi et al. 2002; Nandi et al. 2005). The rhLF purified from rice, with no iron content modification, was partially iron-saturated (pis-rhLF). Apo-rhLF was generated by the procedure as described (Nandi et al. 2002). Iron-saturated rhLF (holo-rhLF) was prepared by a modification of the procedure in Nandi et al. (2005). Briefly, rice flour containing rhLF was extracted with neutral pH buffer and then clarified by removal of solids. The clarified extract was loaded on to a sulfopropyl protein (SP) Sepharose column. After washing with loading buffer, rhLF was eluted with loading buffer plus 1 M sodium chloride. The purified rhLF from the SP Sepharose resin was approximately 50% iron saturated and termed as pis-rhLF. To generate holo-rhLF, the pis-rhLF was mixed at 4°C with ferric nitrate solution at a molar ratio of 1:6 (1 rhLF:6 iron). The holo-rhLF was concentrated and desalted using Millipore S10 membrane with 30,000 nominal molecular weight cutoff. During this concentration and desalting step, any excess unbound iron was also removed. The protein solution was centrifuged to remove any precipitate and filtered through 0.45 μm and 0.2 μm filters prior to lyophilization. The resulting holo-rhLF has iron saturation level greater than 90% or >1.26 mg iron/gram rhLF. Holo-rhLF is being marketed as Lacromin™ by InVitria.
Analysis of rhLF via gel electrophoresis, Western blot, enzyme-linked immunosorbent assay (ELISA), and total protein assay was carried out as previously described (Nandi et al. 2002; Nandi et al. 2005). Bound iron in LF was determined by A280/A465 ratio (Nandi et al. 2002). Total iron in LF samples was determined by the method of atomic absorption in National Food Lab (Dublin, CA).
Effects of rhLF on HT-29 cell growth. To examine the effect of rhLF on cultured cell proliferation, [3H]-thymidine incorporation was used as a marker of cell growth following the same procedure as in Playford et al. (2006). Briefly, cells that were actively dividing would increase their uptake of [3H]-thymidine for incorporation into DNA in the preparatory stage of cell division. The human colon cancer cell line, HT-29, was maintained in Modified Eagles’ Medium (MEM) containing 10% fetal calf serum (FCS). LF in all three forms (iron saturation levels) and at different concentrations was tested under serum-free conditions (MEM without FCS supplementation). To assess the rate of DNA synthesis, [3H]-thymidine (2 μCi/well) was added 24 h after the addition of rhLF and cells were maintained for an additional 24 h, then [3H]-thymidine incorporation was determined. Each test condition was performed in duplicate in six-well plates. Cell viability was greater than 90% as measured by the ability to exclude trypan blue (0.2%).
Cell number was also measured as a function of rhLF on the same HT-29 cell line. Similarly, HT-29 was maintained in MEM containing 10% fetal calf serum (FCS). To pre-condition the cell line, the cells were seeded at 5 × 105 cells per well, in MEM + 5% FCS in 24-well plates. This resulted in >95% attached cells per well 24 h later (as assessed by trypsinization and direct counting using an improved Neubauer hemocytometer). At that time, the medium was changed to one set containing MEM + 5% FCS and three sets containing MEM + 5% FCS plus the three forms of rhLF at a concentration of 1,000 mg/L. After 48 h, the cell numbers were assessed. To ensure that any cells present in the supernatant were included in the total cell count, supernatants were collected, centrifuged to collect cells, which were added to the wells following trypsinization.
Culture of hybridoma L243 cells. The hybridoma L243 cell line (ATCC HB55) was maintained in AFM6 media (KC Bio, Kansas City, MO), which was a serum-free media containing an iron compound as a replacement for transferrin. The special formulation (AFM6-Fe) was prepared without the iron compound as basal medium, which was also used to test the effect of various concentrations of holo-rhLF or human transferrin (hTF). The concentration of either protein ranged from 5 to 100 mg/L for these studies, and AFM6-Fe was also supplemented with 5% FCS as a positive control. Hybridoma L243 cells were seeded at 2 × 105 cells/mL and cultured as a stationary suspension. Each test point was performed in triplicate and at day 4, the cells were sub-cultured, and a total of four passages (sub-culturing) were performed. At each subsequent passage, the same seeding density and the same media was used as in the original seeding. At day 4 of each passage, samples were removed to determine cell viability and IgG production.
Osteoblast cell culture. Primary rat osteoblast cells were examined for stimulation by holo-rhLF measured by [3H]-thymidine incorporation. The same procedure as in Cornish et al. (Cornish et al. 2004) was followed. The cell culture was performed with supplementation of holo-rhLF at concentrations of 0.1, 1, 10, and 100 mg/L. The basal medium was serum-free supplemented with 1% BSA, and this medium, without holo-rhLF, was used as the negative control.
Culture of HEK cell. Two experiments were carried out with human embryonic kidney cells (HEK293) in serum-free medium. In the first experiment, HEK293 were cultured in serum-free media supplemented with either 250 mg/L hTF or 250 mg/L holo-rhLF. The selection of hTF concentration was based on a prior study (C. Card, unpublished data) that indicated 250 mg/L hTF was the optimal concentration for HEK293 growth in the specified serum-free medium. Six passages (three to 4 d in length) were conducted, using three different lots of holo-rhLF, in the same media with the same supplements. At the seventh passage, the cells were allowed to grow for 8 d with viable cells being counted at various time points. A second experiment was carried out to determine the optimal concentration of holo-rhLF to be used for HEK293 cells. Six concentrations of holo-rhLF were used ranging from 10 to 200 mg/L. Eight passages were performed with each passage lasting three to 4 d. Cells in the ninth passage were allowed to grow for 7 d and viable cells were counted at various time interval.
Measurement of antibody production. At each subculture of hybridoma cells, samples were removed from each of three flasks and pooled as an average for antibody production. The samples were centrifuged to remove cells and the supernatants were stored frozen for determination of IgG production.
Enzyme-linked immunosorbent assay (ELISA) kits were used to quantitatively measure murine IgG production by hybridoma L243 cells (cat no.E90–105; Bethyl Labs, Montgomery, TX). According to the manufacturer’s specification, each well of a 96-well plate (Nunc, Rochester, NY) was coated with 100 μL of a 1:100 dilution of 1 mg/mL goat anti-mouse IgG in coating buffer (0.05 M carbonate-bicarbonate, pH 9.6) for 60 min at room temperature (RT). The wells were then rinsed three times with a wash solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0). A blocking solution consisting of 50 mM Tris, 0.14 M NaCl, and 1% bovine serum albumin (BSA), pH 8.0, was applied to the wells and incubated for 30 min at RT, followed by the wash procedure stated above. One hundred microliters of the standards (0–1,000 ng/mL) and samples (the frozen supernatants from above, diluted 1:1000 in blocking solution containing 0.05% Tween 20) was applied to the wells and incubated for 60 min at RT. All standards and samples were analyzed in duplicate. After incubation, standards and samples were removed and the wells were washed and incubated with 100 μL of goat anti-mouse IgG-horseradish peroxidase conjugate (1 mg/mL) at 1:100,000 dilution in diluent (50 mM Tris, 0.14 M NaCl, 1% BSA, 0.05% Tween 20, pH 8.0) and incubated for 60 min at RT. Following the final wash, the HRP activity was detected by incubating with 100 μL of a hydrogen peroxide solution containing 3,3′,5,5′-tetramethylbenzidine substrate solution (TMB, Bethyl Labs, Inc.) until the color turned blue. To stop the TMB reaction, 100 μL of 2 M H2SO4 was applied to the wells. The absorbance at 450 nm was measured by an ELISA plate reader (BioRad Microplate Reader Model 3550, Hercules, CA) and the concentration of the samples was determined from the standard curve.
Result
Recombinant hLF for use in cell culture study. The rhLF purified from rice flour was partially iron-saturated and designated pis-rhLF. The iron saturation level of pis-rhLF was between 40% and 70%; the iron content ranged from 0.6 to 1.0 mg/g rhLF. Iron removal resulted in apo-rhLF with an iron level of less than 10% saturation or <0.14 mg/gram rhLF. Iron addition increased the iron saturation level to over 90%, or greater than 1.26 mg/gram rhLF which is called holo-rhLF (Table 1). Total saturation of lactoferrin with two molecules of iron for each molecule of LF results in 1.4 mg iron/gram of protein. Total iron was also measured to determine the level of unbound iron. As seen in Table 1, very little unbound iron was present in rhLF preparation. To examine the kinetics of iron uptake by pis-rhLF, a time course of iron saturation was studied (Fig. 1). After 120 min incubation time, iron saturation was reached. Breakpoint analysis indicates that rhLF reaches a full saturation level at 126 min under the conditions studied.
All three forms of rhLF were analyzed for total protein (Bradford), purity (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and LF content (ELISA). All of them have similar protein content and protein purity was greater than 90% (Table 1). For easy storage and shipping, rhLF was lyophilized after a 0.2-μm filtration. Apo-rhLF has an off-white color due to the decreased amount of iron, while both pis-rhLF and holo-rhLF powders are pinkish. The rhLF is now being used in cell culture industry as Lacromin™ and parameters in Table 1 are used as specifications of the products. Stability studies show that rhLF can be stored at 4°C for over 2 y without visible degradation measured by gel electrophoresis and ELISA (data not shown).
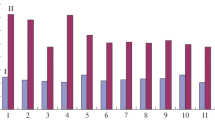
Effect of rhLF on HT-29 cell growth measured by thymidine incorporation and viable cell count. As is often seen of compounds with growth factor activity, proliferation assays of HT-29 cells with rhLF reveal a typical ‘bell shaped’ dose response curve. Figure 2A shows that the maximal stimulatory effect is at a concentration of 1,000 mg/L for all three forms of rhLF when cultured in serum-free media. Of the three rhLF forms, holo-rhLF showed the greatest proliferate activity with [3H]-thymidine incorporation, approximately 3.5 times greater than that of other forms.
Effect of rhLF on intestine (HT-29) cell growth. A. HT-29 cells were grown in MEM serum-free media with rhLF at concentration of 0 to 50,000 mg/L. After a 24-h incubation time with radioisotope, [3H]-thymidine incorporation (3H thymidine uptake) in media containing different levels of rhLF was determined as count per minute (cpm). B. HT-29 cells were grown in MEM + 5% (baseline) or plus supplementation of rhLF at concentration of 1000 mg/L. At the end of experiment (72 h), viable cell count (cell count) was determined with Neubauer hemocytometer.
To support the results and methodology validation, another study was carried out with rhLF at a concentration of 1,000 mg/L in MEM media containing 5% FCS (Fig. 2 B). FCS was needed to maintain basic cell viability for longer experimental time (72 h). Viable cell counts were used to determine the growth effect. These results confirmed those seen in Fig. 2 A and also showed that all three forms of rhLF promoted cell proliferation (Fig. 2 B), with the strongest proliferate activity using holo-rhLF. There are twice as many cells in the medium with holo-rhLF as in the baseline (MEM + 5% FCS).
Effect of rhLF on hybridoma cell growth and monoclonal antibody production. The studies using HT-29 cells with three forms of rhLF indicated that holo-rhLF was the most effective on cell growth. This was true with other cell lines, and thus only studies using holo-rhLF are reported. Holo-transferrin (holo-TF) is commonly used in cell culture media in cell culture industry, and since LF is similar in structure and iron-binding to TF, some studies included holo-TF for comparison.
Table 2 shows the effect of holo-rhLF and holo-hTF on hybridoma cell growth. Hybridoma cell line, L243, grew moderately in animal-free medium without iron supplement (control). The cell number reached 0.39 × 106 cells per milliliter after growth for 4 d (Table 2). With the addition of holo-rhLF or holo-hTF, the cell numbers were significantly increased to 0.7 or 0.8 × 106 cells per milliliter. The cell numbers were similar but slightly less than the same medium, but supplemented with 5% FCS. Three concentrations of holo-rhLF and holo-hTF (5, 25 and 100 mg/L) were used in this study. When comparing LF and TF under the same concentrations, the cell numbers were approximately the same. There was a slight reduction in cell number in 5 mg/L holo-rhLF but not statistically significant, indicating that 5 to 25 mg/L of holo-rhLF is sufficient to promote growth of L243 cells.
To examine the effect of holo-rhLF and holo-hTF on IgG production in L243 hybridoma cells, samples were removed and analyzed for IgG concentrations. The average concentration of IgG from four passages is shown in Table 2. Similar to cell growth data, there was a significant increase of IgG production in all treatments with holo-rhLF or holo-hTF compared to that in animal-free medium without iron supplement (control) (Table 2). The highest IgG level (43 μg/ml) was seen at 5 mg/L of holo-rhLF and was significantly higher than that of the 5 mg/L of holo-hTF (P < 0.05). For concentrations of 25 and 100 mg/L, the level of IgG was the similar between holo-rhLF and holo-hTF. Given variability in cell culture, further studies are needed to confirm the effect of 5 mg/L holo-rhLF on IgG production. The level of IgG in the positive control (with 5% FCS) was lower than all treatments though higher than that in animal-free medium without iron supplement (control), indicating LF promotes IgG production more than FCS (Table 2).
Effect of rhLF on osteoblast growth. Recently, LF was shown to promote osteoblast growth (Cornish et al. 2004). To examine if holo-rhLF from rice had cell growth promoting activity, primary osteoblast cultures from rats were examined for growth stimulation by holo-rhLF in dose response at 0.1, 1, 10 and 100 mg/L as measured by [3H]-thymidine incorporation (Fig. 3). At a concentration of 100 mg/L, [3H]-thymidine incorporation was approximately two times higher than in the same culture media without supplementation of holo-rhLF. A significant increase in cell growth, as measured by [3H]-thymidine incorporation, was also observed in 10, 1 and even 0.1 mg/L. This strong stimulatory effect is similar to that observed with other LF (Cornish et al. 2004).
Effect of holo-rhLF on rat osteoblast growth. [3H]-thymidine incorporation in basal medium containing no rhLF is set as 1. Media containing various concentration of holo-rhLF is presented as compared to basal medium control, no holo-rhLF control. The basal medium was serum-free media supplemented with 1% BSA.
Effect of holo-rhLF on HEK293 cell growth. At concentration of 250 mg/L, both hTF and holo-rhLF are able to support HEK293 cell growth over six passages. On the seventh passage, HEK293 cells reached maximal cell density of 7.0 to 9.3 × 106 cells per milliliter in media supplemented with holo-rhLF (Fig. 4A). Within the same study using the same cells and the same media but supplemented with holo-hTF, the maximal cell density is only 50% of that supplemented with holo-rhLF (Fig. 4A). The cell doubling time is 23 h for cells cultured with holo-rhLF, which is shorter than that with holo-hTF, indicating that holo-rhLF is capable of promoting HEK293 cell growth better than holo-hTF.
Effect of holo-rhLF on human embryonic kidney cell (HEK293) growth. A. HEK293 was cultured in serum-free media supplemented with three lots of holo-rhLF at 250 or 250 mg/L of holo-hTF. Viable cells were determined at time as indicated in the graph. B. HEK293 was cultured in serum-free media supplemented with various concentrations of holo-rhLF. Viable cells were determined at the time indicated in the graph.
To determine the optimal concentration of holo-rhLF, a dose response study was carried out using concentrations from 10 to 200 mg/L and HEK293 cells were cultured for eight passages. All concentrations were capable of supporting HEK293 cell growth except at 10 mg/L; the cells with 10 mg/L were collapsed at the fourth passage. In the final ninth passage, maximal cell densities were similar for concentrations ranging from 50 to 200 mg/L holo-rhLF (Fig. 4 B). Cell doubling time was also similar within these concentrations. On the other hand, at 25 mg/L, maximal cell density is much lower. This study indicated that the optimal concentration of holo-rhLF for use in HEK293 cell culture was between 50 and 100 mg/L.
Discussion
In the present paper, we describe the production of rhLF and show that rhLF has a strong stimulatory effect on various cell lines, including intestinal cells, hybridoma cells, osteoblast cells, and embryonic kidney cells. Since these cell lines represent very different cell types, the stimulatory effect by rhLF is not cell-specific. The growth-promoting effect of rhLF over the negative control was very significant when measured by [3H]-thymidine incorporation or cell proliferation, with holo-rhLF having the strongest stimulatory effect of all three forms of rhLF on cell growth. Table 3 provides a summary of holo-rhLF effects on cell growth. Overall, a 200% cell growth were seen for all four cell lines tested in medium supplemented with holo-rhLF compared to that in the same medium without holo-rhLF supplementation. Our observations of the effect of rhLF on cell growth agrees with some studies with nLF (Hashizume et al. 1983; Nichols et al. 1987; Azuma et al. 1989; Loo et al. 1989; Nichols et al. 1989; Hagiwara et al. 1995; Yanaihara et al. 2000; Cornish et al. 2004). However, not all studies in the literature support the concept that LF is a growth factor (Amouric et al. 1984; Hurley et al. 1994). Even within the same study, the results vary from cell line to cell line (Hashizume et al. 1983). The variations in these studies can be complicated; the source of lactoferrin might be a major factor, along with the method of preparation. Different preparations of LF have different qualities as they are prepared by various methods and from various sources. Some LF preparations are purified by the use of chromatography media while others are not. Some LF is dried by lyophilization while other preparations are spray-dried (see Wakabayashi et al. 2006 for a review of LF production). Different methods of LF preparation could impact the stimulatory effect on cells. Recombinant hLF provides a consistent preparation of lactoferrin and thus expect to generate a more consistent result.
We found that rhLF can function as cell growth factor for various cell lines, but the concentration of rhLF required can be cell-line dependent. We observe that the optimal concentration for intestinal cells is between100 and 1,000 mg/L (Fig. 2 A), while the optimal concentration for HEK293 is between 50 and 100 mg/L, with little variation between 100 and 200 mg/L (Fig. 4 B). In hybridoma cell culture, using L243, the difference between 5 and 100 mg/L is not obvious (Table 2). On the other hand, concentrations of 200 to 250 mg/L seem necessary for another hybridoma cell line, AE1, to achieve a proliferative effect (unpublished data). Thus, when a new cell line is tested, it is critical to test a wide range of rhLF concentrations (from 5 to 1,000 mg/L) for optimal cell growth and production of either antibody or protein.
Two hypotheses have been proposed to explain the cell growth stimulatory property of LF: the iron-nutrient hypothesis and the regulation of gene expression hypothesis. As we observed (Fig. 2), many studies mentioned above show that holo-LF has a stronger growth-promoting effect than apo-LF. Growth promoting activity of LF is possibly associated with its iron-binding and transport ability. Holo-rhLF had higher proliferate activity than pis-rhLF, which in turn had higher proliferative activity than apo-rhLF, indicating that iron content plays a critical role (Fig. 2 A). Typical mammalian cell culture media contains holo-TF as an iron source. LF is structurally similar to TF but has a stronger iron binding constant. In the human body, LF is responsible for iron uptake via intestinal cells and TF brings the iron into circulation. It is believed that through LF’s ability to bind and deliver iron, cells are stimulated to proliferate (Kohno et al. 1993).
The second hypothesis is based on the fact that apo-LF also promotes cell growth (Fig. 2) (Shinoda et al. 1994) and LF binds to receptors on the cell surface, enters the cell cytoplasm, and enters the nuclei to interact with DNA (Grey et al. 2004; Baumrucker et al. 2005; Naot et al. 2005). It is proposed that LF acts in a signal transduction pathway to regulate gene expression and cell development via LF receptors. It is important to note that the stimulatory effect of LF might not require the entry of LF into cells or nuclei (Baumrucker et al. 2005). The actual molecular signal transduction of this gene-expression regulation hypothesis remains to be studied. It is possible that both mechanisms, iron-nutrient hypothesis and gene-expression regulation hypothesis, act simultaneously to promote cell growth. The mechanism of LF stimulation of cell growth is different from that of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) because LF has a synergistic effect on cell growth with both EGF (Hagiwara et al. 1995) and bFGF (Shinoda et al. 1994). Finally, recent studies indicate that LF not only promotes cell growth, but also reduces apoptosis, resulting in a higher percentage of viable cells (Cornish et al. 2004; Baumrucker et al. 2005).
Recombinant human lactoferrin from rice provides a consistent, non-animal source of lactoferrin and is likely to find broad applications in cell culture to promote cell growth and production. Hybridoma cells have been used to produce monoclonal antibodies used in therapeutics, and rhLF can be used to obtain faster cell growth and increased antibody production as illustrated in Table 2. Human embryonic kidney cells are often used to produce vaccines; the use of rhLF in the media could enhance vaccine production. With the testing of other cells, tissues or organs, rhLF will find applications in stem cell culture, in vitro fertilization, skin substitute, tissue engineering, and autologous transplantation. With the increased concern of viral, prion, and other infectious agents in animal-derived products, our effort in generating potent and cost-effective rhLF (Lacromin™) as a non-animal growth factor will contribute to a safer environment and products for consumers.
Reference
Amouric M.; Marvaldi J.; Pichon J.; Bellot F.; Figarella C. Effect of lactoferrin on the growth of a human colon adenocarcinoma cell line—comparison with transferrin. In Vitro 20: 543–548; 1984. doi:10.1007/BF02639770.
Azuma N.; Mori H.; Kaminogaea S.; Yamauchi K. Stimulatory effect of human lactoferrin on DNA synthesis in BALB/c 3T3 cells. Agric Biol Chem 53: 31–35; 1989.
Baumrucker C. R.; Schanbacher F.; Shang Y.; Green M. H. Lactoferrin interaction with retinoid signaling: cell growth and apoptosis in mammary cells. Domest Anim Endocrinol 30: 289–303; 2005. doi:10.1016/j.domaniend.2005.07.009.
Cornish J.; Callon K. E.; Naot D.; Palmano K. P.; Banovic T.; Bava U.; Watson M.; Lin J. M.; Tong P. C.; Chen Q.; Chan V. A.; Reid H. E.; Fazzalari N.; Baker H. M.; Baker E. N.; Haggarty N. W.; Grey A. B.; Reid I. R. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 145: 4366–4374; 2004. doi:10.1210/en.2003-1307.
Grey A.; Banovic T.; Zhu Q.; Watson M.; Callon K.; Palmano K.; Ross J.; Naot D.; Reid I. R.; Cornish J. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol 18: 2268–2278; 2004. doi:10.1210/me.2003-0456.
Hagiwara T.; Shinoda I.; Fukuwatari Y.; Shimamura S. Effects of lactoferrin and its peptides on proliferation of rat intestinal epithelial cell line IEC-18, in the presence of epidermal growth factor. Biosci Biotechnol Biochem 59: 1875–1881; 1995.
Hashizume S.; Kuroda K.; Murakami H. Identification of lactoferrin as an essential growth factor for human lymphocytic cell lines in serum-free medium. Biochim Biophys Acta 763: 377–382; 1983. doi:10.1016/0167-4889(83)90099-X.
Hurley W. L.; Hegarty H. M.; Metzler J. T. In vitro inhibition of mammary cell growth by lactoferrin: a comparative study. Life Sci 55: 1955–1963; 1994. doi:10.1016/0024-3205(94)00528-1.
Kohno Y.; Shiraki K.; Mura T.; Ikawa S. Iron-saturated lactoferrin as a co-mitogenic substance for neonatal rat hepatocytes in primary culture. Acta Paediatr 82: 650–655; 1993.
Kovar J.; Franek F. Hybridoma cultivation in defined serum-free media: growth-supporting substances. I. Transferrin. Folia Biol (Praha) 31: 167–175; 1985.
Lonnerdal B.; Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr 15: 93–110; 1995. doi:10.1146/annurev.nu.15.070195.000521.
Loo D.; Rawson C.; Helmrich A.; Barnes D. Serum-free mouse embryo cells: growth responses in vitro. J Cel Physiol 139: 484–491; 1989. doi:10.1002/jcp.1041390306.
Nandi S.; Suzuki A.; Huang J.; Yalda D.; Pham P.; Wu L.; Bartley G.; Huang N.; Lonnerdal B. Expression of human lactoferrin in transgenic rice grains for the application in infant formula. Plant Sci 163: 713–722; 2002. doi:10.1016/S0168-9452(02)00165-6.
Nandi S.; Yalda D.; Lu S.; Nikolov Z.; Misaki R.; Fujiyama K.; Huang N. Process development and economic evaluation of recombinant human lactoferrin expressed in rice grain. Transgenic Res 14: 237–249; 2005. doi:10.1007/s11248-004-8120-6.
Naot D.; Grey A.; Reid I. R.; Cornish J. Lactoferrin—a novel bone growth factor. Clin Med Res 3: 93–101; 2005.
Nichols B. L.; McKee K. S.; Henry J. F.; Putman M. Human lactoferrin stimulates thymidine incorporation into DNA of rat crypt cells. Pediatr Res 21: 563–567; 1987. doi:10.1203/00006450-198706000-00011.
Nichols B. L.; McKee K.; Putman M.; Henry J. F.; Nichols V. N. Human lactoferrin supplementation of infant formulas increases thymidine incorporation into the DNA of rat crypt cells. J Pediatr Gastroenterol Nutr 8: 102–109; 1989.
Playford R. J.; Belo A.; Poulsom R.; Fitzgerald A. J.; Harris K.; Pawluczyk I.; Ryon J.; Darby T.; Nilsen-Hamilton M.; Ghosh S.; Marchbank T. Effects of mouse and human lipocalin homologues 24p3/lcn2 and neutrophil gelatinase-associated lipocalin on gastrointestinal mucosal integrity and repair. Gastroenterology 131: 809–817; 2006. doi:10.1053/j.gastro.2006.05.051.
Shinoda I.; Takase M.; Fukuwatari Y.; Shimamura S. Lactoferrin promotes nerve growth factor synthesis/secretion in mouse fibroblast L-M cells. Adv Exp Med Biol 357: 279–285; 1994.
Testa U. Proteins of iron metabolism. CRC, New York; 2002.
Wakabayashi H.; Yamauchi K.; Takase M. Lactoferrin research, technology and applications. Int Dairy J 16: 1241–1251; 2006. doi:10.1016/j.idairyj.2006.06.013.
Yamada K.; Ikeda I.; Sugahara T.; Hashizume S.; Shirahata S.; Murakami H. Stimulation of proliferation and immunoglobulin M production by lactoferrin in human–human and mouse–mouse hybridomas cultures in serum-free conditions. Cytotechnology 3: 123–131; 1990. doi:10.1007/BF00143674.
Yanaihara A.; Toma Y.; Saito H.; Yanaihara T. Cell proliferation effect of lactoferrin in human endometrial stroma cells. Mol Hum Reprod 6: 469–473; 2000. doi:10.1093/molehr/6.5.469.
Acknowledgment
This work was partially supported by a grant from NIH 1 R43 AG026206-01.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Huang, N., Bethell, D., Card, C. et al. Bioactive recombinant human lactoferrin, derived from rice, stimulates mammalian cell growth. In Vitro Cell.Dev.Biol.-Animal 44, 464–471 (2008). https://doi.org/10.1007/s11626-008-9136-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-008-9136-7