Abstract
Background
Herpes zoster vaccination rates remain low despite longstanding national recommendations to vaccinate immunocompetent adults aged ≥ 50 years. The Advisory Committee on Immunization Practice (ACIP) updated its recommendations for recombinant zoster vaccine (RZV) in October 2021 to include immunocompromised adults aged ≥19 years.
Objective
To assess practices, attitudes, and knowledge about RZV, barriers to recommending RZV, and likelihood of recommending RZV to patients with various immunocompromising conditions.
Design
Mail and internet-based survey conducted from May through July 2020.
Participants
General internists and family physicians throughout the USA.
Main Measures
Survey responses.
Key Results
The response rate was 66% (632/955). Many physicians were already recommending RZV to immunocompromised populations, including adults ≥50 years with HIV (67% of respondents) and on recombinant human immune modulator therapy (56%). Forty-seven percent of respondents both stocked/administered RZV and referred patients elsewhere, frequently a pharmacy, for vaccination; 42% did not stock RZV and only referred patients. The majority agreed pharmacies do not inform them when RZV has been given (64%). Physicians were generally knowledgeable about RZV; however, 25% incorrectly thought experiencing side effects from the first dose of RZV that interfere with normal activities was a reason to not receive the second dose. The top reported barrier to recommending RZV was experience with patients declining RZV due to cost concerns (67%). Most physicians reported they would be likely to recommend RZV to immunocompromised patients.
Conclusion
Most primary care physicians welcome updated ACIP RZV recommendations for immunocompromised adults. Knowledge gaps, communication issues, and financial barriers need to be addressed to optimize vaccination delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Nearly all middle aged and older adults in the USA had chickenpox in childhood, putting them at risk for herpes zoster (HZ) later in life.1 Immunocompromised people, regardless of age, are also at increased risk for HZ compared to immunocompetent people.2,3,4 Further contributing to the scope of the problem, HZ incidence in the USA appears to be increasing among middle-aged adults.5,6 HZ is a costly medical condition resulting in approximately $1.3 billion in medical costs and $1.7 billion in indirect costs in the USA annually.7
Pain is the cardinal symptom of both the dermatomal blistering rash of HZ and its most common complication, postherpetic neuralgia, defined as pain in the area of the rash persisting for 3 months or greater after the rash resolves. Effective management of these pain conditions is often elusive, making prevention even more compelling.8
Zoster vaccine live (ZVL) was the only vaccine available in the USA for the prevention of HZ from 2006 to 2017; however, ZVL was contraindicated in immunocompromised individuals, including those on moderate- to high-dose immunosuppressive medications, with HIV CD4 counts <200, or undergoing cancer treatment. Recombinant zoster vaccine (RZV), licensed in 2017, demonstrated greater (>90% vs. 53%) and longer-lasting efficacy than ZVL.9,10,11 Therefore, in 2017, the Advisory Committee on Immunization Practices (ACIP) recommended that 1) all immunocompetent adults aged ≥50 years receive the newer two-dose RZV, 2) all adults who had received ZVL receive RZV, and 3) RZV should be given preferentially over ZVL.12 An initially constrained supply of RZV limited implementation of the ACIP recommendation through 2019 and then the COVID-19 pandemic curtailed demand for RZV through 2020.13,14 Although RZV is a non-live recombinant vaccine and is therefore potentially safe to use in immunocompromised people, these individuals were excluded from pivotal efficacy studies9,11 and initial ACIP recommendations included only immunocompetent adults ≥50 years.
Since 2017, various studies have evaluated RZV use in immunocompromised populations. RZV was found to be immunogenic and safe in patients with HIV,15 with hematologic malignancies,16 with solid tumors,17 after renal transplant,18 and after autologous hematopoietic cell transplant.19 A single-institution chart review study among patients with rheumatoid arthritis or other systemic rheumatic disease, most of whom were on immunosuppressants (78%), did not find RZV to trigger rheumatic disease flares.20 To date, there are two published efficacy trials in the immunocompromised population: a trial of 1,846 autologous hematopoietic cell transplant patients ≥18 years who received the RZV vaccine series found an efficacy of 68%,21 and a post hoc analysis of 569 patients ≥18 years with hematologic malignancies found an efficacy of 87%.16 Based on data from these trials,16,21 FDA expanded the indication for RZV to immunocompromised adults aged ≥18 years in July 2021.22 In October 2021, ACIP recommended RZV use in immunocompromised adults aged ≥ 19 years.23
Given the established influence of provider recommendation on patient receipt of vaccination,24,25,26,27 in anticipation of ACIP guidance on RZV use in immunocompromised adults, and in the context of only 34.5% of adults aged ≥60 years having received a zoster vaccine as of 2018 despite longstanding national recommendations,28 we sought to assess among primary care physicians for adults (1) practices, attitudes, and knowledge about RZV, (2) barriers to recommending RZV, and (3) likelihood of recommending RZV to patients with various immunocompromising conditions when the new guidelines are released.
METHODS
Study Setting
From May through July 2020, a survey was administered to a national network of physicians who spent at least 50% of their time practicing primary care. The human subjects review board at the University of Colorado Anschutz Medical Campus approved this study as exempt research.
Study Population
The Vaccine Policy Collaborative Initiative,29,30 a survey mechanism to assess physician attitudes toward vaccine issues in collaboration with the Centers for Disease Control and Prevention (CDC), conducted the survey. We developed two sentinel networks of primary care physicians by recruiting family physicians (FP) in 2019 from the American Academy of Family Physicians (AAFP) and general internists (GIM) in 2018 from the American College of Physicians (ACP). We conducted quota sampling29 to ensure that sentinel networks of physicians were representative of ACP and AAFP memberships by region, by location (urban vs. rural), and by practice setting. Network physicians have been shown to be similar to physicians randomly sampled from the American Medical Association Masterfile with respect to demographics and attitudes regarding vaccines.29
Study Design
The survey is provided as an online appendix. It asked about recommending RZV to certain patient groups including various immunocompromised populations, and if so, whether certain patients were prioritized when they had a limited supply. Additional questions asked about use of reminder/recall, patient barriers, and their likelihood of recommending the vaccine for immunocompromised patients upon ACIP approval. The survey was pretested in 8 primary care physicians and pilot-tested with 63 prior to finalizations for national distribution.
Survey Administration
Per individual physician preference reported at network enrollment, the survey was sent by e-mail or US mail. The e-mail group was sent an initial e-mail and up to 8 e-mail reminders, and the US mail group was sent an initial mailing and up to 2 additional reminders. Non-respondents in the e-mail group were also sent up to 2 surveys by US mail. The mail protocol was patterned on Dillman’s Tailored Design Method.31
Statistical Analysis
Results were largely similar by specialty so are presented together, with any significant differences between specialties noted. Respondents and non-respondents were compared on available characteristics using t-tests, chi-squared tests, and Wilcoxon rank sum tests, as appropriate. FP and GIM respondents were compared using Fisher’s exact and chi-squared tests as appropriate. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
The overall response rate was 66% (632/955), 69% (327/477) for FP and 63% (305/484) for GIM. Respondents and non-respondents did not differ by location, region of the country, or whether decisions regarding purchasing and handling of vaccines by the practice were made independently or at a larger system level (Table 1). Male physicians, GIM physicians, and physicians from smaller practices were less likely to respond than their counterparts.
Physician Practices Regarding RZV
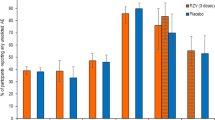
Physician recommendations for RZV in various patient groups are presented in Fig. 1. For patient groups covered by 2017 guidelines, 70% or more currently were recommending the vaccine. For patient groups not under the 2017 guidelines, depending on the group, 32–67% recommended the vaccine.
Physician strength of recommendation for RZV in different types of patients, USA, 2020 (n=632; some percentages do not add up to 100% because of rounding; removed respondents who reported not seeing a particular type of patient for this analysis). ‡, Advisory Committee on Immunization Practices RZV recommendation at the time of the survey. §, p<0.05 for differences between GIM and FP (Fisher's exact chi-squared test or chi-squared test, as appropriate) with GIM more likely to recommend to patients with HIV.\
Forty-seven percent of physicians currently both stocked/administered RZV and referred patients elsewhere for vaccination, 42% did not stock RZV and only referred patients elsewhere, 7% percent stocked and administered and did not refer patients elsewhere for vaccination, and 4% did not stock/administer or refer patients to receive RZV. Physicians from smaller practices (median: 5 providers), private practices, and practices in the South were less likely to stock RZV than larger practices, HMOs or hospital-based clinics, or practices in other regions of the country (p<0.001). Of the 89% who referred patients to receive RZV outside of the practice, the majority referred patients to a pharmacy (80% “often/always,” 19% “sometimes”) and a few reported referring to the public health department (6% “often/always,” 18% “sometimes”).
Among physicians who currently stocked RZV (n=350), the following methods were used to bring patients back for their second dose: giving patients an appointment for the second dose (76%), conducting a reminder/recall for patients to come back for the second dose (51%), relying on the patient to remember to come back to receive the second dose (41%), generating a list of patients who are due for the second dose and giving it to the provider (22%), and referring patients to the vaccine manufacturer for their reminder/recall system (1%). Thirty-three percent reported using one method, 46% percent reported two methods, 19% reported three methods, and 2% reported using four or more of these methods. Eleven percent exclusively relied on the patient to remember to come back to receive the second RZV dose.
Of 350 physicians who stocked RZV, 81% reported their practice had experienced a limited supply or run out of RZV in the 12 months prior to the survey; 48% estimated their practice had been out of RZV for greater than 3 months. Among those physicians who had experienced a shortage of RZV (n=283), the majority prioritized available vaccine for patients receiving their second dose (72%), for patients anticipating immunosuppression in the future (54%), and for patients ≥60 years (52%); 45% prioritized it for immunocompromised patients. GIM prioritized patients getting their second dose (80% GIM vs. 65% FP, p=0.005) and vaccinating immunocompromised patients (52% GIM vs. 38% FP, p= 0.02) more than FP.
Among physicians not stocking RZV (n=267), the most common reasons for not stocking included the practice deciding it was easier to refer patients to receive it (72%), the up-front costs to the practice to purchase the vaccine (70%), inadequate reimbursement to the practice for the vaccination (59%), and the practice not being set up to bill Medicare Part D (42%). Seventeen percent had not stocked RZV because their practice had been unable to secure a steady supply.
Physician Experience with Patients Declining RZV
Of physicians who recommended RZV (n=607), 28% reported patients decline it after it is recommended >25% of the time, 45% reported patients decline it 5-25% of the time after it is recommended, and 19% reported patients decline it less than 5% of the time after it is recommended. Physicians reported patients most frequently decline RZV due to insurance not covering the cost of the vaccine (38% “Often/always,” 42% “Sometimes”), being unable to afford the vaccine (29% “Often/always,” 44% “Sometimes”), fears of immediate side effects (15% “Often/always,” 54% “Sometimes”), and previous frustration with being unable receive RZV at a retail pharmacy (7% “Often/always,” 42% “Sometimes”).
Physician Attitudes Regarding RZV Delivery
Figure 2 shows physician attitudes regarding RZV delivery. Sixty-four percent agreed pharmacists do not inform them when patients get Shingrix at a pharmacy and 40% agreed pharmacists do not put Shingrix doses they administer in the state IIS.
Physician attitudes regarding the delivery of RZV to adult patients, USA, 2020 (n=632; some percentages do not add up to 100% because of rounding. †, p<0.05 for differences between GIM and FP (Fisher's exact chi-squared test or chi-squared test as appropriate) with more GIM agreeing that that patients report retail pharmacies have run out of their RZV supply and therefore they were unable to receive it, that they are comfortable deciding which immunocompromised patients should receive RZV, and that pharmacists do not have adequate vaccination history information to determine if an adult needs RZV.
Physician Knowledge of RZV
Physician responses to various knowledge questions related to RZV are presented in Table 2. Physicians were generally knowledgeable about RZV; however, 25% thought that experiencing side effects from the first dose of RZV that interfere with normal activities was a reason to not receive the second dose. Over 20% were unsure about giving RZV to patients on low-dose methotrexate or immune modulators.
Physician Reported Barriers to Recommending RZV
The most common reported barriers to recommending RZV included the limited supply of RZV (35% “Major,” 32% “Moderate” barrier), experience with patients declining RZV due to cost concerns (29% “Major,” 38% “Moderate” barrier), more pressing medical issues taking precedence (7% “Major,” 27% “Moderate” barrier), and patients having a severe reaction to the first dose (5% “Major,” 15% “Moderate” barrier).
Likelihood of Recommending RZV to Immunosuppressed Patients
As shown in Fig. 3, 12 to 24% reported they would be somewhat or very unlikely to recommend RZV to various types of immunocompromised patients even if it were licensed, recommended, and covered by insurance.
Likelihood of recommending RZV to different types of immunocompromised patients if licensed and approved for these populations. Survey was conducted prior to recent ACIP RZV recommendations in the immunocompromised. Some percentages do not add up to 100% because of rounding. Removed respondents who reported not seeing a particular type of patient or who responded they did not know what they would do for this analysis.
DISCUSSION
To our knowledge, this represents the first assessment of the physician perspective of RZV post-licensure and of initial ACIP recommendations for immunocompetent adults ≥50 years. Despite a lack of recommendation from ACIP at the time of the survey, many physicians were already recommending RZV to some patients with immunocompromising conditions, and most indicated needing more direction on which immunocompromised patients should optimally receive RZV. A sizeable minority were not recommending RZV to adults eligible for the vaccine according to initial recommendations, like adults aged ≥50 years anticipating a transplant or on low-dose methotrexate. Physicians were generally knowledgeable about RZV, although less so in relation to using it in immunocompromised patients. A substantial minority reported being unlikely to recommend it to various immunocompromised patients even if it were licensed, recommended, and covered by insurance. As observed previously,32 RZV vaccination continues to be complicated by delivery inside and outside the medical home. The limited supply of vaccine (now resolved) and cost concerns for patients were the major barriers to recommending RZV.
Like a prior study, which found physicians were recommending ZVL in the immunocompromised,32 we found physicians were very interested in using RZV in immunocompromised patients and, when faced with short supply, often prioritized second doses for immunocompromised patients. This is understandable as immunocompromised patients are at greater risk not only for HZ, but also for disseminated HZ, a life-threatening disease. At the time of the survey, “Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV” recommended RZV use in adults with HIV ≥50 years, regardless of CD4 count,33 possibly explaining the high proportion of physicians who reported already recommending RZV to patients with HIV.
Most physicians agreed they needed more direction about which immunocompromised patients are eligible for RZV and preferred for a subspecialist to decide if an immunocompromised patient should get RZV even though vaccinations are often under primary care’s purview. Admittedly, the population that comprises the immunocompromised is heterogenous. To date, not all categories of immunocompromised patients have been included in RZV clinical trials or effectiveness studies. In addition to clinical trials evaluating RZV efficacy in autologous transplant patients and patients with hematologic malignancies,16,21 a post hoc analysis of the pivotal clinical trials evaluated efficacy in participants with at least one potential immune-mediated disease but not taking immunosuppressive medication, and found an efficacy against HZ of 90.5% and no difference in serious adverse events between vaccinated and unvaccinated groups.34 A post-licensure cohort study35 among Medicare beneficiaries with a wide range of immunocompromising conditions found a vaccine efficacy of 64.1%. These latter studies provide aggregate data and lack specificity regarding RZV performance in patients with particular immunocompromising conditions; such research could inform future guidance for RZV use in these populations.
While it is reassuring that physicians were generally very knowledgeable about RZV, 25% incorrectly thought experiencing side effects from the first dose of RZV that interfere with normal activities was a reason to not receive the second dose. In pooled data from the pivotal clinical trials, 16.5% of RZV recipients experienced such reactions.36 In addition to an initial limited vaccine supply that has since recovered,37 this knowledge gap might contribute to the 26% and 22% incomplete vaccine series within 6 months observed by the Kaiser Family Foundation38 and in a Medicare beneficiary cohort study, respectively.35 Forty percent of physicians expressed difficulty convincing patients to get the second dose after experiencing side effects from the first dose. Relying on patients to remember to come back for the second dose of RZV, as a substantial minority of physicians reported, might also factor into the notable proportion of RZV recipients who have an incomplete vaccination series within 6 months of the first dose. Broader use of evidence-based approaches like reminder/recall, which half of respondents reported using, would likely help. The rates of incomplete vaccination series are concerning because the efficacy of one dose of RZV was found to be considerably less than two doses in an observational study (56.9% for 1 dose vs. 70.1% for 2 doses).35
Administration of RZV continues to be complicated by frequent delivery outside the medical home. More physicians reported not stocking HZ vaccine (46%) than on a comparable previous survey (37%)32 in 2016, thereby relying on outside sources, most often a pharmacy, to provide HZ vaccination. A majority of physicians report that pharmacists do not inform them when they give RZV to one of their patients. Immunization information systems (IISs) are confidential, population-based, computerized systems that collect and consolidate vaccination data for people living in a given geopolitical area and have the potential to facilitate communication about vaccination delivery.39 During this survey in the summer of 2020, several physicians reported that pharmacists do not upload vaccination information to IISs; however, pharmacies have been integral to the US COVID-19 vaccination program which requires providers to upload vaccination information to an IIS. Therefore, pharmacies may be uploading more vaccination information to IISs now compared to prior to the COVID-19 vaccination program.40 A previous study found significant knowledge gaps regarding IISs among primary care physicians, particularly among general internists.41
With supply issues resolved, the most important barriers to overcome for physicians to recommend RZV are financial. RZV costs $162 per dose in the private sector.42 Physicians reported up-front vaccine purchase costs impacted their willingness to stock and recommend RZV and that out-of-pocket costs were a primary factor in patients declining the vaccine. Under the Affordable Care Act, all non-grandfathered private insurance plans are required to cover the vaccine with no copay or coinsurance when administered by an in-network provider; for patients enrolled in grandfathered private insurance plans, costs may be incurred with vaccination. For the 23% of Medicare beneficiaries with Medicare Part B alone,43 RZV vaccination is not covered. For the 77% who are enrolled in a Medicare Part D plan through a prescription drug plan or a Medicare Advantage plan,43 benefits coverage can be variable. Previous studies demonstrate cost-sharing requirements for zoster vaccination are high for Medicare Part D beneficiaries44 and higher than for younger, non-Medicare patients.45 Medicaid coverage is also variable.46,47 Our study suggests addressing these voids in coverage will be important to improving national RZV vaccination rates.
Although this survey includes nationally representative samples of primary care physicians for adults in the USA and the response rate was high, the findings are subject to some limitations. Results are self-reported; actual practice was not observed. Males, general internists, and physicians from smaller practices are slightly underrepresented in these findings. From our results, we cannot distinguish whether some physicians truly do not support using the vaccine in the immunocompromised or whether they would prefer to defer the decision to a subspecialist.
This study indicates the updated ACIP recommendations for RZV use in immunocompromised adults aged ≥ 19 years23 will be welcomed by many primary care physicians and are congruent with what many are already doing. However, knowledge gaps, communication issues, and financial barriers need to be addressed to optimize vaccination delivery and improve national RZV vaccination rates.
References
Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70 Suppl 1:S111-8.
Pergam SA, Forsberg CW, Boeckh MJ, et al. Herpes zoster incidence in a multicenter cohort of solid organ transplant recipients. Transpl Infect Dis. 2011;13(1):15-23.
McKay SL, Guo A, Pergam SA, et al. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125-e134.
Yun H, Yang S, Chen L, et al. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68(9):2328-37.
Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52(3):332-40.
Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis. 2019;69(2):341-344.
McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36(4):259-73.
Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44 Suppl 1:S1-26.
Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019-32.
Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-84.
Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087-96.
Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103-108.
Milly Dawson. Shingrix shortage: Advice for physicians. Available from: https://www.medicaleconomics.com/view/shingrix-shortage-advice-physicians. Published 7-11-2019. Accessed 12-4-21.
Hong K, Zhou F, Tsai Y, et al. Decline in Receipt of Vaccines by Medicare Beneficiaries During the COVID-19 Pandemic — United States, 2020. MMWR Morb Mortal Wkly Rep 2021;70:245–249.
Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279-87.
Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988-1000.
Vink P, Delgado Mingorance I, Maximiano Alonso C, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer 2019;125(8):1301-1312.
Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase 3, randomized clinical trial. Clin Infect Dis. 2020;70(2):181-190.
Stadtmauer EA, Sullivan KM, El Idrissi M, et al. Adjuvanted recombinant zoster vaccine in adult autologous stem cell transplant recipients: polyfunctional immune responses and lessons for clinical practice. Hum Vaccin Immunother. 2021;17(11):4144-4154.
Stevens E, Weinblatt ME, Massarotti E, et al. Safety of the zoster vaccine recombinant adjuvanted in rheumatoid arthritis and other systemic rheumatic disease patients: a single center's experience with 400 patients. ACR Open Rheumatol. 2020;2(6):357-361.
Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA 2019;322(2):123-133.
Jennifer Barrett. FDA OKs Shingrix for Immunocompromised Adults 18 Years and Older. Available from: https://www.drugtopics.com/view/fda-oks-shingrix-for-immunocompromised-adults-18-years-and-older. Published 2021. Accessed 9-23-2021.
Anderson TC, Masters NB, Guo A, et al. Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80-84.
Nichol KL, Mac Donald R, Hauge M. Factors associated with influenza and pneumococcal vaccination behavior among high-risk adults. J Gen Intern Med. 1996;11(11):673-7.
Nichol KL, Lofgren RP, Gapinski J. Influenza vaccination. Knowledge, attitudes, and behavior among high-risk outpatients. Arch Intern Med. 1992;152(1):106-10.
Armstrong K, Berlin M, Schwartz JS, et al. Barriers to influenza immunization in a low-income urban population. Am J Prev Med. 2001;20(1):21-5.
Winston CA, Wortley PM, Lees KA. Factors associated with vaccination of medicare beneficiaries in five U.S. communities: results from the racial and ethnic adult disparities in immunization initiative survey, 2003. J Am Geriatr Soc. 2006;54(2):303-10.
Lu PJ, Hung MC, Srivastav A, et al. Surveillance of Vaccination Coverage Among Adult Populations -United States, 2018. MMWR Surveill Summ. 2021;70(3):1-26.
Crane LA, Daley MF, Barrow J, et al. Sentinel physician networks as a technique for rapid immunization policy surveys. Eval Health Prof. 2008;31(1):43-64.
Brtnikova M, Crane LA, Allison MA, et al. A method for achieving high response rates in national surveys of U.S. primary care physicians. PLoS One. 2018;13(8):e0202755.
Don A. Dillman JDS, Christian LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method, 4th Edition. 2014.
Hurley LP, Allison MA, Dooling KL, et al. Primary care physicians' experience with zoster vaccine live (ZVL) and awareness and attitudes regarding the new recombinant zoster vaccine (RZV). Vaccine. 2018;36(48):7408-7414.
HIV.gov CI. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/varicella-zoster-virus-disease. Accessed 9-23-21.
Dagnew AF, Rausch D, Hervé C, et al. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: a pooled post hoc analysis on two parallel randomized trials. Rheumatology (Oxford). 2021;60(3):1226-1233.
Izurieta HS, Wu X, Forshee R, et al. Recombinant zoster vaccine (Shingrix): real-world effectiveness in the first 2 years post-licensure. Clin Infect Dis. 2021;73(6):941-948.
Centers for Disease Control and Prevention. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE): Recombinant Zoster Vaccine (RZV) and Herpes Zoster Live-Attenuated Vaccine (ZVL). https://www.cdc.gov/vaccines/acip/recs/grade/herpes-zoster.html. Published 2018. Accessed 9-29-2021.
Sagonowsky E. GSK's Shingrix supply recovers thanks to drop in vaccinations, uninterrupted production. Available from: https://www.fiercepharma.com/pharma/gsk-s-shingrix-supply-recovers-due-to-drop-vaccinations-uninterrupted-production. Published 2020. Accessed 9-29-2021.
Cubanski J. Who Didn’t Get a Second Shingrix Shot? Implications for Multidose COVID-19 Vaccines. Available from: https://www.kff.org/medicare/issue-brief/who-didnt-get-a-second-shingrix-shot-implications-for-multidose-covid-19-vaccines/. Published 2020. Accessed 9-22-2021.
Centers for Disease Control and Prevention. Immunization Information Systems (IIS). Available from: https://www.cdc.gov/vaccines/programs/iis/. Published 2019. Accessed 9-29-2021.
Centers for Disease Control and Prevention. CDC COVID-19 Vaccination Program Provider Requirements and Support. Available from: https://www.cdc.gov/vaccines/covid-19/vaccination-provider-support.html Published 2021. Accessed 9-29-2021.
Kempe A, Hurley LP, Cardemil CV, et al. Use of immunization information systems in primary care. Am J Prev Med. 2017;52(2):173-182.
Centers for Disease Control and Prevention. CDC Vaccine Price List. Available from: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html Published 2021. Accessed 10-03-2021.
Cubanski J. and Damico A. Medicare Part D: A First Look at Medicare Prescription Drug Plans in 2022. Available from: https://www.kff.org/medicare/issue-brief/medicare-part-d-a-first-look-at-medicare-prescription-drug-plans-in-2022/#:~:text=In%202021%2C%2048%20million%20Medicare,in%20Medicare%20Advantage%20drug%20plans. Published 2021. Access 2-25-22.
Carr T. Why Does My Shingles Vaccine Cost So Much? Available from: https://www.consumerreports.org/health/why-the-shingles-vaccine-cost-so-much. Published 2017. Accessed 10-6-21.
Tak CR, Kim J, Gunning K, et al. Cost-sharing requirements for the herpes zoster vaccine in adults aged 60+. J Pharm Technol. 2019;35(6):258-269.
Centers for Disease Control and Prevention. Shingrix Vaccination: What Everyone Should Know. Available at: https://www.cdc.gov/vaccines/vpd/shingles/public/shingrix/index.html.Published January 24, 2022. Accessed 2-25-22.
Granade CJ, McCord RF, Bhatti AA, et al. State policies on access to vaccination services for low-income adults. JAMA Netw Open. 2020;3(4):e203316.
Acknowledgements
The authors would like to thank Selam Wubu, MPH, and Darilyn Moyer, MD, from the ACP and Amy Mullins, MD, and Bellinda Schoof, MHA, from the AAFP for collaborating in the establishment of the sentinel networks in general internal medicine and family medicine. We would also like to thank all general internists and family physicians in the networks for participating and responding to this survey.
Funding
This publication was supported by Cooperative Agreement Number 1 U01 IP000849-03, funded by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 99 kb)
Rights and permissions
About this article
Cite this article
Hurley, L.P., O’Leary, S.T., Dooling, K. et al. Survey of Physician Practices, Attitudes, and Knowledge Regarding Recombinant Zoster Vaccine. J GEN INTERN MED 38, 986–993 (2023). https://doi.org/10.1007/s11606-022-07721-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-022-07721-z