Abstract
Insulin autoimmune syndrome (IAS) is an uncommon cause of hyperinsulinemic hypoglycemia characterized by autoantibodies to endogenous insulin in individuals without previous exposure to exogenous insulin. IAS is the third leading cause of spontaneous hypoglycemia in Japan, and is increasingly being recognized worldwide in non-Asian populations. We report a case of IAS in a Caucasian woman with recurrent complaints of hypoglycemia, with laboratory findings of serum glucose 2.5 mmol/L (45 mg/dL), insulin 54,930 pmol/L (7,909 μIU/mL), connecting peptide (C-peptide) 4,104 pmol/L (12.4 ng/mL), and a corresponding insulin to C-peptide molar ratio of 13.4 during a spontaneous hypoglycemic event. Autoantibodies to insulin were markedly elevated at > 50 kU/L (> 50 U/mL). IAS should be considered in the differential diagnosis of hypoglycemia in non-diabetic individuals. Distinction from insulinoma is especially crucial to prevent unwarranted invasive procedures and surgical interventions in hypoglycemic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
CASE REPORT
A 45-year-old Caucasian woman presented to clinic with a 6-week history of recurrent hypoglycemic symptoms consisting of fatigue, lightheadedness, blurry vision, and diaphoresis. The episodes were triggered by fasting and exercise, and alleviated with food intake. She also reported a 10-pound weight gain during this period. There was no history of diabetes mellitus in the patient or her family, and she had no access to insulin and/or insulin secretagogues. Past medical history was significant for endometriosis requiring multiple pelvic surgeries, von Willebrand disease, hepatic steatosis, gastroesophageal reflux disease, and patellofemoral syndrome. The patient took esomeprazole occasionally but no other prescription or over-the-counter medications. She did not smoke, drink alcohol, or use recreational drugs. Family history was negative for any endocrine tumors or autoimmune diseases. Vital signs were normal and her physical examination was non-contributory.
Laboratory investigations revealed normal renal and liver function. Hemoglobin A1c was 5.4 %. Adrenocorticotropic hormone (ACTH) stimulation test indicated an adequate cortisol response. Blood work was collected during a spontaneous symptomatic hypoglycemic event (Table 1). The markedly increased insulin level and the non-suppressed connecting peptide (C-peptide) result, along with the corresponding insulin to C-peptide molar ratio of 13.4, were incompatible with exogenous insulin administration as the cause of hypoglycemia.
Computed tomography (CT) of the abdomen did not identify any masses in the pancreas or in the retroperitoneum. Magnetic resonance imaging (MRI) of the abdomen was aborted due to feelings of claustrophobia in the patient. Selective arterial calcium stimulation was performed to differentiate between focal (e.g. insulinoma) and diffuse (e.g. islet cell hypertrophy) pancreatic pathologies, and to localize the source of hyperinsulinism. Regardless of the sampling site, all specimens had similarly raised insulin and C-peptide levels (Table 1).
Due to the magnitude of insulin elevation and the insulin to C-peptide molar ratio of > 1—which was physiologically impossible—an interference with the laboratory assay was suspected. However, pre-incubation of the patient’s serum samples with heterophilic blocking tubes1 did not alter the results. An autoimmune form of hypoglycemia was thereby considered, and autoantibodies to insulin were found to be markedly increased at > 50 kU/L (> 50 U/mL) (reference range: < 0.4 kU/L).
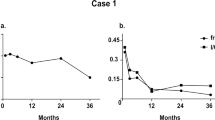
Workup for autoimmune diseases (including anti-nuclear antibodies and rheumatoid factor) and monoclonal gammopathy did not reveal any significant findings. The patient was advised to follow a low glycemic index diet with frequent small meals. The occurrence of hypoglycemic episodes decreased, but continued to manifest during exertion. Unfortunately, the patient did not tolerate acarbose and refused a trial of corticosteroids. Her most recent laboratory investigations demonstrated improved, but persistently raised, insulin and C-peptide levels of 778 pmol/L (112 μIU/mL) and 1,167 pmol/L (3.5 ng/mL) respectively.
DISCUSSION
Insulin autoimmune syndrome (IAS), or Hirata disease, is a rare cause of hyperinsulinemic hypoglycemia characterized by autoantibodies to endogenous insulin in individuals without previous exposure to exogenous insulin.2 First described by Hirata et al. in 1970,3 it is the third leading cause of spontaneous hypoglycemia in Japan following insulinoma and extrapancreatic neoplasms.4 Over 380 cases have been reported in the medical literature since,5 with the majority (90 %) depicted in the Japanese population. Regardless, IAS is also recognized in other countries, including Europe and the United States (US).6 A compilation of all IAS cases described in the US is shown in Table 2. IAS should be considered in any hypoglycemic patient, especially in those with a suspected insulinoma, so as to avoid any unnecessary, invasive and costly procedures and surgical interventions.
Patients with IAS usually present in adulthood (typically after age 40) with postprandial hypoglycemia, although fasting and exercise-induced hypoglycemia have also been described.6 The disease shows no overall predilection to gender. Affected individuals may complain of marked neuroglycopenic symptoms of confusion and an altered level of consciousness, and may even be in a comatose state on initial presentation.7 Autoimmune comorbidities—such as Graves’ disease, systemic lupus erythematosus, and rheumatoid arthritis—and/or monoclonal gammopathy may be elicited on history.6 About half of IAS patients report recent exposure to medications, with over 90 % of offending agents containing a sulfhydryl group.8 Methimazole is the most commonly implicated drug; others include carbimazole, glutathione, tiopronin, tolbutamide, gold thioglucose, interferon-α, captopril, diltiazem, hydralazine, procainamide, isoniazid, D-penicillamine, imipenem and penicillin G.6,7,9 Alpha-lipoic acid, a popular health supplement for the treatment of diabetic neuropathy and obesity, has been linked to IAS in recent years.5
Although the precise mechanism for hypoglycemia in IAS is unknown, the most widely accepted hypothesis is a mismatch between blood glucose and free insulin concentration, secondary to the binding and release of secreted insulin by autoantibodies.7 Following a meal or oral glucose load, glucose concentration in the bloodstream rises, providing a stimulus for insulin secretion. Autoantibodies bind to these insulin molecules, rendering them unavailable to exert their effects. The resultant hyperglycemia not only promotes further insulin release, but may also explain the increased hemoglobin A1c often seen in IAS patients.6 As glucose concentration eventually falls, insulin secretion also subsides, and the total insulin level decreases. Insulin molecules spontaneously dissociate from the autoantibodies at this time, giving rise to a raised free insulin level inappropriate for the glucose concentration, evoking hypoglycemia.7 Insulin autoantibodies with a high binding capacity and a low affinity are more likely to bring about hypoglycemic symptoms.10 Medications containing a sulfhydryl group have been proposed to induce autoantibody formation by interacting with the disulfide bonds of the insulin molecule and augmenting its immunogenicity;11 however, the true underlying pathophysiology remains unclear at this time. Rarely, the co-existence of both insulin autoantibodies and insulin receptor autoantibodies within the same patient has been described.12
A striking feature of IAS is the magnitude of insulin elevation—with results generally above 1,000 pmol/L13—secondary to the insulin assay reacting with the autoantibody-bound insulin molecules. Increased insulin level to this extent is rarely seen in insulinomas.7 If free insulin levels are determined, they may be normal or raised. C-peptide and proinsulin concentrations are increased, and autoantibody titers indicate a high percentage of binding to insulin.6 Hyperplasia of islet β-cells is observed on histological studies.11
Immunoassays are commonly used for the analysis of various hormones, including insulin. Although robust, immunoassays are subjected to interference from endogenous anti-reagent antibodies found in the serum of certain individuals. These antibodies, known as human anti-animal antibodies and heterophile antibodies, may bind to animal immunoglobulins in the assay and produce falsely high or low results.1,14 A method to confirm the presence of anti-reagent antibodies is to treat the patient sample in question with heterophilic antibody blocking tubes; a significant difference in hormone level pre-incubation and post-incubation supports the presence of anti-reagent antibodies, and helps differentiate such antibodies from anti-analyte antibodies (e.g. insulin autoantibodies).1,14
C-peptide and insulin are co-secreted from pancreatic β-cells into the portal circulation in equimolar proportions. Whereas insulin is principally cleared by the liver, C-peptide is mainly metabolized by the kidneys at a substantially slower rate, resulting in a difference in circulating half-life of 5–10 minutes versus 30–35 minutes, respectively.15 Thus, despite equimolar secretion, the insulin to C-peptide molar ratio is normally < 1. The ratio may be reversed to > 1 in two settings: the first is IAS, as demonstrated by our patient; the second is factitious hypoglycemia from exogenous insulin administration, in which insulin concentration is raised with a suppressed C-peptide level.15 It is important to note that different commercial insulin assays exhibit variable cross-reactivities with synthetic insulin analogues.16 Any dialogue regarding the cross-reactivity of the laboratory’s insulin method is best initiated with the local clinical laboratory staff. Expected patterns of insulin, C-peptide, and insulin to C-peptide molar ratio for different causes of hypoglycemia are summarized in Table 3.
In 80 % of patients, IAS is a transient condition with spontaneous resolution within 3–6 months of diagnosis.17 For those with intractable hypoglycemia, small frequent meals low in carbohydrates remain the first line of treatment; the rationale for this is to avoid postprandial hyperglycemia, and thereby the stimulus for insulin secretion.7 Glucocorticoid therapy (e.g. oral prednisone 30–60 mg/day) may be useful as an adjunct therapy.7 Any potentially incriminating medication should be discontinued. Other therapeutic options, such as acarbose (to decrease carbohydrate digestion and absorption), diazoxide, octreotide and partial pancreatectomy (to restrict insulin release), and plasmapheresis (to reduce insulin autoantibody titers) have demonstrated varying success in the management of IAS.6
CONCLUSION
IAS should be considered in any patient undergoing evaluation for hypoglycemia. Discrepancies between an unusually high insulin concentration and only moderately raised proinsulin and C-peptide levels, with a concurrent insulin to C-peptide molar ratio of > 1, are suggestive of IAS. Involving local laboratory physicians in the workup for IAS, especially in the exclusion of spurious insulin results from heterophilic interference, is strongly encouraged. Misdiagnosis may lead to unwarranted and inappropriate patient therapy, with considerable implications for clinical care.
Key Points
-
1.
Insulin autoimmune syndrome (IAS) is an uncommon and mostly transient condition that should be considered in the differential diagnosis of hypoglycemia in non-diabetic patients.
-
2.
Laboratory investigations suggestive of IAS include a significantly elevated insulin level, an increased C-peptide concentration, and an insulin to C-peptide molar ratio of > 1.
-
3.
A detailed drug history should be taken, and co-existing autoimmune disorders and/or monoclonal gammopathy sought, although these are not required for the diagnosis of IAS.
REFERENCES
Sturgeon CM, Viljoen A. Analytical error and interference in immunoassay: minimizing risk. Ann Clin Biochem. 2011;48:418–32.
Uchigata Y, Hirata Y. Insulin autoimmune syndrome (IAS, Hirata disease). Ann Med Interne (Paris). 1999;150:245–53.
Hirata Y, Ishizu H, Ouchi N, et al. Insulin autoimmunity in a case of spontaneous hypoglycemia. J Jpn Diab Soc. 1970;13:312–20.
Takayama-Hasumi S, Eguchi Y, Sato A, Morita C, Hirata Y. Insulin autoimmune syndrome is the third leading cause of spontaneous hypoglycemic attacks in Japan. Diabetes Res Clin Pract. 1990;10:211–4.
Uchigata Y, Hirata Y, Iwamoto Y. Drug-induced insulin autoimmune syndrome. Diabetes Res Clin Pract. 2009;83:e19–20.
Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore). 2009;88:141–53.
Redmon JB, Nuttall FQ. Autoimmune hypoglycemia. Endocrinol Metab Clin North Am. 1999;28:603–18.
Yamada T, Imai J, Ishigaki Y, Hinokio Y, Oka Y, Katagiri H. Possible relevance of HLA-DRB1*0403 haplotype in insulin autoimmune syndrome induced by alpha-lipoic acid, used as a dietary supplement. Diabetes Care. 2007;30:e131.
Lidar M, Rachmani R, Half E, Ravid M. Insulin autoimmune syndrome after therapy with imipenem. Diabetes Care. 1999;22:524–5.
Eguchi Y. Scatchard analysis of insulin autoantibodies in the insulin autoimmune syndrome. J Tokyo Wom Med Univ. 1989;59:1286–305.
Benson EA, Ho P, Wang C, Wu PC, Fredlund PN, Yueng RT. Insulin autoimmunity as a cause of hypoglycemia. Arch Intern Med. 1984;144:2351–4.
Bortolotti D, Mothe-Satney I, Ferrari P, et al. Spontaneous hypoglycaemia in the presence of both anti-insulin antibody and anti-insulin receptor antibody. Diabetes Metab. 2006;32:598–603.
Virally ML, Guillausseau PJ. Hypoglycemia in adults. Diabetes Metab. 1999;25:477–90.
Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004;25:105–20.
Lebowitz MR, Blumenthal SA. The molar ratio of insulin to C-peptide. An aid to the diagnosis of hypoglycemia due to surreptitious (or inadvertent) insulin administration. Arch Intern Med. 1993;153:650–5.
Owen WE, Roberts WL. Cross-reactivity of three recombinant insulin analogs with five commercial insulin immunoassays. Clin Chem. 2004;50:257–9.
Paiva ES, Pereira AE, Lombardi MT, et al. Insulin autoimmune syndrome (Hirata disease) as differential diagnosis in patients with hyperinsulinemic hypoglycemia. Pancreas. 2006;32:431–2.
Waldron-Lynch F, Inzucchi SE, Menard L, et al. Relapsing and remitting severe hypoglycemia due to a monoclonal anti-insulin antibody heralding a case of multiple myeloma. J Clin Endocrinol Metab. 2012;97:4317–23.
Gomez Cruz MJ, Jabbar M, Saini N, et al. Severe hypoglycemia secondary to methimazole-induced insulin autoimmune syndrome in a 16 year old African-American male. Pediatr Diabetes. 2012;13:652–5.
Khoo TK, Service FJ. Hyperinsulinemic hypoglycemia. Endocr Pract. 2007;13:424–6.
Basu A, Service FJ, Yu L, Heser D, Ferries LM, Eisenbarth G. Insulin autoimmunity and hypoglycemia in seven white patients. Endocr Pract. 2005;11:97–103.
Yaturu S, DePrisco C, Lurie A. Severe autoimmune hypoglycemia with insulin antibodies necessitating plasmapheresis. Endocr Pract. 2004;10:49–54.
Shah P, Mares D, Fineberg E, et al. Insulin autoimmune syndrome as a cause of spontaneous hypoglycemia in alcoholic cirrhosis. Gastroenterology. 1995;109:1673–6.
Burch HB, Clement S, Sokol MS, Landry F. Reactive hypoglycemic coma due to insulin autoimmune syndrome: case report and literature review. Am J Med. 1992;92:681–5.
Redmon B, Pyzdrowski KL, Elson MK, Kay NE, Dalmasso AP, Nuttall FQ. Hypoglycemia due to an insulin-binding monoclonal antibody in multiple myeloma. N Engl J Med. 1992;326:994–8.
Benson EA, Healey LA, Barron EJ. Insulin antibodies in patients receiving penicillamine. Am J Med. 1985;78:857–60.
Blackshear PJ, Rotner HE, Kriauciunas KA, Kahn CR. Reactive hypoglycemia and insulin autoantibodies in drug-induced lupus erythematosus. Ann Intern Med. 1983;99:182–4.
Goldman J, Baldwin D, Rubenstein AH, et al. Characterization of circulating insulin and proinsulin-binding antibodies in autoimmune hypoglycemia. J Clin Invest. 1979;63:1050–9.
Acknowledgments
Contributors
None.
Funding
None.
Prior Presentations
None.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, S.L., Priestman, A. & Holmes, D.T. Recurrent Hypoglycemia from Insulin Autoimmune Syndrome. J GEN INTERN MED 29, 250–254 (2014). https://doi.org/10.1007/s11606-013-2588-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-013-2588-9