Abstract
Background
Comorbidity—a condition that co-exists with a primary illness—is common among older persons with heart failure and can complicate the overall management of this population.
Objectives
To determine the relationship between advancing age and the prevalence and patterns of comorbidity among older persons with heart failure.
Design
Retrospective longitudinal cohort study
Participants
A total of 201,130 Medicare beneficiaries with heart failure stratified into three age strata in 2001: 66–75, 76–85, and 86+ years, and followed over 5 years.
Measurements
(1) Prevalence of 19 conditions as identified by the Chronic Conditions Warehouse from Medicare claims data, characterized as concordant (related to heart failure) or discordant (unrelated to heart failure), and (2) overall comorbidity burden, defined as count of conditions.
Results
The median number of comorbidities rose from four (IQR: 2–5) to five (IQR: 4–7) among the young-old, and from 4 (IQR: 3–6) to 6 (IQR: 5–8) among the middle-old and oldest-old between 2001 and 2006. In 2001, the majority of concordant conditions were more prevalent among the youngest than oldest beneficiaries (e.g., diabetes 46.2% vs 26.9%; kidney disease 21.8% vs 18.4%), while the majority of discordant conditions were more prevalent among the oldest-old than youngest-old beneficiaries (e.g., dementia 39.6% vs 9.9%; hip fracture 9.5% vs 1.9%). Discordant conditions increased in prevalence faster among the oldest than youngest beneficiaries (e.g., dementia 13% points versus 9% points).
Conclusion
Among older Medicare beneficiaries with heart failure, there is a higher overall burden of comorbidity and greater prevalence of discordant comorbidity among the oldest old. Comorbidity prevalence increases over time, with discordant comorbidity increasing at the fastest rate among the oldest old. This comorbidity burden highlights the challenge of effectively treating heart failure while simultaneously managing co-existing and unrelated conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The increase in the prevalence of chronic conditions with advancing age, coupled with a rapidly aging US population, makes comorbidity—a chronic condition that co-exists with a primary illness1—a critical public health issue for older persons. This is of particular relevance to older adults with heart failure, as comorbidity in this population increases symptomatology2, polypharmacy3, risk of hospital readmissions4,5, and mortality6–10. Moreover, the management of comorbidity, particularly those conditions that are not directly related to heart failure in either pathogenesis or management, can significantly complicate the overall care needed by these patients3. A thorough understanding of the prevalence and patterns of comorbidity among the elderly is critical to health care planning and quality improvement efforts.
Population-based data on the prevalence of comorbid conditions among older adults with heart failure have indicated a large burden of comorbidity; for example, Braustein et al. (2003) reported that approximately 72% of Medicare beneficiaries with heart failure have at least five comorbid conditions9. These studies have examined older persons as a single cohort9,11,12, which may obscure important age-dependent differences in disease prevalence. There has been little empiric investigation of changes in comorbidity prevalence as older adults with heart failure survive into very old age. Existing data support two potentially opposing hypotheses regarding the relationship between comorbidity and advancing age. On the one hand, evidence linking certain comorbidities in heart failure to increased mortality6–10 suggests that persons who survive to very old age with heart failure are less likely to have those comorbidities. On the other hand, the increase in prevalence with advancing age of several chronic diseases unrelated to heart failure, such as dementia13–16, suggests that older adults with heart failure may accumulate comorbidity as they survive into old age. An existing framework developed by Piette and Kerr (2006) characterizes comorbid conditions as either concordant with the primary disease—related in pathophysiology or management and on the same treatment pathway—or discordant with the primary disease—unrelated in pathophysiology or management and not likely to be part of the same treatment plan17,18. This framework helps to unify the two hypotheses by suggesting that the comorbidity profiles of older adults with heart failure may change with increasing age such that they have a lower prevalence of concordant conditions but a higher prevalence of discordant conditions. In contrast to prior work that characterized comorbidity as cardiac or non-cardiac9, the use of this framework may provide additional insight into the care of older persons with multiple conditions, because comorbidites are characterized in terms of whether they are likely to be the focus of the same treatment plan.
The purpose of this study was to determine the relationship between aging and comorbidity among a population-based sample of older adults (>65 years) with heart failure by (1) examining the cross-sectional prevalence of a broad range of both concordant and discordant comorbid conditions across age groups, and (2) determining changes in the prevalence of comorbid conditions over time.
METHODS
We conducted a retrospective longitudinal cohort study of Medicare beneficiaries with congestive heart failure. Participants were selected from the Center for Medicare and Medicaid Services (CMS) Chronic Conditions Warehouse (CCW), a 5% random sample of Medicare beneficiaries providing beneficiary, assessment, and claims data across the continuum of care. The CCW includes 21 chronic condition variables indicating the presence of a condition as defined by evidence-based algorithms that specify (1) a minimum number of diagnoses/procedure codes, (2) occurring within a specific time frame (either 1, 2, or 3 years), and (3) within certain care settings. For example, the variable indicating the presence of congestive heart failure is derived from the occurrence of at least one of the following International Classification of Diseases, Ninth revision (ICD-9) codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.11, 404.91, 404.03, 404.13, 404.9, or 428.xx, occurring within a 2-year period in either the inpatient or outpatient setting.
Study Population
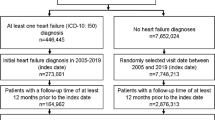
The initial study cohort included all living CCW beneficiaries who were identified as having heart failure as of January 1, 2001 (n = 241, 254). From this cohort, we excluded (1) all beneficiaries age ≤65 years (n = 20,938) to ensure that all included beneficiaries were at least 65 years of age during the lookback period; (2) all beneficiaries who received care outside traditional fee-for-service (FFS) Medicare at any point between January 1, 2000, and December 31, 2006 (n = 18,852), and (3) any beneficiaries with missing data during the study period (n = 334). The final study sample included 201,130 Medicare beneficiaries with heart failure.
Chronic Condition Measures
We examined 19 additional chronic condition variables, which we characterized as either concordant or discordant comorbidities. Concordant comorbidities included acute myocardial infarction (AMI), atrial fibrillation (AFIB), chronic kidney disease (CKD), diabetes, ischemic heart disease (IHD), and stroke/TIA. Discordant comorbidities included Alzheimer’s disease and related disorders or senile dementia (dementia), cataracts, depression, glaucoma, hip/pelvic fracture, osteoporosis, rheumatoid arthritis/osteoarthritis (arthritis), and cancer (female breast, colorectal, prostate, lung, and endometrial). The characterization of chronic obstructive pulmonary disease was problematic. Although COPD is not pathophysiologically related to heart failure, the two conditions have highly overlapping signs and symptoms, and COPD has been shown to increase mortality risk in heart failure7. We therefore characterized COPD as concordant. We considered these conditions to be present from the time they were identified through the data set timeframe. We examined comorbidity both in terms of individual conditions as well as comorbidity burden, defined as a count of conditions.
Analysis
We stratified our study cohort into three subcohorts according to beneficiaries’ ages as of January 1, 2001 (young-old: 66–75 years, middle-old: 76–85 years, and oldest-old: 86+ years). We followed these participants through 2006 without replacement for deaths. We used univariate statistics to describe the demographics of the study cohort at the beginning of 2001. We defined comorbidity burden as the total number of comorbid conditions. Because burden was not normally distributed, we used two non-parametric approaches to examine this variable. First, we characterized burden according to age strata in 2001 and 2006 using the median and interquartile range (IQR). Second, we used the Kruskal-Wallis test to determine the significance of the association between comorbidity burden and age strata. We determined the prevalence of each condition at the end of 2001 and 2006 within the three age strata. Prevalence was calculated as the number of beneficiaries who had the specified comorbidity at the end of a year, over the total number of beneficiaries alive at the beginning of that year. We calculated the change in prevalence over time as the difference between the prevalence of a given condition in 2001 and its prevalence in 2006.
RESULTS
Of the 201,130 Medicare beneficiaries in the CCW identified as having heart failure in 2000, the majority were women (60.6%) and white (86.5%),with a median age of 80 years (Table 1). By the end of the study period in 2006, 62.7% of the initial sample had died. Beneficiaries in our study had a high burden of comorbidity, with 40.6% of the sample having five or more chronic conditions in addition to heart failure as of 2001. In the overall cohort, IHD (74.9%) and cataracts (50.9%) were the two most prevalent conditions. Diabetes, COPD, AFIB, and arthritis had a prevalence of 30–40%, while stroke, CKD, dementia, depression, and osteoporosis had a prevalence of 20–25%. Glaucoma was prevalent among 15.1% of the sample, AMI among 8.4%, and hip fracture among 5.1%, while the prevalence of all cancers was less than 5%.
For a majority of the concordant comorbidities, prevalence decreased across all three age strata, from the youngest to oldest beneficiaries, with no overlap in the corresponding 95% confidence intervals (Fig. 1 and Table 2). For example, prevalence of CKD was 21.8% among the young-old, 21.1% among the middle-old, and 18.4% among the oldest-old. The prevalence of diabetes was 46.2% among the young-old, 38.1% among the middle-old, and 26.9% among the oldest-old. Stroke and AFIB were two concordant conditions that increased rather than decreased in prevalence across all three age strata, from the young-old to the oldest-old.
Prevalence of comorbidity by age group, 2001: ■ 66–75 years, □ 76–85 years  , 86+ years. *No overlap in 95% confidence intervals (CI) across all three age groups. †No overlap in 95% CI between the youngest (66–75 years) and oldest (86+ years) age group. ‡No overlap in 95% CI between the youngest (66–75 years) and middle (76–85 years) age group.
, 86+ years. *No overlap in 95% confidence intervals (CI) across all three age groups. †No overlap in 95% CI between the youngest (66–75 years) and oldest (86+ years) age group. ‡No overlap in 95% CI between the youngest (66–75 years) and middle (76–85 years) age group.
With the exception of cataracts and cancer, the discordant comorbidities increased in prevalence across all three age strata, from the youngest to oldest beneficiaries, with no overlap in the corresponding confidence intervals. For example, the prevalence of dementia was 9.9% among the young-old, 21.3% among the middle-old, and 39.6% among the oldest-old. The prevalence of hip fracture, while low overall, increased from 1.9%) among the young-old, to 4.6% among the middle-old, and to 9.5% among the oldest-old.
The burden of comorbidity assessed as number of comorbid conditions was higher among those who survived through 2006 than among each of the original three age-stratified subcohorts in 2001 (Fig. 2). In addition, the comorbidity burden significantly increased across the three age strata in both 2001 and 2006. In 2001, the median number of comorbidities was four among each age strata (IQR 2–5 among the young-old; IQR 3–6 among the middle- and oldest-old) (Kruskal-Wallis chi-square = 2,345.34, 2 df, p < 0.0001). In 2006, the median number of comorbidities rose to five (IQR 4–7) among the young-old and six (IQR 5–8) among the middle- and oldest-old (Kruskal-Wallis chi-square = 1,336.21, 2 df, p < 0.0001).
By the end of the study period in 2006, the prevalence of all conditions had increased among those who survived as compared to the prevalence in the original three age-stratified subcohorts (Fig. 3). The pattern of comorbidity prevalence for discordant conditions remained similar in 2006 as compared to 2001 (Fig. 4 and Table 2). For example, while discordant conditions increased the most in prevalence over the study period among the oldest-old compared to the young-old, many of these conditions were already significantly more prevalent among the oldest-old at the start of the study period in 2001. Hip fracture increased by 7.7 percentage points among the oldest-old compared to only 2.2 percentage points among the young-old. Dementia increased in prevalence by 12.8 percentage points among the oldest beneficiaries between 2001 and 2006, compared to only 8.9 percentage points over the same period among the youngest beneficiaries. Because many of the concordant conditions also had the greatest increase in prevalence among the oldest-old, the differences in prevalence across the age strata were narrower in 2006 as compared to 2001. For example, COPD, which was more prevalent among the young-old compared to the oldest-old in 2001, increased in prevalence by 10.5 percentage points among the oldest-old and by 8.5 percentage among the young-old between 2001 and 2006.
Prevalence of Comorbidity by Age Group, 2006: ■ 66–75 years, □ 76–85 years,  86+ years. *No overlap in 95% confidence intervals (CI) across all three age groups. †No overlap in 95% CI between the youngest (66–75 years) and oldest (86+ years) age group. ‡No overlap in 95% CI between the youngest (66–75 years) and middle (76–85 years) age group.
86+ years. *No overlap in 95% confidence intervals (CI) across all three age groups. †No overlap in 95% CI between the youngest (66–75 years) and oldest (86+ years) age group. ‡No overlap in 95% CI between the youngest (66–75 years) and middle (76–85 years) age group.
DISCUSSION
This study of Medicare beneficiaries age 66 years and older confirms the high overall burden of comorbid disease among older persons with heart failure, which becomes even higher among those who survive with the disease. By examining comorbidity according to different age groups (66–75, 76–85, and 86+), the results demonstrate significant differences in comorbidity prevalence across these age strata. Several concordant comorbidities were more prevalent than discordant comorbidities among younger beneficiaries, while the majority of discordant conditions were more prevalent among the oldest beneficiaries. Over time, comorbidity increased the fastest among the oldest beneficiaries compared to the youngest beneficiaries, with the greatest prevalence increases in those discordant conditions already more common among the oldest beneficiaries at the start of the study period. These findings support dual hypotheses regarding the relationship between comorbidity and advancing age among older adults with heart failure, namely, that even as the prevalence of comorbidity overall increases over time, the oldest-old continue to be more likely than the young-old to have more discordant conditions and less likely to have concordant comorbidities, particularly those shown to be associated with mortality6–8,10. Overall, the rate of accumulation of most discordant conditions (with the exception of cancer) exceeded the rate of accumulation of concordant comorbidities.
Our findings regarding the overall prevalence of comorbidity among older persons with heart failure are consistent with previous studies. The almost 41% of beneficiaries in the current study who had ≥5 comorbid conditions is similar to the 37% in an earlier study of 122,630 Medicare beneficiaries with heart failure with ≥5 comorbid conditions9. In contrast, among the general Medicare population, it has been estimated that only 7.6% have ≥3 conditions overall19. Our estimates of comorbidity prevalence across age strata are also consistent with existing data; for example, findings from the National Heart Failure Project (NHFP) indicated that diabetes and COPD were more prevalent among adults aged 65–74, while stroke and dementia were more prevalent among adults aged 86 years and over20. While the NHFP examined the cross-sectional prevalence of a small set of comorbidities, the current study provides a more comprehensive evaluation of comorbidity by examining the change in prevalence of a broad range of comorbidities over time. The 5-year mortality rate within our study cohort is similar to a previous report examining mortality after initial diagnosis of heart failure as ascertained by chart review21 and is lower than mortality rates reported among patients recently hospitalized with heart failure22,23. However, our findings suggest that heart failure remains a high-risk diagnosis despite the many therapeutic advances aimed at improving survival24–29.
Our findings highlight some of the challenges to providing optimal care for patients with heart failure. First, the high burden of comorbidity within our study population suggests that the prevailing disease-oriented model of care may not adequately address the health care needs of older adults with heart failure. Clinical guidelines for heart failure focus primarily on interventions directed at the heart failure itself30,31. However, the associations between concordant conditions and quality of life outcomes among persons with heart failure2,3,32–34 suggest that optimizing the health of these patients requires explicit assessment and treatment directed at the comorbid conditions while simultaneously addressing the heart failure.
Second, the finding that the comorbidity profiles of older adults with heart failure shift towards a greater burden of discordant conditions with advancing age suggests that providers will be increasingly challenged to manage a heavy burden of conditions unrelated to heart failure. Successful treatment of co-existing yet unrelated conditions requires considerable time and expertise on the part of the provider and increases the overall complexity of care17. The presence of discordant conditions may increase the risk that the patient may receive lower quality care18,35. A prior study found that patients with a recent AMI who had discordant comorbid conditions were less likely to receive guideline-recommended post-AMI care than patients with concordant comorbidities18. In addition, the application of multiple disease-based guidelines to the individual patient results in complicated and burdensome treatment regimens and is associated with risks of drug-drug and drug-disease interactions36. The complexity of managing heart failure in the face of several co-existing conditions calls for a coordinated, comprehensive system of care that can successfully address the multiple and potentially competing needs of patients with heart failure, yet our existing infrastructure remains fragmented and disorganized with poorly defined roles for non-heart failure specialists37. A team-based approach to heart failure care led by family physicians and supported by heart failure specialists has been suggested as a possible model for heart failure care37; however, to date, the issue of how best to deliver comprehensive care for complex heart failure patients remains unresolved. As the elderly population with heart failure continues to age, it will become of critical importance to determine how best to deliver high quality care by adequately managing both discordant and concordant conditions in a single patient.
Our study has some limitations. We are limited by the use of population-based data to providing associations rather than describing causality between comorbidity and age. The use of administrative data sources for estimating the prevalence of conditions is at risk for underestimating the true burden of disease38; however, we believe that our use of the CCW, designed specifically to mitigate the risk of underestimation through the use of evidence-based algorithms to indicate the presence of a disease, facilitated the accurate reporting of disease prevalence. Moreover, a prior study that utilized chart review methods to identify comorbid conditions among older adults with heart failure resulted in similar prevalence estimates20, providing support for the CCW algorithms as an alternative to more costly and time-consuming methods for identifying comorbidity. Future and ongoing evaluation of the accuracy of CCW-defined conditions will be important in determining the full utility of this data source. We defined conditions as present once the criteria for the algorithm had been met and for all subsequent years that the beneficiary was alive. This raises the possibility that we may have over-reported the prevalence of cataracts within our study sample, since cataracts can be removed and thereby cured. Because we compared the prevalence of 19 different comorbid conditions, it is possible that the significance of our results might be due to chance variation arising from making multiple comparisons. We only have descriptive statistics for characterizing the changes in prevalence over time. We were unable to distinguish between systolic and diastolic heart failure using the CCW data set. As the proportion of diastolic versus systolic heart failure increases with increasing age39–41, it is possible that comorbidity profiles might also differ according to the type of heart failure. Finally, because we focused on Medicare beneficiaries >65 years of age and excluded beneficiaries enrolled for any period of time in managed care, our findings may not be generalizable to the larger Medicare population.
In conclusion, among older Medicare beneficiaries with heart failure examined according to three age strata, the prevalence of comorbidity differed by age, with a higher overall burden of comorbidity and greater prevalence of discordant comorbid conditions among the oldest-old. The prevalence of comorbidity increased over time across all strata, with discordant comorbidities increasing at the fastest rate among the oldest-old. A model of multimorbidity may be the most appropriate approach to care for the elderly population with heart failure. The increasing prevalence of discordant conditions over time, particularly among those who survive to very old age, highlights the challenge facing providers of effectively treating heart failure while simultaneously managing several co-existing and unrelated conditions.
References
Lang CC, Mancini DM. Non-cardiac comorbidities in chronic heart failure. Heart. 2007;93:665–71.
Walke LM, Byers AL, Tinetti ME, Dubin JA, McCorkle R, Fried TR. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch Intern Med. 2007;167:2503–8.
Masoudi FA, Krumholz HM. Polypharmacy and comorbidity in heart failure. BMJ. 2003;327:513–4.
Tsuchihashi M, Tsutsui H, Kodama K, et al. Medical and socioenvironmental predictors of hospital readmission in patients with congestive heart failure. Am Heart J. 2001;142:E7.
Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139:72–7.
Bruch C, Rothenburger M, Gotzmann M, et al. Chronic kidney disease in patients with chronic heart failure–impact on intracardiac conduction, diastolic function and prognosis. Int J Cardiol. 2007;118:375–80.
Rusinaru D, Saaidi I, Godard S, Mahjoub H, Battle C, Tribouilloy C. Impact of chronic obstructive pulmonary disease on long-term outcome of patients hospitalized for heart failure. Am J Cardiol. 2008;101:353–8.
From AM, Leibson CL, Bursi F, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–9.
Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–33.
Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–23.
Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225–30.
Senni M, Santilli G, Parrella P, et al. A novel prognostic index to determine the impact of cardiac conditions and co-morbidities on one-year outcome in patients with heart failure. Am J Cardiol. 2006;98:1076–82.
Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–76.
Marengoni A, Winblad B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008;98:1198–200.
Lee SJ, Go A, Lindquist K, Bertenthal D, Covinsky KE. Chronic conditions and mortality among the oldest old. AJPH. 2008;98:1209–1214.
Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67:114–21.
Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–31.
Sales AE, Tipton EF, Levine DA, et al. Are co-morbidities associated with guideline adherence? The MI-Plus study of Medicare patients. J Gen Intern Med. 2009;24:1205–10.
Schneider KM, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7:82.
Havranek EP, Masoudi FA, Westfall KA, Wolfe P, Ordin DL, Krumholz HM. Spectrum of heart failure in older patients: results from the National Heart Failure Project. Am Heart J. 2001;143:412–7.
Senni M, Tribouilloy C, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–9.
Goldberg RJ, Ciampa J, Lessard D, Meyer TE, Spencer FA. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med. 2007;167:490–6.
Curtis LH, Greiner MA, Hammill BG, et al. Early and long-term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168:2481–8.
The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302.
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II). Lancet. 1999;353:9–13.
Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol. 1988;62:60A–66A.
Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–10.
Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55.
Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–7
Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235.
Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016.
Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34.
Suwanno J, Petpichetchian W, Riegel B, Issaramalai SA. A model predicting health status of patients with heart failure. J Cardiovasc Nurs. 2009;24:118–26.
Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35:594–603.
Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–20.
Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–24.
Cleland JG, Clark AL. Delivering the cumulative benefits of triple therapy to improve outcomes in heart failure: too many cooks will spoil the broth. J Am Coll Cardiol. 2003;42:1234–7.
Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(IV):26–35.
Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. Jama. 2006;296:2209–16.
Ilksoy N, Hoffman M, Moore RH, Easley K, Jacobson TA. Comparison of African-American patients with systolic heart failure versus preserved ejection fraction. Am J Cardiol. 2006;98:806–8.
Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–93.
Acknowledgements
Dr. Ahluwalia was supported by a training grant from the National Institute on Aging (T32AG1934) and is currently supported by an Office of Academic Affiliation’s VA Associated Health Postdoctoral Fellowship Program at the VA Greater Los Angeles HSR&D Center of Excellence. Dr. Fried is a recipient of a Midcareer Investigator Award in Patient-Oriented Research from the National Institute on Aging (K24 AG028443). Dr. Chaudhry is the recipient of a Paul Beeson/K23 Career Development Award (K23AG030986) from the National Institute on Aging. Supported by the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (#2P30AG021342-06 NIH/NIA).
Presented as a poster at the 2010 Society of General Internal Medicine Annual Meeting, April 28–May 1, 2010, Minneapolis, MN.
Conflict of Interest
None disclosed
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahluwalia, S.C., Gross, C.P., Chaudhry, S.I. et al. Change in Comorbidity Prevalence with Advancing Age Among Persons with Heart Failure. J GEN INTERN MED 26, 1145–1151 (2011). https://doi.org/10.1007/s11606-011-1725-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-011-1725-6






 2001, ■ 2006.
2001, ■ 2006.
 86+ years. *Change in prevalence reported in absolute numbers.
86+ years. *Change in prevalence reported in absolute numbers.