Abstract
Background
Clinical staging guides decisions about optimal treatment sequence in patients with gastric cancer, although the preoperative accuracy is not strongly established. This study investigates concordance of clinical and pathologic stage as well as its impact on the survival of patients with gastric adenocarcinoma.
Methods
Patients with clinical stage T2-4, N0, M0 gastric adenocarcinoma who underwent surgery without neoadjuvant therapy were identified from the National Cancer Database (2010–2015). The primary outcome was up-staging, defined as cT < pT, pN1-3, and/or pM1 (AJCC 7th edition). Multivariable logistic regression analysis was performed to predict up-staging. Survival analysis was performed using the Kaplan-Meier method.
Results
In total, 2254 patients were identified. cTNM staging was discordant with pTNM staging in 65.6% of cases, with 50.4% up-staged and 15.2% down-staged. On multivariable logistic regression, younger age (OR 0.991, 95% CI 0.984–0.999, p=0.0188), male sex (versus female; OR 1.392, 95% CI 1.158–1.673, p=0.0004), poor or undifferentiated tumor grade (versus well differentiated or moderately differentiated; OR 2.399, 95% CI 1.987–2.896; p<0.0001), positive margin status (versus negative; OR 4.575, 95% CI 3.360–6.230; p<0.0001), and days from diagnosis to surgery (15–32 days versus ≤ 14 days; OR 1.411, 95% CI 1.098–1.814, p=0.0072) were predictive of up-staging. Patients who were up-staged had a decreased survival compared to patients who were accurately staged (median survival 27.9 months versus 67.6 months; log-rank p<0.0001).
Conclusion
This study found a substantial discordance between clinical and pathologic staging of resectable locally advanced gastric adenocarcinoma. These data support that patients may have more advanced disease at presentation than reflected in clinical staging and may benefit from improved diagnostic modalities and neoadjuvant chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gastric cancer is the fifth most common cancer worldwide, representing approximately 28,000 new cases and 11,000 deaths per year in the USA.1, 2 Although its incidence is declining, largely due to Helicobacter pylori screening and treatment, gastric cancer maintains a poor prognosis with a 5-year survival of 32%.2, 3 Approximately 30% of patients present with surgically resectable disease, and as such, the majority of patients are diagnosed at later stages, especially in underserved populations, thus leading to worse outcomes.2, 4, 5
The treatment of gastric cancer is dependent on staging guidelines. The American Joint Committee on Cancer (AJCC) staging of gastric cancer is the accepted international standard for classification of this malignancy.6, 7 This system includes both clinical and pathologic stages and undergoes periodic evidence-based revisions.8, 9 It is generally agreed upon that accurate preoperative staging is critical in guiding the treatment of these patients. However, the evidence from other solid tumors suggests that clinical staging may have a significant discrepancy from pathological staging, and this staging discordance is not well studied in gastric cancer.10, 11
Curative treatment for gastric cancer requires complete oncologic resection.12 Working in conjunction with surgery, chemotherapy and radiation therapy are also utilized in the treatment of gastric cancer. More recently, multiple studies have demonstrated that the use of neoadjuvant therapy results in a survival benefit in patients with resectable gastric cancer.13,14,15,16 In addition, neoadjuvant therapy has a potential advantage for patients who are initially non-resectable but may achieve “down-staging” of their tumor that allows for surgical intervention.17 Despite these findings, neoadjuvant chemotherapy is not universally utilized in gastric cancer in the USA.
Given the important role that accurate staging plays in the optimization and sequence of multimodality treatment, this study aims to investigate the concordance of clinical and pathologic stage as well as its impact on overall survival in patients with locally advanced gastric adenocarcinoma.
Materials and Methods
Data Source
Patient data were obtained from the National Cancer Database (NCDB). This database is a program of the American College of Surgeons and the American Cancer Society, representing more than 70% of newly diagnosed cancer cases nationwide.18, 19 All NCDB data are de-identified, and thus, this study was deemed exempt from review by the Boston University Institutional Review Board.
Patient Selection
Patients with gastric adenocarcinoma who underwent gastrectomy were identified from the NCDB (2010–2015). These patients were identified based on International Classification of Disease for Oncology, 3rdedition (ICD-O-3) using topography codes C160–C166 and C168–169 and histology codes 8000, 8010, 8012, 8020, 8021, 8050, 8051, 8140–8145, 8210, 8211, 8230, 8243–8245, 8255, 8260–8263, 8310, 8323, 8480, 8481, 8490, 8560, 8570, 8572, and 8574–8576.20 Patients were also selected based on Facility Oncology Registry Data Standard (FORDS) procedure codes 30–33, 40–42, 50–52, and 60–63. Patients with clinical stage T2 and greater, N0 and M0 disease were included. All pathological stages, including missing pathologic M stage, were included. Both clinical and pathologic staging were based upon AJCC 7th Edition staging criteria. Patients with multiple malignancies were excluded (n=6586), as were patients who underwent neoadjuvant therapy so as not to impact pathologic staging (n=2640). Patients 90 years or older were excluded (n=43) along with patients not treated at the reporting facility (n=53). Missing or unknown data for any of the following variables was excluded: sex, race, Charlson-Deyo comorbidity score, grade, facility type, insurance status, vital status, days from diagnosis to definitive surgery, examination of regional lymph nodes, margin status, TNM edition, pathologic T stage, and pathologic N stage (n=397). All exclusions were done so sequentially.
Data Elements
Independent variables in this analysis included patient, tumor, and treatment characteristics. Patient characteristics included age, sex, race, insurance status, and Charlson-Deyo comorbidity score. Tumor characteristics included grade (well and moderately differentiated versus poorly differentiated or undifferentiated) and location (proximal, body, distal, overlapping, and other). Treatment characteristics included facility variables: type (academic versus non-academic center), volume (defined as the number of gastrectomy operations per year divided at the 50th percentile: low [0–11] and high [>11]), and therapy: receipt of adjuvant chemotherapy (defined as any systemic therapy), radiation, lymph node examination (for which the number of nodes greater or equal to 90 is categorized as “90 nodes”), time to surgery (defined in days and divided into quartiles [0–14, 15–32, 33–52.5, >52.5), and margin status (defined as negative or positive).21 The primary outcome was up-staging as compared to concordant staging or down-staging, as defined by differences in clinical T, N, and M stage as compared to pathologic T, N, and M stage following surgery. The secondary outcome was overall survival (OS), defined from the date of diagnosis to death date or last contact.18
Statistical Analysis
Summary statistics were reported as percentages for categorical variables and as medians with interquartile ranges (IQR) for continuous variables. Multivariable logistic regression analysis was performed, based on univariate logistic regression analysis (significance set at ≤ 0.05). The following variables were included in the final multivariable logistic regression model: age, sex, grade, margin status, and days to surgery, with tumor location forced into the model. Survival curves and median survival estimates were obtained using the Kaplan-Meier method and compared using the log-rank test. Statistical significance was set at ≤ 0.05. All data analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
In total, 2254 patients with gastric adenocarcinoma were identified who met inclusion criteria. Patient, hospital, tumor, and treatment information are displayed in Table 1. Most patients were male (60.4%), white (71.8%), and insured by Medicare (56.5%). The median number of gastrectomies performed each year was 11 [IQR, 6–22], the majority of which occurred at non-academic centers (53.4%). In addition, most tumors were poorly differentiated or undifferentiated (64.8%) with a relatively even distribution between tumor location. The median time to surgery was 32.5 days [IQR, 14–53], and the median number of lymph nodes examined was 17 [IQR, 10–24]. The majority of tumor resections had negative margins (86.4%).
Staging
The breakdown of clinical and pathologic staging by AJCC 7th edition TNM staging is presented in Table 2. The breakdown by cT stage was as follows: cT2 49.8%, cT3 37.6%, and cT4 12.6%. cT stage was discordant with pT stage in 44.5% of cases, with 24.7% clinically under-staged and 19.8% clinically over-staged (Fig. 1). Of cN0 patients, 46.9% demonstrated positive nodes on pathological examination (Fig. 2). Distant metastases were discovered on pathology evaluation in 3.0% of cases (Fig. 3).
Overall, cTNM staging was discordant with pTNM staging in 65.6% of cases, with 50.4% up-staged and 15.2% down-staged (Fig. 4). Of early-stage disease, defined as clinical stage IB, IIA, or IIB (n=2186), 50.6% were up-staged. Of late-stage disease, defined as clinical stage IIIB (n=68), 45.6% were up-staged.
When comparing high and low volume centers, there was a significant difference in staging (p<0.0001). In high volume centers, staging was discordant in 68.8% of cases, with 48.7% up-staged and 20.1% down-staged. In low volume centers, staging was discordant in 62.6% of cases, with 52.1% up-staged and 10.5% down-staged.
On multivariable logistic regression, younger age (OR 0.991, 95% CI 0.984–0.999, p=0.0188), male sex (versus female; OR 1.392, 95% CI 1.158–1.673, p=0.0004), poor or undifferentiated tumor grade (versus well differentiated or moderately differentiated; OR 2.399, 95% CI 1.987–2.896; p<0.0001), positive margin status (versus negative; OR 4.575, 95% CI 3.360–6.230; p<0.0001), and days from diagnosis to surgery (15–32 days versus ≤ 14 days; OR 1.411, 95% CI 1.098–1.814, p=0.0072) were predictive of up-staging. Tumor location did not impact up-staging.
Survival
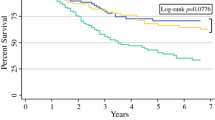
There were survival differences between patients who were accurately staged versus those with stage discordance. Patients who were up-staged had a median overall survival of 27.9 months, while those who were accurately staged had a median survival of 67.6 months, and those who were down-staged had a median survival that was not yet reached (Fig. 5; log-rank, p<0.0001).
When comparing patients whose clinical and pathologic staging was concordant to those who were up-staged, there is a difference in survival at 6 months, 1 year, and 3 years. For those who were up-staged, survival was 86.6% at 6 months, 74.3% at 1 year, and 42.3% at 3 years. This is in comparison to those who were appropriately staged: 92.3% at 6 months, 85.5% at 1 year, and 65.9% at 3 years.
Discussion
This study demonstrates that there is significant discordance between the clinical and pathologic staging of patients with gastric adenocarcinoma. Approximately half of all patients, and the majority of patients with early clinical stage tumors, were ultimately up-staged on pathology. For patients who were up-staged on pathologic examination, there was a clear survival disadvantage.
The importance of appropriate staging in cancer care is widely accepted given its role in treatment and prognosis. The discrepancy between clinical and pathologic staging, and the associated differences in survival, has been previously demonstrated in other cancer types, including renal cell carcinoma and bladder cancer.22, 23 In gastric cancer, patients are clinically staged through a combination of imaging modalities, including CT and EUS, but these are not without limitations. For example, EUS has a high specificity and sensitivity for later stage disease but is less accurate in diagnosing superficial tumors and lymph nodes.24 More recent studies have evaluated the use of MRI, FDG PET/CT, and techniques such as radiomics, which has shown some improvement in clinical staging of gastric adenocarcinoma.25,26,27 Therefore, there may be value in combining diagnostic techniques. Further study is required to evaluate diagnostic methods and the discordance of staging due to variation in diagnostic protocols.
The importance of accurate staging is particularly relevant today as the use of neoadjuvant therapy in gastric adenocarcinoma is increasing in the USA. Multiple studies have demonstrated that the use of neoadjuvant therapy results in improved resection rates and surgical outcomes.14, 15, 17, 28 In our study, 50.4% of patients were up-staged after pathologic evaluation and had a survival disadvantage of 39.7 months as compared to those were accurately staged. This survival difference may be explained not only by a large proportion of patients who were clinically under-staged but also by the omission of neoadjuvant chemotherapy. Interestingly, only 44% of patients received adjuvant chemotherapy, despite that the majority of patients were eligible. However, it is not uncommon to see low levels of adjuvant therapy completion in gastric cancer.29 Still, we would expect that many more patients would benefit from adjuvant therapy after definitive surgery, than are currently completing it.
Previous clinicopathologic profiles of gastric cancer have demonstrated the effect of grade on disease spread and the impact of margin status on survival.30, 31 Our study confirms these findings as higher grade tumors and positive margins were predictive of up-staging. It is possible that these characteristics also impacted the survival differences seen in this study. When considering treatment modifications for these patients, those with more aggressive tumor characteristics or those with more challenging planned resections may benefit from neoadjuvant therapy.
Location of gastric adenocarcinoma was not associated with tumor up-staging in this study. Interestingly, previous studies have demonstrated differences in outcomes based on tumor location in gastric cancer, particularly when comparing proximal to distal tumors.32 Although our study did not find this difference, location is an important consideration in treatment planning. For example, the support for neoadjuvant therapy in locally advanced distal esophageal adenocarcinoma has been extended to proximal gastric tumors.33
Despite significant differences in staging discordance between high and low volume centers, this factor was ultimately not predictive of up-staging. As volume is a well-established predictor of quality in surgical cancer care, it should be considered, in conjunction with patient and tumor characteristics, for staging and treatment planning.34
Surgery within 2 weeks to a month of diagnosis also predicted up-staging but was not significant beyond 33 days. This is likely related to the disease, and previous studies have demonstrated that prolonged time to gastrectomy does not impact overall survival.35 In addition, this could be reflective of the extent of the staging work up, and a more thorough evaluation may result in better accuracy. Timing can also guide therapy, with early initiation of neoadjuvant chemotherapy to halt progression.
This study has several limitations. The NCDB is subject to possible misclassification and is limited by its predefined variables. As such, there is no information available regarding preoperative staging methods or variations in pathologic or radiographic examination, which could provide estimations of discordance due to diagnostic protocol variation. Although the NCDB represents a large proportion of cancers in the USA, it is hospital-based rather than population-based and represents only commission on cancer approved sites. Therefore, it may not generalize to all patients and centers. In addition, we included clinical stage T4 disease in our analysis to determine if patients had been down-staged but recognize that these patients cannot be up-staged by T stage. Lastly, though the most recent AJCC guidelines are currently the 8th edition, we used the AJCC 7th edition to reflect the staging guidelines in use during the time period of our NCDB data collection.
Conclusion
Our study demonstrates a substantial discordance in clinical and pathologic staging in gastric adenocarcinoma, thus potentially misguiding treatment and impacting overall survival. We must improve diagnostic and treatment guidelines and factor in patient and tumor specific variables as well as institutional limitations. Our findings provide evidence that patients often present with more advanced disease than clinically appreciated and lend support to the need for improvement and better utilization of diagnostic techniques. We hope our findings will also encourage the use of neoadjuvant therapy in resectable gastric cancer.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359-86.
Cancer Stat Facts: Stomach Cancer National Cancer Insitute: Surveillance, Epidemiology, and End Results Program [Available from: https://seer.cancer.gov/statfacts/html/stomach.html.
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet (London, England). 2016;388(10060):2654-64.
Dikken JL, van de Velde CJH, Coit DG, Shah MA, Verheij M, Cats A. Treatment of resectable gastric cancer. Therap Adv Gastroenterol. 2012;5(1):49-69.
Morgan R, Cassidy M, DeGeus SWL, Tseng J, McAneny D, Sachs T. Presentation and Survival of Gastric Cancer Patients at an Urban Academic Safety-Net Hospital. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2019;23(2):239-46.
What is TNM? UICC [Available from: https://www.uicc.org/resources/tnm.
Cancer Staging System: AJCC: American Joint Committee on Cancer; [Available from: https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx.
Dikken JL, van de Velde CJ, Gönen M, Verheij M, Brennan MF, Coit DG. The New American Joint Committee on Cancer/International Union Against Cancer staging system for adenocarcinoma of the stomach: increased complexity without clear improvement in predictive accuracy. Ann Surg Oncol. 2012;19(8):2443-51.
In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol. 2017;24(12):3683-91.
Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. Journal of Thoracic Oncology. 2016;11(3):300-11.
Dehal AN, Graff-Baker AN, Vuong B, Nelson D, Chang S-C, Lee DY, et al. Correlation Between Clinical and Pathologic Staging in Colon Cancer: Implications for Neoadjuvant Treatment. Journal of Gastrointestinal Surgery. 2018;22(10):1764-71.
Johnston FM, Beckman M. Updates on Management of Gastric Cancer. Current Oncology Reports. 2019;21(8):67.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. New England Journal of Medicine. 2006;355(1):11-20.
Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World journal of gastroenterology. 2015;21(24):7343-8.
Greenleaf EK, Hollenbeak CS, Wong J. Trends in the use and impact of neoadjuvant chemotherapy on perioperative outcomes for resected gastric cancer: Evidence from the American College of Surgeons National Cancer Database. Surgery. 2016;159(4):1099-112.
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet (London, England). 2019;393(10184):1948-57.
Menges M, Hoehler T. Neoadjuvant Therapy of Gastric Cancer: A Decisive Step Forward. Gastrointest Tumors. 2014;1(2):99-104.
National Cancer Database [09/23/2020]. Available from: https://www.facs.org/quality-programs/cancer/ncdb.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Annals of Surgical Oncology. 2008;15(3):683-90.
Fisher BW, Fluck M, Young K, Shabahang M, Blansfield J, Arora TK. Urgent Surgery for Gastric Adenocarcinoma: A Study of the National Cancer Database. The Journal of surgical research. 2020;245:619-28.
Bachmann MO, Alderson D, Edwards D, Wotton S, Bedford C, Peters TJ, et al. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. The British journal of surgery. 2002;89(7):914-22.
Svatek RS, Shariat SF, Novara G, Skinner EC, Fradet Y, Bastian PJ, et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU international. 2011;107(6):898-904.
Svatek RS, Lotan Y, Herman MP, Duchene DA, Sagalowsky AI, Cadeddu JA. The influence of clinical and pathological stage discrepancy on cancer specific survival in patients treated for renal cell carcinoma. The Journal of urology. 2006;176(4 Pt 1):1321-5; discussion 125.
Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;2015(2):CD009944-CD.
Qiao X, Li Z, Li L, Ji C, Li H, Shi T, et al. Preoperative T(2)-weighted MR imaging texture analysis of gastric cancer: prediction of TNM stages. Abdominal radiology (New York). 2020.
Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, et al. Comparison of (68)Ga-FAPI and (18)F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology. 2020:203275.
Li J, Zhang C, Wei J, Zheng P, Zhang H, Xie Y, et al. Intratumoral and Peritumoral Radiomics of Contrast-Enhanced CT for Prediction of Disease-Free Survival and Chemotherapy Response in Stage II/III Gastric Cancer. Frontiers in oncology. 2020;10:552270.
Mezhir JJ, Tang LH, Coit DG. Neoadjuvant therapy of locally advanced gastric cancer. Journal of Surgical Oncology. 2010;101(4):305-14.
Fuentes E, Ahmad R, Hong TS, Clark JW, Kwak EL, Rattner DW, et al. Adjuvant Therapy Completion Rates in Patients with Gastric Cancer Undergoing Perioperative Chemotherapy Versus a Surgery-First Approach. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2016;20(1):172-9; discussion 9.
Saitoh T, Takamura A, Watanabe G. Endoscopic and clinicopathological features of intramucosal, histologically mixed-type, low-grade, well-differentiated gastric tubular adenocarcinoma with the potential for late-onset lymph node metastasis. BMC gastroenterology. 2018;18(1):189.
Nagata T, Ichikawa D, Komatsu S, Inoue K, Shiozaki A, Fujiwara H, et al. Prognostic impact of microscopic positive margin in gastric cancer patients. J Surg Oncol. 2011;104(6):592-7.
Zhao L, Huang H, Zhao D, Wang C, Tian Y, Yuan X, et al. Clinicopathological Characteristics and Prognosis of Proximal and Distal Gastric Cancer during 1997-2017 in China National Cancer Center. J Oncol. 2019;2019:9784039-.
Platz TA, Nurkin SJ, Fong MK, Groman A, Flaherty L, Malhotra U, et al. Neoadjuvant chemoradiotherapy for esophageal/gastroesophageal carcinoma. Journal of gastrointestinal oncology. 2013;4(2):137-43.
Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Annals of surgery. 2007;245(5):777-83.
Brenkman HJF, Visser E, van Rossum PSN, Siesling S, van Hillegersberg R, Ruurda JP. Association Between Waiting Time from Diagnosis to Treatment and Survival in Patients with Curable Gastric Cancer: A Population-Based Study in the Netherlands. Annals of surgical oncology. 2017;24(7):1761-9.
Funding
Marianna Papageorge is supported by a T32 grant through Boston University School of Medicine (award #T32HP10028). Alison Woods is supported by a T32 grant through Johns Hopkins University School of Medicine, from the National Institutes of Health (National Cancer Institute award #T32CA126607).
Author information
Authors and Affiliations
Contributions
Each author meets authorship criteria per the guidelines of the ICMJE and the Definition of Authorship as outlined by JOGS.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and supporting institutions.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Papageorge, M.V., de Geus, S.W.L., Zheng, J. et al. The Discordance of Clinical and Pathologic Staging in Locally Advanced Gastric Adenocarcinoma. J Gastrointest Surg 25, 1363–1369 (2021). https://doi.org/10.1007/s11605-021-04993-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-04993-4