Abstract
Background
The link between smoking and poor postoperative outcomes is well established. Despite this, current smokers are still offered bariatric surgery. We describe the risk of postoperative 30-day complications and readmission following laparoscopic sleeve gastrectomy and laparoscopic Roux-En-Y gastric bypass in smokers.
Methods
The National Surgical Quality Improvement Program database was queried to identify patients who underwent laparoscopic sleeve gastrectomy and Roux-En-Y gastric bypass from 2012 to 2017. Patient outcomes were compared based on smoking status. Primary outcomes included 30-day readmission and death or serious morbidity. Secondary outcomes included wound and respiratory complications. Multivariable logistic regression was used to determine the association between smoking status and measured outcomes.
Results
Of the 133,417 patients who underwent bariatric surgery, 12,424 (9.3%) were smokers. Smokers more frequently experienced readmission (4.9% v 4.1%, p < 0.001), death or serious morbidity (3.8% v 3.4%, p = 0.019), wound complications (2% v 1.4%, p < 0.001), and respiratory complications (0.8% v 0.5%, p < 0.001). The likelihood of death or serious morbidity (OR 1.13, 95% CI 1.01–1.26), readmission (OR 1.21, 95% CI 1.10–1.33), wound (OR 1.44, 95% CI 1.24–1.68), and respiratory complications (OR 1.69, 95% CI 1.34–2.14) were greater in smokers. The adjusted ORs remained significant on subgroup analysis of laparoscopic sleeve gastrectomy and Roux-En-Y gastric bypass patients, with the exception of death or serious morbidity in laparoscopic Roux-En-Y gastric bypass (OR 1.04, 95% CI 0.89–1.24).
Conclusions
Smokers undergoing bariatric surgery experience significantly worse 30-day outcomes when compared with non-smokers. There should be a continued emphasis on perioperative smoking cessation for patients being evaluated for bariatric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery remains one of the most effective methods of achieving sustained long-term weight loss and improvement in obesity-related comorbidities.1,2 The rigorous preoperative evaluation process, consisting of appropriate screening for medical and psychological comorbidities, has made bariatric surgery an especially safe procedure.3,4 This is particularly notable, given the inherently higher risk of complications associated with operating on morbidly obese patients. However, areas for improvement in outcomes following bariatric surgery remain. One key risk factor for poor surgical outcomes is smoking. The link between smoking and poor postoperative outcomes has been well established. Smoking represents an independent predictor of postoperative complications and is associated with higher healthcare costs.5,6,7,8,9 Despite this, current smokers continue to be offered elective bariatric surgery.
Smoking cessation prior to surgery has been associated with a significant improvement in both postoperative outcomes and healthcare expenditures.8,10 To date, the association between smoking and postoperative outcomes following bariatric surgery has only been explored in the context of limited case series.11,12,13 However, the generalizability of these studies has been limited by their small sample size and inclusion of only single institution or single state cohorts. In addition, the single national study evaluating the association between smoking and outcomes following bariatric surgery was performed using data prior to the adoption of laparoscopic sleeve gastrectomy (LSG).11 As a result, the impact of smoking on one of the most common bariatric procedures currently performed is poorly understood.
As bariatric surgery, in particular LSG, continues to serve a key role in the treatment of obesity, it is important to evaluate the association between the modifiable risk factor of smoking and postoperative outcomes. In this retrospective cohort study, data from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) was used to describe the risk of postoperative 30-day complications and readmission following LSG and laparoscopic Roux-En-Y gastric bypass (LRYGB) in smokers.
Materials and Methods
Data Source
Data from the 2012–2017 ACS NSQIP was used to identify all laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en Y gastric bypass (LRYGB) patients. ACS NSQIP sampling strategy, data abstraction, variables collected, and outcomes are detailed elsewhere.14,15 Briefly, the ACS NSQIP database maintains prospectively collected data on several clinical and pathological characteristics. These include patient demographics, comorbidities, operative details, and 30-day postoperative outcomes. Data are collected by highly trained surgical clinical reviewers in a standardized fashion. This study was deemed exempt by the Institutional Review Board of Northwestern University.
Study Population
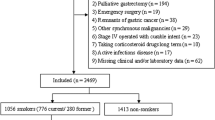
Current Procedural Terminology (CPT) codes were used to identify patients who underwent LSG or LRYGB in the ACS NSQIP database (CPT codes 43644 and 43775). Patients who underwent revision surgery (n = 1016), were classified as emergent (n = 1737), or had an American Society of Anesthesiologist (ASA) class of V or missing were excluded (n = 117). Smoking status was determined based on patient self-report of smoking cigarettes within the last year and coded as “current smoker” vs “non-smoker.” ACS NSQIP does not collect data regarding patients who were “former” smokers.
Outcomes
The primary outcomes studied include 30-day readmission and death or serious morbidity (DSM). DSM included death, deep surgical site infection, organ space surgical site infection, wound dehiscence, pneumonia, reintubation, pulmonary embolism, acute kidney injury, myocardial infarct, cardiac arrest, sepsis, septic shock, return to OR, deep venous thrombosis, requiring ventilator support for 48 h, or bleeding requiring transfusion. Secondary outcomes included wound (defined as superficial, deep, and organ space surgical site infection [SSI]) and respiratory (pneumonia, reintubation, and failure to wean from mechanical ventilation within 48 h) complications.
Covariates
Patient-specific demographic (age, sex, race/ethnicity), comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease [COPD], dyspnea, steroid use, and functional status), and operative characteristics (ASA, wound class, and operative time) were available within the dataset.
Statistical Analysis
Bivariate associations between demographic characteristics and smoking status were evaluated using descriptive statistics. Categorical variables were evaluated using chi-squared tests, while continuous variables were evaluated with either a Student t test or Mann-Whitney test. The incidence of postoperative readmission and complications by smoking status was evaluated with chi-squared test. Multivariable logistic regression was used to assess for associations between the outcomes of interest and smoking status. The multivariable model adjusted for age, sex, race (Non-Hispanic White, Non-Hispanic Black, Hispanic, Other/Unknown), BMI, and pre-existing comorbidities (diabetes, hypertension, COPD, and dyspnea). Subgroup analyses, evaluating LSG and LRYGB individually, were performed to assess for differential associations based on type of surgery. All tests were two sided and the level of significance was set at 0.05. Statistical analysis was performed using STATA v15.1 (College Station, TX).
Results
Among 133,417 patients who underwent bariatric surgery from 2012 to 2017, 12,424 (9.3%) self-reported as smokers (Table 1). Current smokers were more likely to be younger (24.3 v 27.9 years old, p < 0.001), male (21.5% v 20.3%, p = 0.001), have a higher BMI (46.2 v 45.5, p < 0.001), have COPD (3.9% v 1.5%, p < 0.001), and experience dyspnea with moderate exertion or at rest (15.6% v 12.2%, p < 0.001). Current smokers were less likely to have diabetes (23.4% v 27.1%, p < 0.001) and hypertension (43.0% v 49.1%, p < 0.001). There were no significant differences between type of bariatric surgery and smoking status.
The overall rate of DSM following bariatric surgery was 3.5% while the overall rate of 30-day readmission was 4.2%. On unadjusted analysis, a total of 608 (4.9%) current smokers were readmitted within 30 days, compared with 4934 (4.1%) non-smokers (p < 0.001; Table 2). Rates of DSM were higher in current smokers (475 [3.8%] v 4136 [3.4%], p = 0.019). Current smokers were also more likely to experience wound (251 [2.0%] v 1712 [1.4%], p < 0.001) and respiratory (95 [0.8%] v 622 [0.5%], p < 0.001) complications when compared with non-smokers. Analysis of individual complications revealed no differences in the rates of septic complications, venous thromboembolism, cardiac complications, or transfusion requirements. Similar trends were noted on subgroup analysis of LSG and LRYGB patients.
Following adjustment for patient demographic, clinical factors, and surgery type, current smokers were more likely to experience DSM (odds ratio [OR] 1.13, 95% confidence interval [CI] 1.01–1.26) and 30-day readmission (OR 1.21, 95% CI 1.10–1.33; Table 3). In addition, current smokers were more likely to experience wound (OR 1.44, 95% CI 1.24–1.68) and respiratory (OR 1.69, 95% CI 1.34–2.14) complications. However, current smokers were no more likely to return to the operating room (OR 1.15, 95% CI 0.98–1.34) when compared with non-smokers. The adjusted ORs remained significant on subgroup analysis of LSG and LRYGB patients, with the exception of death or serious morbidity in LSG (OR 1.04, 95% CI 0.89–1.24).
Discussion
In this study, a national cohort of patients undergoing elective bariatric surgery was analyzed to compare outcomes between smokers and non-smokers. Smoking was associated with increased DSM and 30-day readmission following surgery after adjusting for patient comorbidities. Patients who were smokers were particularly more likely to experience wound and respiratory complications. Similar findings were noted when evaluating LSG and LRYGB individually.
The association between smoking and poor postoperative outcomes is well established. Previous studies have shown smokers undergoing major oncologic, cardiovascular, or thoracic operations to have higher rates of infectious, wound, and pulmonary complications.7,16 Similarly, recent work by our group has demonstrated that smokers undergoing even minor elective operations, such as hernia repairs, also experience worse postoperative outcomes when compared with nonsmokers.17 The results of this study similarly demonstrate the adverse impact of smoking at the time of bariatric surgery in a large-scale, national patient cohort. While previous studies have evaluated the association between smoking and bariatric surgery outcomes, they have been limited by small sample size, single institutions or state cohorts, or the lack of data on the most common bariatric surgery currently performed. Thus, the current study provides the most generalizable results reported to date by using a national cohort consisting of patients who underwent either LSG or LRYGB. In particular, we found that smokers undergoing LSG experience higher rates of DSM and readmission when compared with smokers undergoing LRYGB.
While complications following bariatric surgery are rare, the number of bariatric surgical procedures performed each year in the USA continues to increase.18 Therefore, even a small reduction in postoperative complications and readmissions related to smoking would represent an important opportunity to improve patient outcomes and reduce healthcare costs. Along these lines, certain insurance policies mandate that patients remain nicotine free during the preoperative period. Furthermore, past work has shown that the likelihood of sustained abstinence from smoking at 1 year was greatest if performed preoperatively.19,20,21 The extended preoperative workup associated with bariatric surgery provides a unique opportunity for surgeons to target a modifiable risk factor. Despite these findings, several studies have shown surgeons are less likely to address smoking cessation preoperatively.22,23 Therefore, this study adds to the body of literature indicating that surgeons should take a leading role in addressing smoking cessation.
This study should be interpreted within the context of the following limitations. First, for data collection purposes, NSQIP classifies smokers as any patient who reported smoking within the past year even if they may have quit within that time frame. While this produces a broad definition of “smoker,” the resulting decrease in effect size indicates that the estimated association between smoking and outcomes of interest would be biased towards the null hypothesis. Second, the NSQIP definition of smoking also does not take into account previous history of smoking. Several studies have shown that those with a history of smoking have higher risks for postoperative complications when compared with patients who have never smoked. As in the previous case, this limitation would likely produce a conservative estimate of the association between smoking and outcomes of interest. Third, NSQIP only gathers outcomes up to 30 days following surgery. This precludes any evaluation of long-term associations between smoking and postoperative outcomes.
Conclusion
Smokers undergoing bariatric surgery experience significantly worse 30-day outcomes when compared with non-smokers. These findings further underscore the importance of smoking cessation counseling prior to bariatric surgery. Given the large volume of bariatric procedures performed annually, counseling regarding preoperative smoking cessation process may decrease poor postoperative outcomes, reduce healthcare costs, and encourage patients to sustain smoking cessation.
Abbreviations
- LSG:
-
Laparoscopic sleeve gastrectomy
- LRYGB:
-
Laparoscopic Roux-En-Y gastric bypass
References
Courcoulas, A.P., et al., Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg, 2014. 149(12): p. 1323–9.
Maciejewski, M.L., et al., Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg, 2016. 151(11): p. 1046–1055.
Longitudinal Assessment of Bariatric Surgery, C., et al., Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med, 2009. 361(5): p. 445–54.
Birkmeyer, N.J., et al., Hospital complication rates with bariatric surgery in Michigan. JAMA, 2010. 304(4): p. 435–42.
Hawn, M.T., et al., The attributable risk of smoking on surgical complications. Ann Surg, 2011. 254(6): p. 914–20.
Kamath, A.S., et al., Hospital costs associated with smoking in veterans undergoing general surgery. J Am Coll Surg, 2012. 214(6): p. 901–8 e1.
Schmid, M., et al., Impact of smoking on perioperative outcomes after major surgery. Am J Surg, 2015. 210(2): p. 221–229 e6.
Warner, D.O., et al., Smoking status and health care costs in the perioperative period: a population-based study. JAMA Surg, 2014. 149(3): p. 259–66.
Nolan, M.B., et al., Association Between Smoking Status, Preoperative Exhaled Carbon Monoxide Levels, and Postoperative Surgical Site Infection in Patients Undergoing Elective Surgery. JAMA Surg, 2017. 152(5): p. 476–483.
Lindstrom, D., et al., Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg, 2008. 248(5): p. 739–45.
Haskins, I.N., R. Amdur, and K. Vaziri, The effect of smoking on bariatric surgical outcomes. Surg Endosc, 2014. 28(11): p. 3074–80.
Inadomi, M., et al., Effect of patient-reported smoking status on short-term bariatric surgery outcomes. Surg Endosc, 2018. 32(2): p. 720–726.
Kowalewski, P.K., et al., Cigarette smoking and its impact on weight loss after bariatric surgery: A single center, retrospective study. Surg Obes Relat Dis, 2018. 14(8): p. 1163–1166.
Cohen, M.E., et al., Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg, 2013. 217(2): p. 336–46 e1.
Ingraham, A.M., et al., Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg, 2010. 44: p. 251–67.
Mason, D.P., et al., Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg, 2009. 88(2): p. 362–70; discussion 370-1.
DeLancey, J.O., et al., The effect of smoking on 30-day outcomes in elective hernia repair. Am J Surg, 2018. 216(3): p. 471–474.
American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011–2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers . Accessed on 4/3/19.
Goodney, P.P., et al., Feasibility and pilot efficacy of a brief smoking cessation intervention delivered by vascular surgeons in the Vascular Physician Offer and Report (VAPOR) Trial. J Vasc Surg, 2017. 65(4): p. 1152–1160 e2.
Sadr Azodi, O., et al., The efficacy of a smoking cessation programme in patients undergoing elective surgery: a randomised clinical trial. Anaesthesia, 2009. 64(3): p. 259–65.
Thomsen, T., N. Villebro, and A.M. Moller, Interventions for preoperative smoking cessation. Cochrane Database Syst Rev, 2010(7): p. CD002294.
Krupski, W.C., et al., Smoking cessation counseling: a missed opportunity for general surgery trainees. J Vasc Surg, 2002. 36(2): p. 257–62; discussion 262.
Thorndike, A.N., et al., National patterns in the treatment of smokers by physicians. JAMA, 1998. 279(8): p. 604–8.
Funding
TKY (Agency for Healthcare Research and Quality [AHRQ] 5T32HS000078) and RK (National Institutes of Health [NIH] 5T32HL094293) were supported by a postdoctoral research fellowship. DDO receives support from the National Cancer Institute of the National Institutes of Health under Award Number K07CA216330.
Author information
Authors and Affiliations
Contributions
TKY, RK: data analysis, interpretation of results, writing.
NJS, ESH, APN, ENT, KYB, DDO: conceptualization, interpretation of results, methodology, writing, editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
Views expressed in this work represent those of the authors only.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meeting Information
This work was presented as a plenary at the 2019 Digestive Disease Week Meeting as well as the 2019 SSAT Residents and Fellow Research Conference.
Rights and permissions
About this article
Cite this article
Yuce, T.K., Khorfan, R., Soper, N.J. et al. Post-Operative Complications and Readmissions Associated with Smoking Following Bariatric Surgery. J Gastrointest Surg 24, 525–530 (2020). https://doi.org/10.1007/s11605-019-04488-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04488-3