Abstract
Objectives
The benefits of curative or palliative gastrectomy for Borrmann type IV (B-IV) gastric cancer remain controversial. This study was conducted to investigate whether or not gastrectomy could benefit prognosis of patients with Borrmann type IV gastric cancer.
Material and Methods
A cohort of 469 B-IV gastric cancer patients from January 2001 to September 2017 was retrospectively reviewed. Survival analysis was used to investigate the prognosis of patients with or without gastrectomy.
Results
Among this cohort, the average age was 55 years and the median follow-up time was 12 months. One hundred and forty-six (31%) patients underwent curative resection, 187 (40%) patients underwent palliative resection, and the remaining 136 (29%) patients were judged unresectable. During the follow-up, a total of 294 (63%) patients died. Cox multivariate analysis showed that Tumor Node Metastasis (TNM) stage (p = 0.002), grade (p = 0.033), and gastrectomy (p < 0.001) were independent predictors of overall survival. Kaplan–Meier analysis revealed that, no matter in total group or subgroup stratified by tumor stage and grade, overall survival rates at 1 year, 2 years, and 5 years in patients with palliative resection were significantly worse than those in patients with curative resection (all p < 0.05), but significantly better than those in patients with no resection (all p < 0.05).
Conclusions
Curative or palliative gastrectomy could increase the survival rate for B-IV gastric cancer patients. In the absence of alternative effective therapies, surgical resection remains a choice of improved survival or potential cure for B-IV gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is a common malignant disease and remains the third most frequent cause of cancer deaths worldwide.1 As an uncommon variant of this malignancy, Borrmann type IV (B-IV) gastric cancer is characterized by a diffusely infiltrative gastric wall and also referred as linitis plastica.2
Generally, gastrectomy remains to be the mainstay of therapy for other Bormann type gastric cancer; however, the effects of gastrectomy for B-IV gastric cancer have not been investigated thoroughly. Some investigations have reported that curative or non-curative gastrectomy could improve the prognosis and life quality of patients with B-IV gastric cancer.3,4,5 In contrast, other studies6,7,8 reported a really poor prognosis of B-IV gastric cancer and concluded that non-curative cases would not benefit from gastrectomy. Overall, evidence available to assess the effects of gastrectomy for B-IV gastric cancer is fairly insufficient and limited mostly to small cohort and unsatisfactory statistical methodology.
Thus, based on a database containing a large cohort of B-IV gastric cancer patients, this study aims to better define the prognostic benefits of curative or palliative gastrectomy for B-IV gastric cancer.
Material and Methods
Definition
B-IV gastric cancer is defined as an advanced, flat, non-cratered, and macroscopically widely spreading carcinoma. Involved circumferentially at least one-third of the stomach, it frequently involved the stomach from the fundus to the pylorus.9, 10 Patients were excluded if the presence or absence of B-IV gastric cancer was not clearly stated in the medical history records. In addition, linitis plastica is a term frequently used to describe B-IV gastric cancer in many publications, and we also used this term sometimes to keep consistent with the publications we cited.
Patients Selection
After institutional review board approval, we retrospectively reviewed data of 531 patients with B-IV gastric cancer at our institution between January 2001 and September 2017. After excluding those patients who had incomplete medical data (n = 62), a total of 469 B-IV gastric cancer patients were finally enrolled into this study. These patients constituted 10% of the 4661 patients with gastric cancers who went to our institution during the same period.
Data regarding patients’ demographic, clinicopathological characteristics and multimodality therapies were retrospectively collected. Pathologic classification and TNM staging following surgical resection were defined by AJCC, 7th edition.11 The extent of lymphadenectomy was recorded based on the Japanese Gastric Cancer Association classification system.12
Extent of Gastric Resection and Lymphadenectomy
Patients with a lesion located in the proximal or upper middle portion of the stomach underwent total gastrectomy, whereas those with a gastric carcinoma located in the distal or lower middle portion received subtotal gastrectomy. Essentially, uniform techniques were used for each of the two procedures. Informed consent for surgical treatment was obtained from all patients.
Curative gastrectomy was defined as a resection with no macroscopic residual disease, including an absence of distant metastasis and proximally and distally negative surgical margins, e.g., an R0 resection according to the rules of UICC13. In these cases with distant metastasis or positive surgical margins, the resection was considered palliative. No resection means only supportive therapy with or without laparoscopy or gastrojejunostomy.
Follow-up and Statistical Methods
All patients included in the study were regularly followed up with a standardized protocol.14 Comparisons of clinicopathological features between groups were performed using χ2 tests for categoric variables and Kruskal–Wallis tests for continuous variables. The cause of death was determined by the treating physicians, chart review corroborated, telephone interview, or death certificates alone. Using the Kaplan–Meier method, overall survival (OS) was estimated from the time of surgery to event. An analysis of the difference between the survival rates was performed using the log-rank test. Cox proportional hazard models were used to evaluate risk factors of OS. Only those variables that were univariately significant were entered into the multivariate Cox analysis. All reported p values were two-sided, and statistical significance was set at p < 0.05. Statistical analysis was performed using PASW version 18.0 statistical software (IBM Corp, Somers, NY, USA).
Results
Table 1 reports the main demographic and clinical characteristics of the cohort under study. As depicted, the average age was 55 years (interquartile range [IQR] 44–64) and the median follow-up time was 12 months (IQR 7–20). Of the total cohort, 146 (31%) patients underwent curative resection, 187 (40%) patients underwent palliative resection, and the remaining 136 (29%) patients were judged unresectable. Positive surgical margin was observed in 45 (13.5%) patients. In addition, 294 (63%) patients were diagnosed with metastasis, including 245 (52%) patients with peritoneal dissemination.
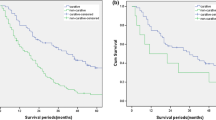
During the follow-up, a total of 294 (63%) patients died. Table 2 shows the univariate and multivariate Cox regression analysis for predicting OS in total cohort. In the multivariate analysis, TNM stage (p = 0.002), grade (p = 0.033), and gastrectomy (p < 0.001) were found to be independent predictors of OS. Furthermore, Figs. 1 and 2 display OS curves stratified by treatment (curative gastrectomy vs palliative gastrectomy vs no resection) separately in total cohort, TNM II~III stage cohort and grade 3 cohort. Table 3 summarizes OS rates stratified by treatment. As depicted, in total cohort, OS rates at 1 year, 2 years, and 5 years in patients with palliative resection were significantly worse than those in patients with curative resection (65.7%, 29.6%, and 9.4% vs 81%, 49.7%, and 23.6%, respectively; p < 0.001), but significantly better than those in patients with no resection (65.7%, 29.6%, and 9.4% vs 45.4%, 14.6%, and 0%, respectively; p < 0.001). Also, in subgroup cohort of TNM II~III stage and grade 3, OS rates at 1 year, 2 years, and 5 years in patients with palliative resection were significantly worse than those in patients with curative resection, but significantly better than those in patients with no resection (all p < 0.05). Figure 3 displays OS curves stratified by treatment (palliative resection vs no resection) in TNM IV stage cohort. As depicted, OS rates at 1 year, 2 years, and 5 years in patients with palliative resection were significantly better than those in patients with no resection (65.2%, 30%, and 8.5% vs 46%, 14.8%, and 0%, respectively; p < 0.001).
Discussion
In this study, we retrospectively evaluated the clinicopathological and prognostic features of B-IV gastric cancer underwent different treatments based on a large cohort. Our results indicated that gastrectomy, along with TNM stage and tumor grade, was the most important predictors of OS in B-IV gastric cancer. Regarding to methodology, we found that nearly all prior studies evaluated the treatment efficiency for B-IV gastric cancer in cohort mixed with different tumor stages and grades. Our study, however, uniquely explored treatment efficiency in subgroup analysis stratified by tumor stage and grade. More specifically, our subgroup analysis demonstrated that overall survival in patients underwent palliative gastrectomy could be worse than in patients underwent curative gastrectomy, but significantly better than in those received no gastric resection (Figs. 1, 2, and 3). In the absence of alternative effective therapies, our result indicated that surgical resection remained a choice of improved survival or potential cure for B-IV gastric cancer. In addition, considering the majority of previous studies were limited to small cohort, this cohort could be one of the largest database focusing on the role of surgical treatment in B-IV gastric cancer.
Nowadays, the classification of advanced gastric carcinoma by Borrmann (types I through IV) is still accepted worldwide by many surgeons. According to Borrmann’s classification, B-IV gastric cancer shows a special morphology both macro- and microscopically. B-IV gastric cancer represents a minor Borrmann types, accounting for around 10~20% of all cases.15, 16 In this study, B-IV gastric cancer constituted 10% of the 4661 patients with gastric cancers who went to our institution during the same period, which was consistent with these studies.
Some investigators have reported that B-IV gastric cancer predominates in females and in undifferentiated histology, invades the serosal surface, involves lymph nodes more frequently, and has a high incidence of peritoneal dissemination.4, 17 Indeed, our results were also consistent with these studies, i.e., 294 (63%) patients were diagnosed with metastasis, including 245 (52%) with peritoneal dissemination (Table 1). Even though there have been substantial advances in the diagnosis of gastric cancer, most of B-IV gastric cancer are still detected at advanced or even end stage. The detection delay presents a great challenge for the surgeon treating this malignancy.
Currently, there have been piles of studies published to explore prognosis of gastric cancer. Overall, those pathological factors, such as tumor stage and grade, have been confirmed to be the most crucial prognostic predictors of postoperative survival.14, 18 The prognosis of B-IV gastric cancer has been believed to be the worst among all Borrmann types without regard to the extent or type of resection.19, 20 In our patients, the 2-year and 5-year OS rates of the total cohort were 32.5% and 13% respectively (Table 3), also reflecting the poor outcome of this disease. Huang et al. even recommended that classifying B-IV gastric cancer as pT4b disease could improve pT classification prediction of prognosis in patients with advanced gastric cancer after radical surgery.21 Specifically designed multimodality treatment protocols should be tested in this setting. Hyperthermic intra-peritoneal chemotherapy could represent an option and should be tested in specifically designed controlled studies.
Though the overall prognosis of B-IV gastric cancer has come to a consensus, the surgical treatment efficiency for this malignancy is still controversial and limitedly reported. In an earlier study from Aranha et al., they observed no significant difference in mean survival time between unresectable and resected linitis plastica, leading them to conclude that linitis plastica was not a surgical disease.8 Results from another study also reported no improvement in survival between palliative (R1, R2) and unresected linitis plastica, highlighting the importance of complete surgical resection.22 Moreover, there are reports showing that gastrectomy should be avoided in B-IV gastric cancer with positive peritoneal cytology.23,24,25 Consequently, it has been hypothesized that gastric linitis plastica, accompanied with liver metastasis, peritoneal dissemination, serosal involvement, or local extension of the tumor, would not benefit from a total gastrectomy, and alternative forms of treatment including chemotherapy and/or radiation therapy should be selected.25, 26
Whereas outcomes in these studies were discouraging, other studies tended to support a positive impact of gastrectomy on B-IV gastric cancer. Based on a cohort containing 370 (9%) B-IV gastric cancer patients, Yook et al. observed a significant 5-year OS difference between curative resection and no resection (38.4 vs 4.5%, respectively).4 Other publications13, 16, 27 based on small cohorts also observed a survival advantage achieved after curable or non-curable gastrectomy. Luo et al. made a meta-analysis and concluded that curative resection may increase the survival rate for B-4 patients. If it is not possible to perform a curative resection, a non-curative resection may improve the prognosis.5 Obviously, our result verified this conclusion again. However, it is noteworthy that nearly all previous works evaluated prognosis in cohorts mixed with different tumor stages and grades. In fact, patients who received palliative or no gastrectomy could have more tumor burden leading to worse prognosis; thus, we believe that it would be more reasonable to use the methodology of subgroup analysis according to tumor stage and grade.
Finally, several limitations of this study merit discussion. The first and foremost, our study represents a retrospective analysis of our single-institutional cohort, resulting in an accumulation of variability and inherent biases. Secondly, patients were not randomized to treatment groups; as a result, we could not ignore the possibility that the palliative resection group had a better prognosis than the no resection group due to difference in patient status. In addition, many patients received perioperative systemic treatment, but the lack of standardized treatment protocols and the bias existing among oncology providers likely led to the variations in treatment regimens. Overall, further study in a randomized controlled trial is warranted to validate our results.
Conclusions
Our data suggest that curative or palliative gastrectomy may increase the survival rate for B-IV gastric cancer patients. In the absence of alternative effective therapies, surgical resection remains a choice of improved survival or potential cure for B-IV gastric cancer.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA: a cancer journal for clinicians65, 5–29, https://doi.org/10.3322/caac.21254 (2015).
Bertuccio, P. et al. Recent patterns in gastric cancer: a global overview. Int J Cancer125, 666–673, https://doi.org/10.1002/ijc.24290 (2009).
Nashimoto, A., Yabusaki, H. & Nakagawa, S. Treatment strategy for the type IV gastric cancer--from the standpoint of the surgery. Gan to kagaku ryoho. Cancer & chemotherapy34, 983–987 (2007).
Yook, J. H., Oh, S. T. & Kim, B. S. Clinicopathological analysis of Borrmann type IV gastric cancer. Cancer research and treatment : official journal of Korean Cancer Association37, 87–91, https://doi.org/10.4143/crt.2005.37.2.87 (2005).
Luo, Y. et al. Clinicopathologic characteristics and prognosis of Borrmann type IV gastric cancer: a meta-analysis. World Journal of Surgical Oncology14 (2016).
Kim, E. Y., Yoo, H. M., Song, K. Y. & Park, C. H. Limited significance of curative surgery in Borrmann type IV gastric cancer. Medical Oncology33 (2016).
Pedrazzani, C. et al. Gastric linitis plastica: which role for surgical resection? Gastric Cancer15, 56–60, https://doi.org/10.1007/s10120-011-0063-z (2012).
Aranha, G. V. & Georgen, R. Gastric linitis plastica is not a surgical disease. Surgery106, 758–762; discussion 762-753 (1989).
Lauren, P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta pathologica et microbiologica Scandinavica64, 31–49 (1965).
Mastoraki, A., Papanikolaou, I. S., Sakorafas, G. & Safioleas, M. Facing the challenge of managing linitis plastica--review of the literature. Hepato-gastroenterology56, 1773–1778 (2009).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology17, 1471–1474, https://doi.org/10.1245/s10434-010-0985-4 (2010).
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer14, 113–123, https://doi.org/10.1007/s10120-011-0042-4 (2011).
Accetta, A. C. et al. Type IV Borrmann gastric adenocarcinoma: analysis of curative resection results. Revista do Colegio Brasileiro de Cirurgioes38, 237–244 (2011).
Marrelli, D. et al. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg241, 247–255 (2005).
Zhu, Y.-l., Yang, L., Sui, Z.-q., Liu, L. & Du, J.-f. Clinicopathological features and prognosis of Borrmann type IV gastric cancer. Journal of Buon21, 1471–1475 (2016).
Kim, D. Y., Kim, H. R., Kim, Y. J. & Kim, S. Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ journal of surgery72, 739–742 (2002).
Dong, R.-Z., Guo, J.-M., Zhang, Z.-W., Zhou, Y.-M. & Su, Y. Prognostic impact and implications of extracapsular lymph node spread in Borrmann type IV gastric cancer. Oncotarget8, 97593–97601 (2017).
Lu, J. et al. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Annals of surgical oncology24, 486–493 (2017).
Yang, B., Wu, G., Wang, X. & Zhang, X. Discussion of modifying stage IV gastric cancer based on Borrmann classification. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine34, 1485–1491, https://doi.org/10.1007/s13277-013-0673-7 (2013).
Chang, J. M., Lara, K. A., Gray, R. J., Pockaj, B. A. & Wasif, N. Clinical Outcomes after Surgery for Linitis Plastica of the Stomach: Analysis of a Population Cancer Registry. The American surgeon83, 23–29 (2017).
Huang, J.-y. et al. Borrmann type IV gastric cancer should be classified as pT4b disease. Journal of Surgical Research203, 258–267 (2016).
Kodera, Y. et al. The number of metastatic lymph nodes is a significant risk factor for bone metastasis and poor outcome after surgery for linitis plastica-type gastric carcinoma. World J Surg32, 2015–2020, https://doi.org/10.1007/s00268-008-9672-z (2008).
Kodera, Y. et al. Is Borrmann type IV gastric carcinoma a surgical disease? An old problem revisited with reference to the result of peritoneal washing cytology. J Surg Oncol78, 175–181; discussion 181-172 (2001).
Ikeguchi, M., Yamamoto, O. & Kaibara, N. Management protocol for scirrhous gastric cancer. In vivo (Athens, Greece)18, 577–580 (2004).
Schauer, M., Peiper, M., Theisen, J. & Knoefel, W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. European journal of medical research16, 29–33 (2011).
Fujitani, K. et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. The Lancet. Oncology17, 309–318, https://doi.org/10.1016/s1470-2045(15)00553-7 (2016).
Blackham, A. et al. Is Linitis Plastica a Contraindication for Surgical Resection: A Multi-Institution Study of the U.S. Gastric Cancer Collaborative. Ann. Surg. Oncol.23, 1203–1211 (2016).
Author information
Authors and Affiliations
Contributions
Chengcai Liang: Protocol development, data collection and analysis, manuscript writing.
Guoming Chen: Data collection and analysis, manuscript writing.
Baiwei Zhao: Data collection and analysis.
Haibo Qiu: Protocol development and analysis.
Wei Li: Data analysis and manuscript editing.
Xiaowei Sun: Data collection and analysis.
Zhiwei Zhou: Protocol development, data analysis, and manuscript editing Yingbo Chen: Protocol development, data analysis, and manuscript editing.
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, C., Chen, G., Zhao, B. et al. Borrmann Type IV Gastric Cancer: Focus on the Role of Gastrectomy. J Gastrointest Surg 24, 1026–1032 (2020). https://doi.org/10.1007/s11605-019-04236-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04236-7