Abstract
Background
Laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding have been popular alternatives to laparoscopic Roux-en-Y gastric bypass due to their technical ease and lower complication rates. Comprehensive longitudinal data are necessary to guide selection of the appropriate bariatric procedures for individual patients.
Methods
We used the Truven Heath Analytics MarketScan® database between 2000 and 2015 to identify patients undergoing bariatric surgery. Kaplan-Meier and Cox proportional hazard regression analyses were performed to compare complication rates between laparoscopic gastric bypass and laparoscopic sleeve gastrectomy, as well as between laparoscopic gastric bypass and laparoscopic adjustable gastric banding.
Results
256,830 individuals met search criteria. By 2015, laparoscopic sleeve gastrectomy was the most commonly performed bariatric procedure followed by laparoscopic gastric bypass and then laparoscopic adjustable gastric banding. Overall, laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding had fewer complications relative to laparoscopic gastric bypass with the exceptions of heartburn, gastritis, and portal vein thrombosis following sleeve gastrectomy and heartburn and dysphagia following adjustable gastric banding.
Conclusion
Laparoscopic sleeve gastrectomy is now the most commonly performed bariatric procedure in the USA. It is reassuring that its overall postoperative complication rates are lower relative to laparoscopic gastric bypass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity continues to be a significant problem in the USA. Approximately 36% of adults and 17% of younger Americans are estimated to be obese.1 Bariatric surgery has been proven to be a safe and effective treatment for obesity and its associated medical conditions. Laparoscopic sleeve gastrectomy is now the most commonly performed bariatric procedure worldwide, followed by laparoscopic Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding, and laparoscopic biliopancreatic diversion with duodenal switch.2 Roux-en-Y gastric bypass remains the gold standard bariatric procedure due to its proven record of durable weight loss and resolution of obesity-related comorbidities, as well as its low morbidity. Recently, sleeve gastrectomy has gained popularity due to its relative technical ease and long-term data demonstrating its durability. Outcomes have shown similar efficacy to gastric bypass in maintaining weight loss and improving comorbidities.3,4,5 Adjustable gastric banding, which became very popular after the turn of the millennium due to its technical simplicity, has seen dramatically reduced utilization in recent years as longer-term outcomes were not acceptable in most studies.6,7,8 Other bariatric procedures, such as biliopancreatic diversion with duodenal switch, as well as other variations of this procedure, are also performed, but at low frequency. As the trends in bariatric procedures change, new procedures should be compared in relation to gastric bypass.
Overall, mortality and morbidity rates for bariatric procedures are low. Studies report 0 to 0.64% 30-day mortality rate and 0 to 2.5% 30-day serious morbidity.7, 9,10,11 With the recent increase in the rate of laparoscopic sleeve gastrectomy relative to all other bariatric procedures, it is important not only to assess and compare weight loss outcomes between procedures, but also to assess differences in rates of complications. Thus, the purpose of this analysis was (1) to assess temporal trends in bariatric surgery and (2) to measure and compare the cumulative incidence of complications after laparoscopic Roux-en-Y gastric bypass (LRYGB), laparoscopic sleeve gastrectomy (LSG), and laparoscopic adjustable gastric banding (LAGB) in the USA.
Materials and Methods
Study Population
This study was conducted using the Truven Health Analytics MarketScan Commercial Claims and Encounters® database. This administrative database contains individual-level data on clinical utilization, expenditures, and enrollment across inpatient and outpatient services of privately insured individuals. To date, this database includes private sector health data from 350 payors and contains over 20 billion claims for over 148 million unique individuals. It captures all inpatient and outpatient billing claims for individuals and dependents covered by the participating payors. Diagnoses and procedures were captured using International Classification of Diseases, 9th edition, Clinical Modification (ICD-9-CM) codes and Current Procedural Terminology (CPT) codes, respectively.
All patients who underwent gastric bypass (CPT 43644, 43645, 43846, and 43847), laparoscopic vertical sleeve gastrectomy (43775), laparoscopic adjustable gastric banding (43770), vertical banded gastroplasty (VBG, 43842), or biliopancreatic diversion with duodenal switch (BPD/DS, 43845) between June 1, 2000, and December 31, 2014, were eligible for inclusion. Gastric bypass surgeries were also stratified into open (43846 and 43847) and laparoscopic (43644 and 43645) procedures. Patients were required to have at least 180 days of continuous coverage (with 8-day grace periods) prior to their procedure; this window was used to capture patient comorbidities. Patients who had procedure codes for more than one bariatric procedure were excluded (n = 1164). Patients were censored 12 months after their surgery, when they unenrolled from their healthcare plan, or on September 31, 2015 (when ICD-10-CM codes were implemented), whichever came first. Patient complications were identified using ICD-9-CM diagnosis codes and CPT procedure codes, where appropriate. The full list of complications and codes is shown in Table 1. Outpatient visit dates and inpatient admission dates were used to estimate the date of each complication. Due to their acute nature, only the 1-month incidences of acute myocardial infarction (MI)/angina, stroke/transient ischemic attack (TIA), pneumonia, acute kidney failure, and sepsis were estimated and compared. Additionally, the incidence of revision or removal was only estimated in patients undergoing adjustable gastric banding.
Statistical Analyses
Patient characteristics, stratified by procedure type, were described using univariate analyses. The Charlson score was calculated using the methodology described by Deyo et al.12 Trends in the type of bariatric procedures used over time were assessed using Poisson regression.
Inverse probability of treatment (IPT)-weighted Kaplan-Meier curves were used to estimate the stratified 1-month, 6-month, and 12-month cumulative incidences of complications for laparoscopic adjustable gastric banding, laparoscopic sleeve gastrectomy, and laparoscopic gastric bypass surgery, among patients treated between 2005 and 2015.13 Briefly, the propensity or probability of undergoing adjustable gastric banding, sleeve gastrectomy, and gastric bypass was estimated using generalized logistic regression, using year of surgery, patient age (modeled as a restricted cubic spline), sex, each of the Charlson score components (excluding human immunodeficiency virus [HIV]), and whether they had an inpatient or outpatient procedure. IPT weights were stabilized using the marginal (i.e., overall) probability of undergoing each procedure in the cohort and truncated at the 1st and 99th percentiles.
The effect of laparoscopic adjustable gastric banding, compared to laparoscopic gastric bypass, among patients undergoing surgery between 2006 and 2015, was estimated using IPT-weighted Cox proportional hazards regression. Additionally, the effect of laparoscopic sleeve gastrectomy, compared to laparoscopic gastric bypass, among patients undergoing surgery between 2010 and 2015, was estimated using similar methods. In order to account for the weighting, robust sandwich estimators were used to calculate the 95% confidence intervals. Separate IPT weights were calculated for each of the subset analyses using the relevant sub-cohort. All analyses were performed using SAS 9.3 (SAS Inc., Cary, NC).
Results
256,830 individuals met search criteria and were included (Table 2). 162,528 (63%) had complete 12-month follow-up (median follow-up time 365 days, interquartile range [IQR] 222–365). The median age was 44 years (IQR 36–52) and the majority of patients were female (n = 202,183, 79%). Patients with diabetes were more likely to undergo gastric bypass or biliopancreatic diversion with duodenal switch (36% vs. 28%, p < 0.0001). Patients undergoing sleeve gastrectomy and adjustable gastric banding were more likely to have outpatient procedures (54% vs. 12%, p < 0.0001), and when they were admitted as inpatients, their average length of stay (LOS) was shorter (mean LOS 1.6 days vs. 2.5 days, p < 0.0001). All other patient characteristics were relatively consistent across bariatric procedures.
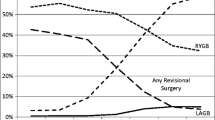
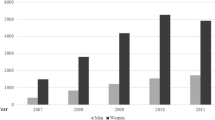
From 2000 to 2015, 92,100 (36%) individuals underwent laparoscopic Roux-en-Y gastric bypass, 68,918 (27%) laparoscopic sleeve gastrectomy, and 69,440 (27%) laparoscopic adjustable gastric banding (Table 2). The remainder underwent open gastric bypass (9%), vertical banded gastroplasty (0.4%), and biliopancreatic diversion with duodenal switch (0.5%). At the beginning of this period in 2000, more than 90% underwent open gastric bypass while the remainder underwent vertical banded gastroplasty (Fig. 1). By 2005, the proportion of open gastric bypass (40%) had substantially decreased in lieu of laparoscopic bypass (58%). While this transition does reflect the growing trend of laparoscopic surgery during this time, the CPT code for LRYGB was not introduced until 2005, which can partly account for the drastic change in the numbers of open versus laparoscopic gastric bypass procedures from 2000 to 2005. Around this same time, laparoscopic adjustable gastric banding was introduced and from 2008 to 2010 the number of procedures exceeded that of laparoscopic gastric bypasses. Laparoscopic sleeve gastrectomy emerged in 2010, and by 2012 had become the most popular bariatric procedure. The proportion of LSG has continued to increase annually, while those of LRYGB and LAGB have decreased. During this time period, vertical banded gastroplasty and biliopancreatic diversion with duodenal switch made up a very small percentage of all bariatric procedures.
The cumulative 1-month, 6-month, and 12-month incidences of complications after laparoscopic gastric bypass, laparoscopic adjustable gastric banding, and laparoscopic sleeve gastrectomy, respectively, can be found in Table 3. Additionally, we compared the incidences of complications of laparoscopic gastric bypass to each of the two procedures that have emerged during different periods of time, namely laparoscopic adjustable gastric banding and laparoscopic sleeve gastrectomy. We compared laparoscopic gastric bypass versus laparoscopic adjustable gastric banding over the years 2006 to 2015, and laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy over the years 2010 to 2015 (Tables 4 and 5). After standardization, patients who underwent LSG and LAGB were overall less likely to have postoperative complications compared to those who had LRYGB.
Patients undergoing laparoscopic adjustable gastric banding, compared to laparoscopic gastric bypass patients, had significantly lower incidences of 1-year all-cause readmission (hazard ratio [HR] 0.47, 95% confidence interval [CI] 0.45, 0.48, p < 0.0001), 30-day all-cause readmission (HR 0.28, 95% CI 0.26, 0.29, p < 0.0001), emergency room visits (HR 0.68, 95% CI 0.66, 0.69, p < 0.0001), leak (HR 0.54, 95% CI 0.41, 0.70, p < 0.0001), stroke/transient ischemic attack (TIA) (HR 0.74, 95% CI 0.56, 0.98, p < 0.04), pulmonary embolism (PE) (HR 0.64, 95% CI 0.57, 0.73, p < 0.0001), deep vein thrombosis (DVT) (HR 0.64, CI 95% 0.63, 0.73, p < 0.0001), 30-day pneumonia (HR 0.47, 95% CI 0.42, 0.53, p < 0.0001), dehydration (HR 0.36, 95% CI 0.35, 0.38, p < 0.0001), gastrointestinal hemorrhage (HR 0.31, 95% CI 0.29, 0.34, p < 0.0001), nausea/vomiting (HR 0.66, 95% CI 0.64, 0.68, p < 0.0001), gallbladder disorders (HR 0.34, 95% CI 0.32, 0.36, p < 0.0001), malabsorption (HR 0.15, 95% CI 0.15, 0.16, p < 0.0001), and vitamin deficiencies (HR 0.32, 95% CI 0.32, 0.34, p < 0.0001) (Table 4). However, adjustable gastric banding patients showed a significantly higher incidence of heartburn (HR 1.73, 95% CI 1.57, 1.91, p < 0.0001) and dysphagia (HR 1.42, 95% CI 1.35, 1.47, p < 0.0001). No differences were seen in the incidences of acute myocardial infarction (MI)/angina or portal vein thrombosis (PVT). By 1 year, 1% of adjustable gastric banding patients had undergone a band removal or revision.
Patients who underwent laparoscopic sleeve gastrectomy, compared to laparoscopic gastric bypass patients, had significantly lower incidences of 1-year all-cause readmission (HR 0.69, 95% CI 0.66, 0.71, p < 0.0001), 30-day all-cause readmission (HR 0.73, 95% CI 0.69, 0.77, p < 0.0001), emergency room visits (HR 0.74, 95% CI 0.72, 0.75, p < 0.0001), acute MI/angina (HR 0.81, 95% CI 0.67, 0.87, p < 0.02), PE (HR 0.86, 95% CI 0.76, 0.97, p < 0.02), DVT (HR 0.92, 95% CI 0.85, 0.99, p < 0.02), 30-day pneumonia (HR 0.70, 95% CI 0.62, 0.79, p < 0.0001), dehydration (HR 0.78, 95% CI 0.76, 0.81, p < 0.0001), gallbladder disorders (HR 0.75, 95% CI 0.72, 0.78, p < 0.0001), gastrointestinal hemorrhage (HR 0.45, 95% CI 0.41, 0.48, p < 0.0001), nausea/vomiting (HR 0.75, 95% CI 0.75, 0.79, p < 0.0001), dysphagia (HR 0.64, 95% CI 0.61, 0.67, p < 0.0001), malabsorption (HR 0.51, 95% CI 0.49, 0.52, p < 0.0001), and vitamin deficiencies (HR 0.82, 95% CI 0.81, 0.84, p < 0.0001) (Table 5). LSG was associated with a higher incidence of portal vein thrombosis (HR 4.07, 95% CI 2.62, 6.33, p < 0.0001), heartburn (HR 1.61, 95% CI 1.47, 1.78, p < 0.0001), gastritis (HR 4.67, 95% CI 4.49, 4.87, p < 0.0001), and esophageal procedures (HR 1.22, 95% CI 1.19, 1.24, p < 0.0001). No differences were seen in leak or stroke/TIA.
Discussion
In this study, we evaluated the complication rates of laparoscopic Roux-en-Y gastric bypass and compared them with those of laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding, two procedures which arose as alternatives to bypass. Overall, we found that laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding have lower postoperative complication rates than laparoscopic gastric bypass, although there were specific complications that were higher in the LSG and LAGB groups.
We also found that between 2000 and 2015, laparoscopic sleeve gastrectomy has become the most commonly performed bariatric procedure. As the number of LSG has continued to increase annually, the proportions of both LRYGB and LAGB have correspondingly decreased. The most recent report from the American Society for Metabolic and Bariatric Surgery (ASMBS) published in June 2018 demonstrated similar findings with the percentage of LSG increasing yearly from 2011 to 2017 while the percentages of LRYGB and LAGB have decreased.14 Laparoscopic sleeve gastrectomy, which initially emerged as the first step in biliopancreatic diversion with duodenal switch,15, 16 has established itself as an effective and durable stand-alone bariatric operation. It has been proven to be a viable alternative to Roux-en-Y gastric bypass, the longtime gold standard in bariatric surgery. With the changing trends in bariatric surgery, it is important to continuously assess the comparative outcomes and complications of the available procedures.
Prior research has shown that laparoscopic adjustable gastric banding has lower rates of complications compared to laparoscopic gastric bypass.7, 17 Our analysis demonstrated similar findings, with most of the complications evaluated having significantly lower incidences following LAGB. In addition, we found significantly higher 12-month incidences of heartburn and dysphagia after LAGB compared to LRYGB. These results have also been described in the literature.18, 19 The overall incidences of these complications vary from study to study. This is likely due to different lengths of follow-up and different definitions of complications.
Many studies evaluating LAGB have concluded that the procedure has lower incidences of early (≤ 30 day) complications, but a higher incidence of late (> 30 day) complications.8, 19 Many of the long-term complications associated with adjustable gastric banding are related to mechanical issues with the band such as slippage, erosion, and port problems.18 Although our study did not assess for these complications, we did find that at 12 months, 1% of patients had undergone band removal or revision.
Laparoscopic sleeve gastrectomy’s initial attractiveness derived from data demonstrating its lower morbidity profile relative to laparoscopic gastric bypass. While our data confirmed an overall lower 1-year incidence of complications after LSG, we found significantly higher occurrences of heartburn and portal vein thrombosis within 1 year of this procedure. These are two notable associations that need further study given the rapid increase in utilization of sleeve gastrectomy.
The incidence of heartburn following LSG is somewhat controversial. Rebecchi et al. performed a prospective study evaluating gastroesophageal reflux disease (GERD) in 65 patients after sleeve gastrectomy.20 The authors used 24-hour pH data and concluded that pre-existing reflux improves in most patients following sleeve gastrectomy. Stenard et al. published a review comparing 13 studies that demonstrated an adverse impact of sleeve gastrectomy on GERD and 12 studies showing a favorable effect of sleeve gastrectomy on GERD, reflecting the disagreement of the topic.21 A recent multicenter randomized controlled trial of 240 patients assessed the incidence of GERD following sleeve gastrectomy and gastric bypass.3 The authors found 5.8% of patients in the sleeve gastrectomy group had GERD after 30 days and seven patients ultimately underwent conversion to Roux-en-Y gastric bypass due to GERD. In our analysis, we also found laparoscopic sleeve gastrectomy was associated with a significantly higher 1-year incidence of heartburn compared to laparoscopic gastric bypass, with 2% of patients having a diagnosis of heartburn within 1 year of surgery.
Portal vein thrombosis (PVT) appears to be a unique complication of laparoscopic sleeve gastrectomy, as it has not been demonstrated after gastric bypass. While the 1-year cumulative incidence of PVT after sleeve gastrectomy was low (1%), this was significantly higher than the incidence after gastric bypass, and it is a potentially devastating complication. There are few studies in the literature regarding PVT after bariatric surgery. Goitein et al. published a retrospective, multicenter study to evaluate the incidence of portomesenteric vein thrombosis in patients undergoing laparoscopic bariatric surgery.22 This group found 17 of 5706 patients (0.3%) suffered portomesenteric vein thrombosis after surgery: 16 after sleeve gastrectomy and 1 after adjustable gastric banding. Our larger analysis showed similar results, with a 0.2% 1-year cumulative incidence after sleeve gastrectomy, and < 0.1% 1-year incidence after both gastric bypass and adjustable gastric banding.
Interestingly, we also found that following LSG, patients had a significantly higher incidence of undergoing esophageal procedures. For the purposes of this study, we did not assess the specific reasons for needing a procedure or the details regarding the type of intervention performed. Studies looking at endoscopic management of complications following sleeve gastrectomy describe various interventions for staple line disruption or leak,23,24,25,26,27 stenosis of the sleeve,28, 29 and intraluminal bleeding.30 These are similar to reasons for LRYGB patients requiring endoscopic therapies;31, 32 thus, it is notable that sleeve gastrectomy patients showed a higher 1-year incidence of esophageal procedures relative to gastric bypass patients, although the significance is still unclear.
Both laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding have been popular alternatives to laparoscopic gastric bypass due to their technical ease and lower morbidity. However, LAGB did not demonstrate sufficient weight loss efficacy and concomitant resolution of obesity-related comorbidities to maintain its position as a viable substitute to LRYGB and is now rarely performed. However, to date, LSG has shown comparable outcomes relative to LRYGB, and therefore has seen dramatically increased utilization because of its relative technical ease and lower complication rates.
This study is one of the largest and most comprehensive analyses of complications after bariatric surgery, to date. Much of the current literature regarding bariatric surgery complication rates is limited to analysis of specific complications or relatively short follow-up periods (e.g., 30 days), or only captures occurrences at the primary treatment hospital. Because of the nature of the MarketScan® claims database, we were able to assess a wide range of complications using diagnosis and procedure codes, follow patients for a full year, and have both inpatient and outpatient records available. Our results confirm many of the findings present in the literature regarding the incidences of complications when comparing LRYGB to LSG and LAGB, as well as highlighting the specific types of complications associated with each individual bariatric procedure. Additionally, due to the large sample size, we were able to evaluate the incidences of minor complications or side effects (e.g., dehydration, nausea/vomiting, difficulty swallowing), as well as rare complications (e.g., portal vein thrombosis). LSG and LAGB had overall lower incidences of major and minor complications compared to LRYGB; however, heartburn and portal vein thrombosis demonstrated higher incidences following LSG, and heartburn and dysphagia were higher following LAGB. This information is critical when it comes to helping educate patients and better prepare them for bariatric surgery.
That being said, this study does have significant limitations. It was performed using an employee-sponsored, private insurance database; thus, results may not generalize to those with Medicare or Medicaid or the uninsured. Additionally, we would miss any complications in which the patient did not seek treatment or the diagnosis was not adequately recorded in the inpatient or outpatient record. While treatment was not randomized, we did attempt to remove confounding by standardizing treatment across year of surgery, patient demographics, comorbidities, and inpatient/outpatient procedure status. However, body mass index (BMI) was not captured using the Truven® database and it may influence a surgeon’s decision-making. Also, higher BMI is associated with increased rates of postoperative mortality.33 Finally, we were unable to obtain complete 1-year follow-up for all patients although the median follow-up time was 1 year.
Conclusions
The field of bariatric surgery continues to change. Although Roux-en-Y gastric bypass remains the “gold standard” for bariatric surgery, newer procedures are continuously being developed to reduce technical complexity and complication rates. While laparoscopic adjustable gastric banding has lost popularity, the use of laparoscopic sleeve gastrectomy has increased dramatically. Given the rise of sleeve gastrectomy relative to gastric bypass in recent years, it is reassuring to confirm that the overall complications of sleeve gastrectomy are lower, with the notable exceptions of heartburn and portal vein thrombosis. Although laparoscopic sleeve gastrectomy has established itself as a safe and effective alternative to laparoscopic gastric bypass, the understanding of higher incidences of certain postoperative complications may help inform patients and surgeons when choosing the optimal bariatric procedure.
References
Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011-2014. National Center for Health Statistics (NCHS) Data Brief. Centers for Disease Control and Prevention. No. 219, November 2015.
English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surgery for Obesity and Related Diseases. 14 (2018) 259–263.
Salminen P, Helmio M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, Hurme S, Soinio M, Nuutila P, Victorzon M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients with Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. The Journal of the American Medical Association. 2018;319(3):241–254.
Peterli R, Borbely Y, Kern B, Gass M, Peters T, Thurnheer M, Schultes B, Laederach K, Bueter M, Schiesser M. Early Results of the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS): A Prospective Randomized Trial Comparing Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass. Annals of Surgery. 2013;258(5):690–695.
Arterburn D, Gupta A. Comparing the Outcomes of Sleeve Gastrectomy and Roux-en-Y Gastric Bypass for Severe Obesity. The Journal of the American Medical Association. 2018;319(3):235–237.
Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term Follow-up After Bariatric Surgery: A Systematic Review. The Journal of the American Medical Association. 2014;312(9):934–942.
Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, Krause KR, Schram JL, Kole KL, Finks JF, Birkmeyer JD, Share D, Birkmeyer NJO. The Comparative Effectiveness of Sleeve Gastrectomy, Gastric Bypass, and Adjustable Gastric Banding Procedures for the Treatment of Morbid Obesity. Annals of Surgery. 2013;257(5):791–797.
Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric Banding or Bypass? A Systematic Review Comparing the Two Most Popular Bariatric Procedures. The American Journal of Medicine. 2008;121(10):885–893.
Morton J, Sherif B, Winegar D, Ngyuen N, Ponce J, Blackstone R. National Comparisons of Bariatric Surgery Safety and Efficacy: Findings from the BOLD Database 2007-2010. American Society for Metabolic & Bariatric Surgery.
Chang SH, Freeman NLB, Lee JA, Stoll CRT, Calhoun AJ, Eagon JC, Colditz GA. Early Major Complications after Bariatric Surgery in the USA, 2003-2014: A Systematic Review and Meta-Analysis. Obesity Reviews. 2018;19(4):529–537.
Chen SY, Stem M, Schweitzer MA, Magnuson TH, Lidor AO. Assessment of postdischarge complications after bariatric surgery: A National Surgical Quality Improvement Program analysis. Surgery. 2015;158(3):777–786.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology. 1992;45(6):613–619.
Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Computer Methods and Programs in Biomedicine. 2004,75(1):45–49.
American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011–2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. 2018.
Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obesity Surgery. 1998; 8: 267–282.
Marceau P, Biron S, Bourque RA, Potvin M, Hould FS, Simard S. Biliopancreatic Diversion with a New Type of Gastrectomy. Obesity Surgery. 1993;3(1):29–35.
Lancaster RT, Hutter MM. Bands and bypasses: 30-day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi-center, risk-adjusted ACS-NSQIP data. Surgical Endoscopy. 2008;22:2554–2563.
Kodner C, Hartmand DR. Complications of Adjustable Gastric Banding Surgery for Obesity. American Family Physician. 2014;89(10):813–818.
Dogan K, Gadiot RPM, Aarts EO, Betzel B, van Laarhoven CJHM, Biter LU, Mannaerts GHH, Aufenacker TJ, Janssen IMC, Berends FJ. Effectiveness and Safety of Sleeve Gastrectomy, Gastric Bypass, and Adjustable Gastric Banding in Morbidly Obese Patients: a Multicenter, Retrospective, Matched Cohort Study. Obesity Surgery. 2015;25:1110–1118.
Rebecchi F, Allaix ME, Giaccone C, Ugliono E, Scozzari G, Morino M. Gastroesophageal Reflux Disease and Laparoscopic Sleeve Gastrectomy A Physiopathologic Evaluation. Annals of Surgery. 2014;260(5):909–915.
Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World Journal of Gastroenterology. 2015;21(36):10348–10357.
Goitein D, Raziel A, Szold A, Sakran N. Assessment of perioperative complications following primary bariatric surgery according to the Clavien-Dindo classification: comparison of sleeve gastrectomy and Roux-en-Y gastric bypass. Surgical Endoscopy. 2016;30:273–278.
Nimeri A, Ibrahim M, Maasher A, Al Hadad M. Management Algorithm for Leaks Following Laparoscopic Sleeve Gastrectomy. Obesity Surgery. 2016;26(1):21–25.
Souto-Rodríguez R, Alvarez-Sánchez MV. Endoluminal solutions to bariatric surgery complications: A review with a focus on technical aspects and results. World Journal of Gastrointestinal Endoscopy. 2017;9(3):105.
Shoar S, Poliakin L, Khorgami Z, Rubenstein R, El-Matbouly M, Levin JL, Saber AA. Efficacy and Safety of the Over-the-Scope Clip (OTSC) System in the Management of Leak and Fistula After Laparoscopic Sleeve Gastrectomy: A Systematic Review. Obesity Surgery. 2017;27:2410–2418.
Smallwood NR, Fleshman JW, Leeds SG, Burdick JS. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surgical Endoscopy. 2016;30(6):2473–80.
Papavramidis ST, Eleftheriadis EE, Papavramidis TS, Kotzampassi KE, Gamvros OG. Endoscopic management of gastrocutaneous fistula after bariatric surgery by using a fibrin sealant. Gastrointestinal Endoscopy. 2004;59(2):296–300.
Parikh A, Alley JB, Petersen RM, Harnisch MC, Pfluke JM, Tapper DM, Fenton SJ. Management Options for Symptomatic Stenosis After Laparoscopic Sleeve Gastrectomy in the Morbidly Obeses. Surgical Endoscopy 2012;(26):738–746.
Shnell M, Fishman S, Eldar S, Goiten D, Santo E. Balloon Dilatation for Symptomatic Gastric Sleeve Stricture. Gastrointestinal Endoscopy. 2014;73:521–524.
Campanile FC, Boru CE, Rizzello M, Puzziello A, Copaescu C, Cavallaro G, Silecchia G. Acute Complications after Laparoscopic Bariatric Procedures: Update for the General Surgeon. Langenbeck’s Archives of Surgery. 2013;398(5):669–686.
Boules M, Chang J, Haskins IN, Sharma G, Froylich D, El-Hayek K, Rodriguez J, Kroh M. Endoscopic management of post-bariatric surgery complications. World Journal of Gastrointestinal Endoscopy. 2016;8(17):591–599.
Walsh, C, Karmali S. Endoscopic management of bariatric complications: A review and update. World Journal of Gastrointestinal Endoscopy. 2015;7(5):518–523.
Sakran N, Sherf-Dagan S, Blumenfeld O, Romano-Zelekha O, Raziel A, Keren D, Raz I, Hershko D, Gralnek IM, Shohat T, Goitein D. Incidence and Risk Factors for Mortality Following Bariatric Surgery: a Nationwide Registry Study. Obesity Surgery. 2018 [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Oral Presentation: Digestive Diseases Week®, June 4, 2018, Washington D.C.
Rights and permissions
About this article
Cite this article
Chung, A.Y., Strassle, P.D., Schlottmann, F. et al. Trends in Utilization and Relative Complication Rates of Bariatric Procedures. J Gastrointest Surg 23, 1362–1372 (2019). https://doi.org/10.1007/s11605-018-3951-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-3951-2