Abstract
Background
Decision-making on invasive intervention in patients with clinical signs of infected necrotizing pancreatitis is often related to the presence of gas configurations and the degree of encapsulation in necrotic collections on imaging. Data on the natural history of gas configurations and encapsulation in necrotizing pancreatitis are, however, lacking.
Methods
A post hoc analysis was performed of a previously described prospective cohort in 21 Dutch hospitals (2004–2008). All computed tomography scans (CTs) performed during hospitalization for necrotizing pancreatitis were categorized per week (1 to 8, and thereafter) and re-assessed by an abdominal radiologist.
Results
A total of 639 patients with necrotizing pancreatitis were included, with median four (IQR 2–7) CTs per patient. The incidence of first onset of gas configurations varied per week without a linear correlation: 2–3–13–11–10–19–12–21–12%, respectively. Overall, gas configurations were found in 113/639 (18%) patients and in 113/202 (56%) patients with infected necrosis. The incidence of walled-off necrosis increased per week: 0–3–12–39–62–76–93–97–100% for weeks 1–8 and thereafter respectively. Clinically relevant walled-off necrosis (largely or fully encapsulated necrotic collections) was seen in 162/379 (43%) patients within the first 3 weeks.
Conclusions
Gas configurations occur in every phase of the disease and develop in half of the patients with infected necrotizing pancreatitis. Opposed to traditional views, clinically relevant walled-off necrosis occurs frequently within the first 3 weeks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis is the most common gastrointestinal disorder requiring hospital admission in the USA and its incidence is rising.1 Necrotizing pancreatitis, defined as necrosis of the pancreatic parenchyma and/or extrapancreatic fat tissue, occurs in around 20% of patients.2,3 Associated collections in necrotizing pancreatitis (necrotic collections) are either called “acute necrotic collections” (not fully encapsulated) or “walled-off necrosis” (fully encapsulated).4 In case of infected necrosis, an invasive intervention is nearly always needed.5,6 Current guidelines advise a step-up approach in patients with infected necrosis, starting with catheter drainage. If the patient does not recover with drainage alone, minimally invasive necrosectomy is performed.5,6 Although overall outcome has improved over the last decade, mortality and morbidity in these patients are still 15 and 40%, respectively.7
Decision-making on invasive intervention is influenced by clinical, biochemical, and imaging features, primarily on computed tomography (CT). Two CT features stand out in the decision-making process. First, the presence of gas configurations within necrotic collections is deemed important as this is regarded pathognomonic for infected necrosis. Second, the degree of encapsulation of necrotic collections is relevant because drainage is typically postponed until necrotic collections are largely or fully encapsulated. The timing of invasive intervention in patients with infected necrotizing pancreatitis, however, remains a topic of debate.8
It is often assumed that gas configurations occur most often between the second and fourth week and that full encapsulation of necrotic collections occurs at least 4 weeks after symptom onset. Accurate data supporting these statements are, however, lacking.4 Improved knowledge about the natural course of necrotic collections might support decision-making on the timing of invasive intervention. Moreover, it can add to the interpretation and further standardization of clinical research in necrotizing pancreatitis.
The main purpose of this study was to evaluate the natural history of gas configurations in and encapsulation of necrotic collections on CT during the disease course of necrotizing pancreatitis. In addition, clinical and radiological factors associated with occurrence of gas and (early) encapsulation in necrotic collections were studied.
Methods
Study Design and Patients
This study is a post hoc analysis of a prospective cohort of patients with necrotizing pancreatitis, collected from 2004 to 2008 in 21 Dutch hospitals of the Dutch Pancreatitis Study Group.9 All contrast-enhanced CTs performed during the index admission and before any kind of invasive (surgical, endoscopic, or percutaneous) intervention were re-assessed by an experienced abdominal radiologist (TLB). Patients with at least one CT confirming the diagnosis of necrotizing pancreatitis were included. Follow-up CTs were performed in case of a lack of clinical improvement according to current standard practice. CTs after any intervention were excluded. CTs were collected from all participating hospitals (including referral hospitals in transferred patients). Different brands of CT scanners were used and CT protocols varied widely among hospitals, varying from a monophasic to four-phasic CT protocol. All CTs, however, were executed with a multislice technique (at least a 16-slice multidetector CT scanner or higher) with 3-mm reconstructions and were contrast-enhanced in the pancreatic and/or portal venous phase. Also, in most cases, reformatted images were available for review. Non-invasive treatment consisted of intravenous fluid therapy, oral or enteral feeding, and adequate pain management. Invasive interventions were performed in cases of (suspected) infected necrosis based on gas configurations on CT, positive culture after fine needle aspiration, or clinical deterioration with no other cause than infected necrosis.10
Data Extraction
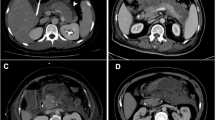
All CTs were categorized into groups according to duration since onset of disease, i.e., weeks 1 to 8, and further. If more than one CT was performed in a week, the last CT was used for assessment. In all CTs, the presence of first onset of gas configurations was evaluated (see Fig. 1a, b for CT examples). Gas configurations depicted on every follow-up CT in patients undergoing a non-invasive treatment were not scored in the incidence assessment. The degree of encapsulation was scored as none (0%), moderately (less than 50%), largely (between 50 and 99%), or fully (100%) encapsulated (see CT examples in Fig. 2a–d). Walled-off necrosis was defined according to the Revised Atlanta Classification as fully encapsulated necrotic collections.4 In clinical practice, however, invasive intervention is contemplated and deemed feasible when infected necrotic collections are largely or fully encapsulated. Hence, besides the original definition of walled-off necrosis, we also assessed a more clinically relevant definition of walled-off necrosis, defined as necrotic collections that are largely or fully encapsulated. In this line of reasoning, we defined “early walled-off necrosis” as largely or fully encapsulated collections occurring within 3 weeks after symptom onset, i.e., before the traditional 4 weeks mentioned in the Revised Atlanta Classification.4 The following clinical baseline data were available: age, sex, disease etiology, and American Society of Anesthesiologists (ASA) classification. Data on type and timing of intervention and clinical outcome in patients with suspected infected necrosis have been published previously.9,10
Statistical Analysis
Data were analyzed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Outcomes were reported as absolute numbers and percentages for categorical variables. Continuous variables were summarized as either means with corresponding standard deviations (SD) or medians with interquartile ranges (IQR) depending on normality of distribution. Univariable logistic regression was performed to identify factors associated with the occurrence of gas configurations and for “early walled-off necrosis”. Factors associated in the univariable analysis (P < 0.1) were entered into a multivariable logistic regressions analysis (backward stepwise elimination method). A two-sided P value below 0.05 was considered statistically significant for all statistical tests.
Results
The study cohort consisted of 639 patients with necrotizing pancreatitis. Median age was 58 years (IQR 45–70) and 62% (398) of patients were male. A median of four (IQR 2–7, range 1–23) CTs was performed per patient (Table 1). The median CT severity index was 4 (IQR 4–8). In 324 (51%) patients, pancreatic parenchymal necrosis was present; in the remainder of 315 (49%) patients, there was extrapancreatic necrosis only. A median of three (IQR 2–5) necrotic collections were observed per patient, mostly centrally located (i.e., predominantly in the lesser sac and/or transverse mesocolon) or left-sided (i.e., predominantly at the left side of the retroperitoneum), in 235 (37%) and 188 (29%) of patients, respectively. Figure 3 depicts the frequency per location of the (extra)pancreatic necrotic collections.
Gas Configurations
In 18% of patients (113 of 639 patients) and in 56% of patients with proven infected necrosis (113 of 202 patients), gas configurations were seen at some point in time. Figure 4 shows the number of patients (in percentage) in whom first onset of gas configurations was seen on CT per week: w1, 2%; w2, 3%; w3, 13%; w4, 11%; w5, 10%; w6, 19%; w7, 12%; w8, 21%; and > w8, 12%. There was no linear correlation. In a multivariable analysis, age (P < 0.001), presence of pancreatic necrosis (P < 0.001), number of necrotic collections (P = 0.018), and left-sided collections (P < 0.001) were independently associated with the occurrence of gas configurations (see Table 2). As described previously, in 184 patients, infected necrosis was confirmed by culture taken at the first intervention.10 In 114 of these 184 patients (62%), the infection was monomicrobial, whereas in 70 patients (38%), two or more bacteria/fungi were cultured. Escherichia coli was most frequently found in patients with gas bubbles on CT (42 patients), whereas in patients without gas bubbles, Staphylococcus aureus was most frequently found (34 patients). No single microorganism was found to be solely responsible for the formation of gas bubbles in necrotic collections.
The degree of encapsulation and the presence of gas configurations in (extra)pancreatic necrotic collection per week. Only patients in whom CT was performed and (extra)pancreatic necrotic collections were seen (total n = 639 patients); first week n = 540 (85%); second week n = 329 (51%); third week n = 195 (31%); fourth week n = 142 (22%); fifth week n = 87 (14%); sixth week n = 59 (9%); seventh week n = 43 (7%); eighth week n = 34 (5%); beyond 8 weeks n = 138 (22%)
Encapsulation
Figure 4 shows the degree of encapsulation related to the number of patients. The incidence of fully encapsulated necrotic collections (walled-off necrosis according to the Revised Atlanta Classification) increased per week: w1, 0%; w2, 3%; w3, 12%; w4, 39%; w5, 62%; w6, 76%; w7, 93%; w8, 97%; and > w8, 100%. Likewise, the incidence of largely or fully encapsulated necrotic collections (i.e., clinically relevant walled-off necrosis) increased per week: w1, 1%; w2, 17%; w3, 61%; w4, 88%; w5, 100%; w6, 99%; w7, 100%; w8, 100%; and > w8, 100%. Early clinically relevant walled-off necrosis (i.e., within the first 3 weeks) was seen in 162 of 379 (43%) patients. Male sex (P = 0.035), pancreatic necrosis (P = 0.014), and the presence of gas configurations (P = 0.044) were independently associated with the occurrence of early clinically relevant walled-off necrosis in a multivariable analysis (see Table 3).
Discussion
This study provides novel information on the natural history of imaging features of first onset of gas configurations and encapsulation in necrotizing pancreatitis. The main findings are that first onset of gas configurations and walled-off necrosis occur in nearly every phase of the disease, well before as after 4 weeks of symptom onset. Although walled-off necrosis becomes more prevalent with time, over 40% of patients already develop clinically relevant walled-off necrosis within the first 3 weeks of disease.
Gas in necrotic collections is thought to be caused by gas-forming bacteria or loss of integrity of the gastrointestinal tract.5 Both are considered pathognomonic for infected necrosis. Infected necrosis is almost always an indication for invasive intervention since only a small subset (< 5%) of patients recover with antibiotic treatment only.9 Little is known about risk factors for gas configurations at imaging or the timing of its occurrence. According to our study, gas configurations are seen in every phase of the disease, i.e., very early as well as late in the disease course. Gas configurations were more often seen in patients with higher age, parenchymal necrosis, multiple collections, and left-sided collections. The association between these factors and gas configurations remains speculative. It is conceivable that the translocation of bacteria is facilitated in the elderly and in cases where necrotic collections are in direct contact over a longer segment of the intestine. This latter phenomenon may be more pronounced in patients with parenchymal necrosis (i.e., more extensive collections) and in those with multiple collections. Furthermore, the greater part of the pancreas is located left of the midline, which likely contributes to the preferential spread of necrotic collections to the left retroperitoneal compartment. More research on this topic is, however, required for verification of this association.
In the current study, different microorganisms were responsible for the occurrence of gas in necrotic collections, including Gram-positive and Gram-negative bacteria as well as yeasts. Also, in a significant number of patients, culture was polymicrobial. This justifies the institution of broad-spectrum antibiotics in patients with infected necrosis based on gas in necrotic collections, as routine narrowing of antibiotic treatment is not supported by our data.
The 2012 Revised Atlanta Classification classifies early pancreatic collections into acute peripancreatic fluid collections and acute necrotic collections that after 4 weeks develop into pancreatic pseudocysts and walled-off necrosis, respectively, when completely encapsulated.4 Necrotic collections may involve the pancreatic parenchyma and/or extrapancreatic tissues and are considered a different clinical entity with a worse clinical outcome as compared with interstitial edematous pancreatitis.4,11 Little is known about the natural history of imaging features of necrotic collections. Previous studies have evaluated the natural clinical history of (extra)pancreatic collections, but did not analyze their imaging characteristics or the timing of encapsulation.12,13,14,15 Some have studied the clinical course (i.e., resolution) of pancreatic fluid collections and risk factors associated with the presence of pancreatic collections,12,13 analyzed clinical and biochemical factors associated with formation of encapsulation (or “pseudocyst formation”),12,14 or evaluated resolution of necrotic collections in the later phase of a disease by means of endoscopic ultrasound or transabdominal ultrasonography (i.e., not by CT) at different time points (i.e., after 4–6 weeks up to 6 months).13,15
The pathophysiology and rate of encapsulation of necrotic collections are as of yet incompletely understood. It is generally assumed that in necrotizing pancreatitis, the premature release of activated pancreatic enzymes and resultant acinar cell injury incites an extensive local and systemic inflammatory response. Locally, this might be regarded as a natural defense mechanism in which the body attempts to contain the area of inflammation. Over time, a capsule of granulation tissue is formed at the periphery to separate the inflamed tissue from healthy tissue to mitigate further spread of toxic enzymes and thus to wall off necrotic collections. This natural process of walling off an inflammatory process is likely analogous to an abscess wall formation. It is often stated (but not studied) that the timing of encapsulation takes about 4 weeks and this timescale is incorporated by the Revised Atlanta Classification. In the current study, however, there was a wide temporal range in which necrotic collections eventually became walled-off. In 85% of patients, it took well over 4 weeks for necrotic collections to become completely walled-off, whereas in 3 and 12%, complete encapsulation was already noted during the second and third weeks, respectively. Moreover, early clinically relevant walled-off necrosis (within the first 3 weeks) occurred in 43% and was more seen in male patients, patients with parenchymal necrosis, and patients with gas configurations (i.e., parameters associated with poorer clinical outcome).16,17 The reason for the wide temporal range and observed associations with early encapsulation remains speculative. Possibly, the magnitude of inflammatory response incited locally together with immune-mediated and patient factors could result in necrotic collections becoming walled-off early or late. More research on this topic is, however, needed.
Our study has several limitations. First, for this study, CTs were assessed by one abdominal radiologist. Therefore, no interobserver agreement could be calculated. Since other studies show good agreement for the type of necrotic collection, presence of intraluminal gas in necrotic collections, and presence of a wall among experienced radiologists,18 we expect our results to be reproducible. Second, full blinding of CTs was unfortunately not feasible. The radiologist was aware of the date and the presence of prior CTs, but was blinded to date of symptom onset and the clinical course. Third, follow-up CTs were not routinely (for example, weekly) performed but rather on the discretion of treating physicians, often based on the change in a patient’s condition. This is in line with standard practice as routinely performing CT is not justifiable out of costs and radiation burden perspectives. Fourth, CTs were executed with varying CT protocols. All CTs, however, were performed with a multislice technique with 3-mm reconstructions and were contrast-enhanced in the pancreatic and/or portal venous phase. Also, in most cases, reformatted images were available for review. Therefore, we feel that the finding of gas within collections was easily visible whenever present. Fifth, we defined “clinically (relevant) walled-off necrosis” as necrotic collections that are largely or fully encapsulated. We feel that in clinical practice, the distinction between collections that are not or only moderately encapsulated is treated differently (non-invasive therapy) compared with those that are largely or fully encapsulated (invasive therapy possible). More data are needed to determine whether this definition more closely relates to clinical management than the original definition.
Results of this study could have therapeutic implications because the knowledge of gas configurations and early encapsulation might influence the timing to proceed to an earlier invasive intervention in a subset of patients with infected necrosis. Current international guidelines, however, advise to postpone invasive intervention for at least 4 weeks in patients with (suspected) infected necrotizing pancreatitis until walled-off necrosis is present because intervention is believed to be safer in walled-off necrosis (e.g., less bleeding).5,6 This advice is primarily based on studies in which patients underwent early primary open necrosectomy (within first 2 weeks) which was associated with worse outcome.19,20,21 Nowadays, standard treatment of infected necrosis is primary catheter drainage. At least 35–50% of patients do not need additional necrosectomy after catheter drainage and this is associated with a lower risk for complications.7,22 Since catheter drainage is the first step of treatment which does not require fully encapsulated necrotic collections, some suggest that early and proactive drainage could prevent clinical deterioration, improve outcome, and shorten hospital stay.23,24 This hypothesis is currently being studied in the Dutch multicenter randomized controlled POINTER trial (ISRCTN33682933). This study compares immediate catheter drainage with postponed catheter drainage in patients with proven or suspected infected necrotizing pancreatitis.
In conclusion, opposed to common views, gas configurations and walled-off necrosis are seen in every phase of the disease in patients with necrotizing pancreatitis. This may have therapeutic implications in a subset of patients with infected necrosis and early walled-off necrosis.
References
Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179-87e1-3
Beger HG, Rau B, Mayer J, et al. Natural course of acute pancreatitis. World J Surg 1997;21:130–135
Bradley EL III. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993;128:586–590
Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111
Working group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1–15
Tenner S, Baillie J, DeWitt J, et al; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400–1415
Van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491–1502
Van Grinsven J, van Brunschot S, Bakker OJ, et al. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study. HPB (Oxford) 2016;18:49–56
Van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011; 141:1254–1263
Van Baal MC, Bollen TL, Bakker OJ, et al. The role of routine fine-needle aspiration in the diagnosis of infected necrotizing pancreatitis. Surgery. 2014;155:442–448
Kwong WT, Ondrejková A, Vege SS. Predictors and outcomes of moderately severe acute pancreatitis - Evidence to reclassify. Pancreatology. 2016;16:940–945
Cui ML, Kim KH, Kim HG, et al. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci. 2014;59:1055–1062
Sarathi Patra P, Das K, Bhattacharyya A, et al. Natural resolution or intervention for fluid collections in acute severe pancreatitis. Br J Surg. 2014;101:1721–1728
Poornachandra KS, Bhasin DK, Nagi B, et al. Clinical, biochemical, and radiologic parameters at admission predicting formation of a pseudocyst in acute pancreatitis. J Clin Gastroenterol. 2011;45:159–163
Rana SS, Bhasin DK, Reddy YK, et al. Morphological features of fluid collections on endoscopic ultrasound in acute necrotizing pancreatitis: do they change over time? Ann Gastroenterol. 2014;27:258–261
Bakker OJ, van Santvoort H, Besselink MG, et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62:1475–1480
Hollemans RA, Bollen TL, van Brunschot S, et al. Predicting Success of Catheter Drainage in Infected Necrotizing Pancreatitis. Ann Surg. 2016;263:787–792
Bouwense SA, van Brunschot S, van Santvoort HC et al. Describing Peripancreatic Collections According to the Revised Atlanta Classification of Acute Pancreatitis: An International Interobserver Agreement Study. Pancreas. 2017;46:850–857
Fernández-del Castillo C, Rattner DW, Makary MA, et al. Debridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228:676–684
Besselink MG, Verwer TJ, Schoenmaeckers EJ, Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142:1194–1201
Mier J, León EL, Castillo A, et al. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173:71–75
Van Baal MC, van Santvoort HC, Bollen TL, et al. Dutch Pancreatitis Study Group. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg 2011;98:18–27
Van Grinsven J, Timmerman P, van Lienden KP, et al. Proactive Versus Standard Percutaneous Catheter Drainage for Infected Necrotizing Pancreatitis. Pancreas. 2017;46:518–523
Sugimoto M, Sonntag DP, Flint GS, et al. Better Outcomes if Percutaneous Drainage Is Used Early and Proactively in the Course of Necrotizing Pancreatitis. J Vasc Interv Radiol 2016;27:418–425
Funding
Parts of this research were funded by Fonds NutsOhra, the Netherlands, grant number 1404-044.
Author information
Authors and Affiliations
Consortia
Contributions
TLB designed the study protocol and evaluated the CTs. MGB and HCvS collected the clinical data. JvG, SvB, and MCvB collected and analyzed the data. JvG drafted and revised the manuscript. SvB, MCvB, MGB, PF, HvG, HCvS, and TLB critically edited the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
van Grinsven, J., van Brunschot, S., van Baal, M.C. et al. Natural History of Gas Configurations and Encapsulation in Necrotic Collections During Necrotizing Pancreatitis. J Gastrointest Surg 22, 1557–1564 (2018). https://doi.org/10.1007/s11605-018-3792-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-3792-z