Abstract
Background
Endoscopic sleeve gastroplasty (ESG) is a novel endobariatric procedure. Initial studies demonstrated an association of ESG with weight loss and improvement of obesity-related comorbidities. Our aim was to compare ESG to laparoscopic sleeve gastrectomy (LSG) and laparoscopic adjustable gastric banding (LAGB).
Methods
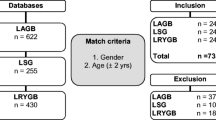
We included 278 obese (BMI > 30) patients who underwent ESG (n = 91), LSG (n = 120), or LAGB (n = 67) at our tertiary care academic center. Primary outcome was percent total body weight loss (%TBWL) at 3, 6, 9, and 12 months. Secondary outcome measures included adverse events (AE), length of stay (LOS), and readmission rate.
Results
At 12-month follow-up, LSG achieved the greatest %TBWL compared to LAGB and ESG (29.28 vs 13.30 vs 17.57%, respectively; p < 0.001). However, ESG had a significantly lower rate of morbidity when compared to LSG or LAGB (p = 0.01). The LOS was significantly less for ESG compared to LSG or LAGB (0.34 ± 0.73 vs 3.09 ± 1.47 vs 1.66 ± 3.07 days, respectively; p < 0.01). Readmission rates were not significantly different between the groups (p = 0.72).
Conclusion
Although LSG is the most effective option for weight loss, ESG is a safe and feasible endobariatric option associated with low morbidity and short LOS in select patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity constitutes a twenty-first century pandemic and public health concern with serious implications for the health and well-being of the population. In 2014, approximately 37% of the worldwide population was overweight (BMI > 25).1 In the USA, over one third of the adult population has a BMI over 30.2,3 Because of its increasing prevalence among adults, the impact of obesity on morbidity, mortality, and healthcare costs is profound.
Bariatric surgery is the most effective treatment for obesity. It is associated with successful long-term maintenance of weight loss and reduction in obesity-related morbidities, as well as improvement in quality of life of these patients.4,5,6,7 The armamentarium of surgical procedures to combat morbid obesity and related comorbidities has expanded. The laparoscopic sleeve gastrectomy (LSG) remains one of the most popular options, while laparoscopic adjustable gastric banding (LAGB) has decreased over time.8,9,10,11,12 Nonetheless, in spite of the efficacy of bariatric surgery in achieving weight loss and resolution of related comorbidities, only 1% of the patients eligible for these procedures select bariatric surgery as their treatment of choice.9,13,14
The increase in the number of patients with obesity has led to the development of innovative treatment strategies to address this disease. Endoscopic bariatric therapy represents a novel, minimally invasive approach to weight loss in patients with obesity that involves restrictive techniques.15,16,17 Endoscopic sleeve gastroplasty (ESG) is a trans-oral endoscopic gastric volume reduction technique that was first reported in 2008 and improved upon in 2013 with the ability to perform full-thickness sutures.18,19 Endoscopically placed sutures extend from the incisura to the greater curvature of the stomach and reduce the size of the stomach. Published experience demonstrates successful short-term weight loss, decrease biomarkers of diabetes, and improvement in hypertension and hypertriglyceridemia, with a favorable side effect profile.20,21,22
Despite initial encouraging results, ESG’s role in weight management remains unclear. While early studies showed safety and efficacy of ESG, there has been no studies comparing it to other minimally invasive surgical bariatric procedures. In this study, we aim to study the effectiveness of ESG and compare it to both the laparoscopic sleeve gastrectomy and the laparoscopic adjustable gastric banding.
Materials and Methods
We conducted a single-center, retrospective cohort study of obese patients who underwent ESG, LSG, or LABG. Consecutive ESG patients were included who had at least a 1 year follow-up between January 2011 and December 2016. The ESG patients were compared to a cohort of patients undergoing LSG from 2013 to 2014 as well as any patient who underwent a LABG over the period of study. All surgical patients were referred to our academic bariatric center of excellence for management of obesity. Indications for weight loss procedures were based on obesity parameters, with a BMI > 30 kg/m2, with previous failed attempts at medical weight loss measures.
Patients were evaluated for candidacy for ESG, LSG, or LABG. The LSG or LAGB was not covered by insurance in patients with a BMI < 40 without comorbidities or a BMI < 35 and comorbidities.23 ESG was contraindicated in patients with gastric lesions with bleeding potential (ulcers and gastritis), neoplastic findings, or family history of gastric cancer.24 Individuals with mental health disorders, coagulopathies, or other significant medical comorbidities precluding anesthesia were also excluded. Choice of weight loss procedure was ultimately made with the patient after consultation with their gastroenterologist or bariatric surgeon. Additional pre-procedural consultations with a primary care physician, cardiologist, endocrinologist, nutritionist, and psychiatrist were provided to ensure multidisciplinary care.
Patient demographics and medical comorbidities were obtained. Variables included age, gender, race, BMI, ASA class,25 diagnosis of hypertension, hyperlipidemia, diabetes, and serologic testing for hemoglobin A1C (HgbA1c). Presence of diabetes was defined as taking diabetes medication (other than metformin prescribed for weight loss) or a HgbA1c ≥ 6.5%.26 Hypertension was defined as systolic blood pressure of ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or taking an anti-hypertensive medication. Hyperlipidemia was defined as currently taking a lipid-lowering medication or an LDL ≥ 160 mg/dL or fasting triglycerides ≥ 200 mg/dL.27 Post procedure data included hospital length of stay, duration of follow-up, and percent total body weight loss (%TBWL). All adverse events and hospital readmission within 90 days of the weight loss procedure were recorded.
Endoscopic and Surgical Techniques
We used a standard technique as previously described.22 A double-channel therapeutic upper endoscope (GIF- 2TH180; Olympus, Center Valley, PA) was outfitted with a cap-based flexible endoscopic suturing system (OverStitch; Apollo Endosurgery, Austin, TX) to perform the procedure. The suturing device consists of a needle driver, a catheter-based suture anchor, and an actuating handle. Sutures were reloaded without endoscope removal. ESG was then created by using an interrupted Z pattern to invaginate the greater curvature of the stomach for formation of the sleeve. The helix device was used to capture the muscularis propria, allowing sequential full-thickness bites. A running stitch was used to oppose the anterior and posterior placement sites. The stitch was then tightened to approximate the opposing gastric walls, creating a full-thickness volume reduction plication. The suture was cut by using a cinch. A second layer of sutures was then placed over the length of the central sleeve in an interrupted stitch pattern to further reduce gastric volume and reinforce the sleeve. The end result of the procedure was a tubular reconfiguration of the gastric lumen. Lavage of the sleeve with topical gentamicin (80 mg in 60 mL normal saline) was performed to reduce risk of infection. The ESG was performed by one experienced endoscopist (RZS).
Laparoscopic Sleeve Gastrectomy
Our technique for laparoscopic sleeve gastrectomy has been previously published.28,29 The procedures were all performed using the same technique by one of the three surgeons skilled with this procedure (AP, GD, CA).
Briefly, the dissection begins 5 cm from the pylorus. The greater curvature of the stomach is completely mobilized. The hiatus of the esophagus is explored posteriorly and any hiatal hernia is repaired posteriorly if ≥ 3 cm. The sleeve is performed on a 40-fr bougie with sequential firings of the laparoscopic stapler. Buttress material is routinely used except for the first firing which is oversewn. An intraoperative dye leak test is routinely performed.
Laparoscopic Adjustable Gastric Banding
For the adjustable gastric banding, patients are placed in reverse Trendelenburg. The abdomen is accessed and pneumoperitoneum established. The lesser omentum is opened via pars flaccida. The angle of His is mobilized. A retro-gastric dissection is performed. The Lap-Band System (Inamed-Allergan, Santa Barbara-Carpinteria, CA) is introduced in this retrogastric tunnel. The band is plicated in place using gastro-gastric sutures. The subcutaneous port is fixed to the anterior fascia of the rectus. All patients had the Lap-Band System placed by one of the two surgeons (AP, GD).
Outcome Measures
Changes in BMI and %TBWL were measured during scheduled follow-up at 3, 6, 9, and 12 months post procedure. The primary outcome was change in BMI and %TBWL at 12 months post procedure. Secondary outcomes included post procedure hospital length of stay and post procedure adverse events, as well as hospital readmission within 90 days. Adverse events were classified according to modified Clavien-Dindo classification of surgical complications.30
Statistical Analysis
Descriptive statistics were calculated for all demographic and clinical variables and reported as median (range), mean ± standard deviation, or proportion where appropriate. Univariate analysis was performed using the χ 2 test and Fisher exact test for categorical variables and the Student t test, Wilcoxon test, or Mann-Whitney U test as required for continuous variables. All variables were tested for normality using the Shapiro-Wilk test. All statistical analysis was conducted using STATA 13.0 (StataCorp LP, College Station, TX). A p value of < 0.05 was considered significant.
Results
Patient Characteristics
We evaluated consecutive patients who have had bariatric procedures at our institution (n = 278) during a 1-year time period. Of those 278 patients, 120 patients had a LSG, 91 had an ESG, and 67 had a LAGB. All patients reached at least 6 months follow-up and were eligible for the study. The mean age was 42 ± 12 years (range 18–77), 76% were female, and the mean baseline BMI was 43.82 ± 0.50 kg/m2. On average, patients who had LSG had higher BMI than LAGB, and patients who had ESG had lower BMI (47.22 ± 7.84 for LSG, 38.61 ± 6.98 for ESG, and 44.98 ± 6.45 for LAGB; p < 0.001). Incidence of hypertension and hyperlipidemia were also higher in the surgical groups compared to the ESG group (p < 0.01) (Table 1).
Impact on Weight Loss
At 6-month follow-up, LSG achieved the greatest %TBWL compared to ESG and LABG (23.48 vs 14.37 vs 12.68%, respectively). At 12-month follow-up, the LSG achieved the greatest BMI decrease (Fig. 1) and %TBWL at 12 months compared to ESG and LABG (29.28 vs 17.57 vs 13.30%, respectively; p < 0.001) (Fig. 2).
In multivariable analysis when controlling for age, gender, and ASA class, LSG was the procedure associated with the most significant weight loss (p value < 0.001). This remained significant when we stratified with BMI > 40 kg/m2. However, when we stratified by BMI < 40 kg/m2, even after adjusting for age, gender, and ASA, there was no significant difference in %TBWL at 12 months when comparing the three different techniques: LSG with ESG or LAGB (p value = 0.21) (Table 2).
Length of Stay
Post-procedure length of stay was significantly less for ESG compared to LSG or LAGB (0.34 ± 0.73 vs 3.09 ± 1.47 vs 1.66 ± 3.07 days, respectively; p < 0.01) (Table 3).
Adverse Events
We noted a statistically significant difference in adverse event rates for ESG compared to LSG or LAGB (2.20 vs 9.17 vs 8.96%, p value < 0.05) (Table 3). In the ESG group, the patient developed a peri-gastric leak 11 days post procedure after eating a solid meal (despite being instructed to maintain a liquid diet for 2 weeks). The patient was managed non-operatively with a percutaneous drain. Another patient was re-admitted with a migraine and was discharged later.
In the LSG group, one patient developed a peri-gastric leak and was taken back to the operating room for drainage of an intra-abdominal abscess and later was readmitted for a gastroesophageal stricture requiring a stent. Another patient demonstrated a peri-gastric leak on upper GI series and was brought back to the operating room; however, no leak was identified. Another patient developed wound dehiscence with herniated viscera requiring reoperation for closure and later developed a serous leakage from the same wound necessitating its opening. One patient developed a pulmonary embolism requiring anticoagulation. One patient developed hypercarbic respiratory failure after a successful LSG, requiring re-intubation. One patient developed a prolonged post-operative ileus. One other patient developed a wound infection requiring antibiotics. One patient developed a urinary tract infection requiring antibiotics. Two patients had to be re-admitted for fluid resuscitation, and one patient was re-admitted to the hospital after 28 days with nausea and vomiting, had an EGD that showed bile reflux.
In the LAGB group, two patients had failure of contrast to pass during upper GI series, requiring re-operation for removal of the band. Two patients developed a pulmonary embolism after discharge, one required readmission for anticoagulation. One patient developed a wound infection requiring antibiotics, and one patient had to be admitted with abdominal pain, but was discharged after a negative workup (Table 4).
Readmissions
There was no statistically significant difference between all readmission events within 90 days between the three groups (LSG 4.17%, ESG 2.20%, and LAGB 2.99%, p value = 0.72) (Table 3).
Discussion
This study highlights the safety and efficacy of ESG in improving weight loss up to 12 months post-procedure. The ESG cohort had a 17.57% decrease in TBWL with a lower rate of post-procedural morbidity as compared to the LSG and LAGB. These results are consistent with previously published studies of ESG with 13–18% TBWL at 12 months.24 There has been increasing literature and experience supporting ESG as an effective nonsurgical approach to gastric remodeling by promoting satiety and impairing gastric emptying, altering potential gut metabolic and neurohormonal signaling vital in regulating weight loss.
Since the first study on ESG demonstrated the feasibility of creating an ESG in 2013, there has been much interest in obtaining long-term outcome data. However, despite the clinical experiences that have been published, the adverse events with ESG have been sparse. In contrast, and in large part attributable to the plethora of data on the surgical techniques for weight loss, the existing surgical literature describes various peri- and post-operative complications of bariatric surgery, ranging from wound infections to death. Our study found that ESG patients had significantly fewer adverse events as compared to those who underwent a LAGB or a LSG. There was only one endoluminal leak readmission out of the 91 patients who underwent an ESG. Not surprisingly, our study found that the average LOS with ESG is significantly less than that of LSG or LAGB groups, with the vast majority of patients going home the same day.
Similar to an algorithmic or stepwise approach to weight loss through promotion of diet and lifestyle modification, we propose that endoscopic therapies be considered in the “bariatric treatment gap” or a body mass index between 30 and 40. Patients with a BMI greater than 40 should be assessed for surgical options such as a LSG, given extensive data to support the greatest weight loss and decrease in weight-related comorbidities from surgery as compared to other therapies. However, for those patients with a BMI less than 40 who have failed an adequate trial of dietary changes, lifestyle modification, and pharmacologic therapy, endoscopic therapies, including intragastric balloon placement should be considered as part of the approach to weight loss. While engaging and activating patients to become active participants in their own care is vital, the advent of weight loss centers can help facilitate a multidisciplinary approach to obesity, individualizing treatment options to each and every patient.
Our study carries several inherent limitations. First, there is an obvious limitation related to the retrospective nature of the study. Second, the follow-up is limited to 12 months. While our study was strengthened by the three arms, there was no control group or randomization among the different cohorts. We found that weight loss in the ESG group was comparable to that from the LAGB cohort with fewer post-operated complications and a shorter inpatient length of stay.
Endoscopic sleeve gastroplasty is a viable and safe weight loss approach for those between a BMI of 30 to 40. Further prospective studies demonstrating long-term weight loss durability and effectiveness are needed.
Abbreviations
- ESG:
-
Endoscopic sleeve gastroplasty
- LSG:
-
Laparoscopic sleeve gastrectomy
- LAGB:
-
Laparoscopic gastric band
- DM:
-
Type II diabetes
- BMI:
-
Body mass index
- LOS:
-
Length of stay
- HgbA1c:
-
Hemoglobin A1C
- %TWBL:
-
Percent total body weight loss
- AE:
-
Adverse events
- RZS:
-
Reem Z. Sharaiha
- CA:
-
Cheguevara Afaneh
- GD:
-
Gregory F. Dakin
- AP:
-
Alfons Pomp
References
M. Ng, T. Fleming, M. Robinson, B. Thomson, N. Graetz, C. Margono, E. C. Mullany, S. Biryukov, C. Abbafati, S. F. Abera, J. P. Abraham, N. M. E. Abu-Rmeileh, T. Achoki, F. S. Albuhairan, Z. A. Alemu, R. Alfonso, M. K. Ali, R. Ali, N. A. Guzman, W. Ammar, P. Anwari, A. Banerjee, S. Barquera, S. Basu, D. A. Bennett, Z. Bhutta, J. Blore, N. Cabral, I. C. Nonato, J. C. Chang, R. Chowdhury, K. J. Courville, M. H. Criqui, D. K. Cundiff, K. C. Dabhadkar, L. Dandona, A. Davis, A. Dayama, S. D. Dharmaratne, E. L. Ding, A. M. Durrani, A. Esteghamati, F. Farzadfar, D. F. J. Fay, V. L. Feigin, A. Flaxman, M. H. Forouzanfar, A. Goto, M. A. Green, R. Gupta, N. Hafezi-Nejad, G. J. Hankey, H. C. Harewood, R. Havmoeller, S. Hay, L. Hernandez, A. Husseini, B. T. Idrisov, N. Ikeda, F. Islami, E. Jahangir, S. K. Jassal, S. H. Jee, M. Jeffreys, J. B. Jonas, E. K. Kabagambe, S. E. A. H. Khalifa, A. P. Kengne, Y. S. Khader, Y. H. Khang, D. Kim, R. W. Kimokoti, J. M. Kinge, Y. Kokubo, S. Kosen, G. Kwan, T. Lai, M. Leinsalu, Y. Li, X. Liang, S. Liu, G. Logroscino, P. A. Lotufo, Y. Lu, J. Ma, N. K. Mainoo, G. A. Mensah, T. R. Merriman, A. H. Mokdad, J. Moschandreas, M. Naghavi, A. Naheed, D. Nand, K. M. V. Narayan, E. L. Nelson, M. L. Neuhouser, M. I. Nisar, T. Ohkubo, S. O. Oti, A. Pedroza, D. Prabhakaran, N. Roy, U. Sampson, H. Seo, S. G. Sepanlou, K. Shibuya, R. Shiri, I. Shiue, G. M. Singh, J. A. Singh, V. Skirbekk, N. J. C. Stapelberg, L. Sturua, B. L. Sykes, M. Tobias, B. X. Tran, L. Trasande, H. Toyoshima, S. Van De Vijver, T. J. Vasankari, J. L. Veerman, G. Velasquez-Melendez, V. V. Vlassov, S. E. Vollset, T. Vos, C. Wang, X. Wang, E. Weiderpass, A. Werdecker, J. L. Wright, Y. C. Yang, H. Yatsuya, J. Yoon, S. J. Yoon, Y. Zhao, M. Zhou, S. Zhu, A. D. Lopez, C. J. L. Murray, and E. Gakidou, Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013, Lancet, vol. 384, no. 9945, pp. 766–781, 2014.
K. M. Flegal, M. D. Carroll, C. L. Ogden, and L. R. Curtin, Prevalence and trends in obesity among US adults, 1999-2008, JAMA, vol. 303, no. 3, pp. 235–241, 2010.
N. Health and N. Examination Survey, National Health and Nutrition Examination Survey Fact Sheet, 2016.
V. L. Gloy, M. Briel, D. L. Bhatt, S. R. Kashyap, P. R. Schauer, G. Mingrone, H. C. Bucher, and A. J. Nordmann, Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials, BMJ, vol. 347, 1, pp. f5934, 2013.
G. Mingrone, S. Panunzi, A. De Gaetano, C. Guidone, A. Iaconelli, G. Nanni, M. Castagneto, S. Bornstein, and F. Rubino, Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 Year follow-up of an open-label, single-centre, randomised controlled trial, Lancet, vol. 386, no. 9997, pp. 964–973, 2015.
G. Mingrone, S. Panunzi, A. De Gaetano, C. Guidone, A. Iaconelli, L. Leccesi, G. Nanni, A. Pomp, M. Castagneto, G. Ghirlanda, and F. Rubino, Bariatric surgery versus conventional medical therapy for type 2 diabetes, N. Engl. J. Med., vol. 366, no. 17, pp. 1577–1585, 2012.
N. Lindekilde, B. P. Gladstone, M. Lübeck, J. Nielsen, L. Clausen, W. Vach, and A. Jones, The impact of bariatric surgery on quality of life: a systematic review and meta-analysis., Obes. Rev., vol. 16, no. 8, pp. 639–51, 2015.
C. J. Neylan, U. Kannan, D. T. Dempsey, N. N. Williams, and K. R. Dumon, The Surgical Management of Obesity, Gastroenterology Clinics of North America, vol. 45, no. 4. pp. 689–703, 2016.
S.-H. Chang, C. R. T. Stoll, J. Song, J. E. Varela, C. J. Eagon, and G. A. Colditz, The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012., JAMA Surg., vol. 149, no. 3, pp. 275–87, 2014.
S. K. H. Wong, A. P. S. Kong, W. L. M. Mui, W. Y. So, B. Y. S. Tsung, P. Y. P. Yau, F. C. C. Chow, and E. K. W. Ng, Laparoscopic bariatric surgery: a five-year review., Hong Kong Med. J., vol. 15, no. 2, pp. 100–9, 2009.
J. L. Colquitt, K. Pickett, E. Loveman, and G. K. Frampton, Surgery for weight loss in adults, in Cochrane Database of Systematic Reviews, no. 8, J. L. Colquitt, Ed. Chichester, UK: John Wiley & Sons, Ltd, 2014, p. CD003641.
L. Sjöström, A.-K. Lindroos, M. Peltonen, J. Torgerson, C. Bouchard, B. Carlsson, S. Dahlgren, B. Larsson, K. Narbro, C. D. Sjöström, M. Sullivan, H. Wedel, and Swedish Obese Subjects Study Scientific Group, Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery., N. Engl. J. Med., vol. 351, no. 26, pp. 2683–93, 2004.
J. I. Mechanick, A. Youdim, D. B. Jones, W. T. Garvey, D. L. Hurley, M. M. Mcmahon, L. J. Heinberg, R. Kushner, T. D. Adams, S. Shikora, J. B. Dixon, and S. Brethauer, Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery, Surg. Obes. Relat. Dis., vol. 9, pp. 159–191, 2013.
L. M. Funk, S. Jolles, L. E. Fischer, and C. I. Voils, Patient and Referring Practitioner Characteristics Associated With the Likelihood of Undergoing Bariatric Surgery: A Systematic Review., JAMA Surg., vol. 53792, no. 10, pp. 999–1005, 2015.
B. K. Abu Dayyeh, S. A. Edmundowicz, S. Jonnalagadda, N. Kumar, M. Larsen, S. Sullivan, C. C. Thompson, and S. Banerjee, Endoscopic bariatric therapies, Gastrointest. Endosc., vol. 81, no. 5, pp. 1073–1086, 2015.
T. Kozlowski, K. Kozakiewicz, J. Dadan, and P. Mysliwiec, Innovative solutions in bariatric surgery., Gland Surg., vol. 5, no. 5, pp. 529–536, 2016.
N. Kumar, Weight loss endoscopy: Development, applications, and current status, World J. Gastroenterol., vol. 22, no. 31, p. 7069, 2016.
R. Fogel, J. De Fogel, Y. Bonilla, and R. De La Fuente, Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients, Gastrointest. Endosc., vol. 68, no. 1, pp. 51–58, 2008.
B. K. Abu Dayyeh, E. Rajan, and C. J. Gostout, Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity., Gastrointest. Endosc., vol. 78, no. 3, pp. 530–5, 2013.
R. Z. Sharaiha, P. Kedia, N. Kumta, E. M. DeFilippis, M. Gaidhane, A. Shukla, L. J. Aronne, and M. Kahaleh, Initial experience with endoscopic sleeve gastroplasty: technical success and reproducibility in the bariatric population, Endoscopy, vol. 47, no. 2, pp. 164–166, 2015.
G. Lopez-Nava, M. P. Galvão, I. Da Bautista-Castaño, A. Jimenez, T. De Grado, and J. P. Fernandez-Corbelle, Endoscopic sleeve gastroplasty for the treatment of obesity, Endoscopy, vol. 47, no. 5, pp. 449–452, 2015.
R. Z. Sharaiha, N. A. Kumta, M. Saumoy, A. P. Desai, A. M. Sarkisian, A. Benevenuto, A. Tyberg, R. Kumar, L. Igel, E. C. Verna, R. Schwartz, C. Frissora, A. Shukla, L. J. Aronne, and M. Kahaleh, Endoscopic Sleeve Gastroplasty Significantly Reduces Body Mass Index and Metabolic Complications in Obese Patients, Clin. Gastroenterol. Hepatol., vol. 15, no. 4, pp. 504–510, 2017.
Z. Hanipah and P. Schauer, Surgical Treatment of Obesity and Diabetes, Gastrointest. Endosc. Clin. N. Am., vol. 27, no. 2, pp. 191–211, 2017.
G. Lopez-Nava, M. Galvao, I. Bautista-Castaño, J. P. Fernandez-Corbelle, and M. Trell, Endoscopic sleeve gastroplasty with 1-year follow-up: factors predictive of success., Endosc. Int. open, vol. 4, no. 2, pp. E222–7, 2016.
American Society of Anesthesiologists, American Society of Anesthesiologists: ASA Physical Status Classification System, American Society of Anesthesiologists Web site. Internet, 2014. [Online]. Available: https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system.
J. B. Buse, S. Caprio, W. T. Cefalu, A. Ceriello, S. Del Prato, S. E. Inzucchi, S. McLaughlin, G. L. Phillips, R. P. Robertson, F. Rubino, R. Kahn, and M. S. Kirkman, How do we define cure of diabetes?, Diabetes Care, vol. 32, no. 11. pp. 2133–2135, 2009.
S. Belle, P. Berk, W. Chapman, N. Christian, A. Courcoulas, G. Dakin, D. Flum, M. Horlick, W. King, C. McCloskey, J. Mitchell, E. Patterson, J. Pender, K. Steffen, R. Thirlby, B. Wolfe, and S. Yanovski, Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study, Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surgery., vol. 9, no. 6, pp. 926–935, 2013.
J. Moy, A. Pomp, G. Dakin, M. Parikh, and M. Gagner, Laparoscopic sleeve gastrectomy for morbid obesity, Am. J. Surg., vol. 196, no. 5, 2008.
C. Afaneh, R. Costa, A. Pomp, and G. Dakin, A prospective randomized controlled trial assessing the efficacy of omentopexy during laparoscopic sleeve gastrectomy in reducing postoperative gastrointestinal symptoms, Surg. Endosc., vol. 29, no. 1, pp. 41–47, 2015.
D. Dindo, N. Demartines, and P.-A. Clavien, Classification of Surgical Complications. A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey, Ann. Surg., vol. 240, no. 2, pp. 205–213, 2004.
Author information
Authors and Affiliations
Contributions
Study conception and design: Drs. Sharaiha, Novikov, Afaneh
Acquisition of data: Drs. Sharaiha, Afaneh, Pomp, Dakin, Shukla, Aronne
Analysis and interpretation of data: Drs. Sharaiha, Afaneh, Novikov, Saumoy
Drafting of manuscript: Drs. Sharaiha, Afaneh, Saumoy, Parra, Novikov, Shah, Dawod
Critical revision: Drs. Novikov, Afaneh, Saumoy, Shukla, Dakin, Pomp, Dawod, Shah, Aronne, and Sharaiha
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Novikov, Afaneh, Saumoy, Parra, Shukla, Dakin, Pomp, Dawod, Shah, and Aronne have nothing to disclose.
Dr. Sharaiha receives grant support from Apollo Endosurgery. The remaining authors have no relevant disclosures.
Rights and permissions
About this article
Cite this article
Novikov, A.A., Afaneh, C., Saumoy, M. et al. Endoscopic Sleeve Gastroplasty, Laparoscopic Sleeve Gastrectomy, and Laparoscopic Band for Weight Loss: How Do They Compare?. J Gastrointest Surg 22, 267–273 (2018). https://doi.org/10.1007/s11605-017-3615-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3615-7