Abstract
Distal pancreatectomy with celiac axis resection is one of the most aggressive approaches for the treatment of locally advanced pancreatic cancer with common hepatic artery and/or celiac axis invasion. However, ischemic complications such as ischemic gastropathy and liver failure are problematic. To avoid these complications, we developed left gastric artery-reconstructing distal pancreatectomy with celiac axis resection. We used the middle colic artery for reconstruction. We performed this procedure in 10 patients, using the middle colic artery in three different ways: left branch reconstruction, right branch reconstruction, and reverse reconstruction. On postoperative images, 90% of the reconstructed left gastric arteries were patent. No complications associated with arterial reconstruction occurred. No patients developed ischemic gastropathy or liver failure. The R0 resection rate was 70%. Nine patients underwent adjuvant chemotherapy and seven patients were able to start it within 90 days. Distal pancreatectomy with celiac axis resection combined with reconstruction of the left gastric artery using the middle colic artery is a feasible option and would enhance the safety for carefully selected patients. Multicenter validation is needed to clarify the benefits of this new procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a malignant cancer associated with a low resection rate of 20% and a very poor prognosis.1 However, with the development of perioperative chemotherapy and radiation therapy in recent years, increasing numbers of patients are being diagnosed with resectable or borderline resectable PDAC, even with an initial status of unresectable locally advanced PDAC. Postoperative adjuvant therapy is imperative to improve overall survival and is now considered the standard treatment protocol. Considering these trends, both surgical radicality and preservation of organ function are important because uneventful recovery directly leads to smooth introduction of adjuvant therapy.
The Appleby operation was initially devised for the treatment of locally advanced gastric cancer with celiac axis (CA) invasion.2 In the 1990s, distal pancreatectomy with CA resection (DP-CAR) was devised as a modified Appleby procedure to resect pancreatic cancer infiltrating the CA.3 – 5 Although DP-CAR has gradually become common in some institutions and countries, this procedure has high morbidity and mortality rates, even in high-volume centers.6 The true nature of the problem regarding DP-CAR is the change in blood flow. This change is characterized by a decrease in the stomach and liver arterial supply secondary to resection of the CA and common hepatic artery (CHA). Reduction of the gastric blood flow is associated with postoperative delayed gastric emptying.7 Left gastric artery (LGA)-preserving DP-CAR was established to minimize this drawback.8 – 10 However, the LGA cannot be preserved if it is involved with the tumor. Therefore, we developed DP-CAR with reconstruction of the LGA using the middle colic arterial branch. We preliminarily reported the first four cases of LGA-reconstructing DP-CAR in a case series of 17 modified DP-CAR procedures.10 We herein describe the technical details of this reconstruction method and surgical outcomes of LGA-reconstructing DP-CAR.
Methods
Patients and Indications for DP-CAR
From April 2011 to December 2015, 10 patients with PDAC in the distal pancreas underwent DP-CAR with reconstruction of the LGA at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research. DP-CAR was indicated in patients with left-sided PDAC showing infiltration or abutment to the CHA, CA, or root of the splenic artery on preoperative thin-slice contrast-enhanced computed tomography (CT). Although the final judgment was dependent upon the intraoperative findings, we usually decided whether to preserve or reconstruct the LGA based on the preoperative CT findings because the extent of cancer invasion is difficult to determine from intraoperative inspection and palpation.
Definitions
Cancer staging was based on the UICC TNM classification of pancreatic cancer. Complications were assessed according to the Clavien–Dindo (CD) classification,11 pancreatic fistula was assessed according to the International Study Group on Pancreatic Fistula criteria,12 and delayed gastric emptying (DGE) was classified according to the International Study Group of Pancreatic Surgery classification.13 Locally advanced or borderline resectable cancer was classified according to the 2015 version of the National Comprehensive Cancer Network guidelines.
Surgical Procedure of LGA-Reconstructing DP-CAR
Preparation of En Bloc Resection of CA System
The basic concept of our procedure is shown in Fig. 1. This figure depicts the right-side view of the sagittal section. Following Kocher’s maneuver, the para-aortic lymph nodes were routinely sampled and examined by frozen section. Para-aortic lymph node dissection was added in cancer-positive cases. The right-sided celiac ganglion and nerve plexus were dissected from the aorta, and the origins of the superior mesenteric artery (SMA) and CA were exposed and taped. The origin of the CA was clamped using a bulldog clamp. The CHA was detected and temporarily clamped immediately proximal to the gastroduodenal artery origin. After confirmation of the hepatic arterial flow using Doppler ultrasonography, the CHA was divided. Generally, if the detected hepatic arterial flow is insufficient, revascularization of the CHA to the CA will be necessary after tumor resection. In such cases, the distal splenic artery is harvested from the resected specimen and used for the conduit as described by Konishi et al.14
Basic concept of the resection. Black arrows with numbers indicate the order of the resection method. The celiac ganglia and nerve plexus around the SMA were resected together with the tumor en bloc. LGA left gastric artery, CA celiac axis, MCA middle colic artery, SMA superior mesenteric artery, IPDA inferior pancreaticoduodenal artery
Exposure of the LGA
The stomach was retracted cranially and the positional relationship between the tumor and the LGA was confirmed. The pedicle of the LGA was dissected and the LGA was exposed and taped on the distal side. The LGV was ligated and cut. The dissection around the LGA was continued toward the origin of the LGA, and the dissection was stopped upon arrival at the main trunk of the LGA (Fig. 2). The LGA was cut and the stump was temporary clamped using a lightweight vessel clamp (TKL; AROSurgical Instruments, Inc., Newport Beach, CA, USA).
Preparation of en bloc resection. The LGA was taped on the peripheral side, and the LGV was dissected at the same level. The connective tissue including the LGV around the LGA was dissected toward the tumor. All double lines indicate the position of the diversion. The right-sided celiac ganglia have already been resected. LGA left gastric artery, LGV left gastric vein, CA celiac axis, CHA common hepatic artery
Preparation of En Bloc Resection Surrounding the SMA
After a wide dissection of the gastrocolic ligament, the superior mesenteric vein was exposed and taped. The dissection along the anterior aspect of the superior mesenteric vein was promoted and connected with the cranial side. Transection of the pancreas was usually performed by a linear stapler with a maximum closed staple height of 1 mm (TL60 Reloadable Linear Stapler; Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). Reinforcement material was not used. The splenic vein was clamped, cut, and closed by a running suture. In patients with tumor invasion to the superior mesenteric vein, wedge-shaped or conduit resection was performed with consequent reconstruction. The SMA then appeared on the left posterior side of the portal vein. The anterior to left side of the nerve plexus around the SMA was dissected proximally up to its origin. The inferior pancreaticoduodenal artery was carefully preserved. Following this procedure, the origin of the CA appeared on the cranial side of the SMA origin. The origin of the CA was triple-ligated with a transfixation ligature technique and divided. The left-sided celiac ganglion and nerve plexus were dissected sequentially.
En Bloc Resection of Retroperitoneum
At this stage, transection of the major vessels and pancreas had already been completed. As the final part of the resection, Gerota’s fascia, the left-sided para-aortic fat and lymph nodes, and the left adrenal gland were resected en bloc, exposing the anterior surface of the left kidney and its pedicle. Figure 3 shows the schema of retroperitoneal resection, and Fig. 4 shows a photograph of a typical case after excision. To facilitate this part of the procedure, we routinely performed full mobilization of the left kidney.
En bloc resection. The stump of the LGA was temporarily clamped. The CA was triple-ligated, and the CHA was double-ligated. The antero-left-sided nerve plexus of the SMA was resected. CA celiac artery, SMA superior mesenteric artery, CHA common hepatic artery, SpV splenic vein, IPDA inferior pancreaticoduodenal artery, MCV middle colic vein, MCA middle colic artery, LGA, left gastric artery
After excision and before reconstruction. The LGA was clamped with a yellow vessel clip. The CA was triple-ligated, and the CHA was double-ligated. CA celiac artery, LGA left gastric artery, LRV left renal vein, CHA common hepatic artery, SMA superior mesenteric artery, SMV superior mesenteric vein, GDA gastroduodenal artery
Reconstruction of the LGA
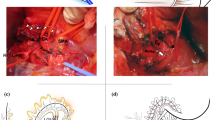
We used three different reconstruction methods (Fig. 5). Caudal retraction of the transverse colon facilitated identification of the origin of the middle colic artery (MCA), the bifurcation of the left and right branches, and the arterial arcade. We then confirmed which part of the MCA to use for reconstruction. In most cases, the left branch of the MCA was suitable for tension-free anastomosis (Fig. 5a). At the proximal side of the marginal arcade, the left branch of the MCA was clamped and the arterial pulsation of the arcade was confirmed. The left branch was then cut, preserving the arterial arcade, and the proximal end was temporarily clamped using an arterial vascular clamp. When performing this procedure, patients who have previously undergone colectomy and those with arteriosclerosis should be given special attention to avoid intraoperative and postoperative colonic ischemia. When the left branch was too short, lacking, or involved by the tumor, the right branch was considered as the next option (Fig. 5b). The third option was reverse reconstruction using the left branch of the MCA. The left branch was cut at the distal point of the tumor infiltration, and arterial pulsation at the distal end was confirmed (Fig. 5c). The colonic splenic flexure was then mobilized into the post-gastric area, facilitating tension-free anastomosis (Fig. 5d). In this method, the arterial supply to the LGA depends on the backflow from the marginal arterial arcade. In each reconstruction, end-to-end anastomosis was performed using microsurgery, with 10 to 12 interrupted 8-0 or 9-0 monofilament sutures. The reconstructed LGA did not need to be wrapped or covered because the anastomosis was usually located far leftward from the pancreatic stump.
Three ways of LGA reconstruction using the middle colic artery. a Left branch reconstruction. b Right branch reconstruction. c A situation for reverse reconstruction. d Reverse reconstruction. The longer branch was used for reconstruction. In cases of tumor invasion to the left branch, the proximal stump was used for reconstruction. Mobilization of the splenic flexure relaxed the anastomotic tension. PHA proper hepatic artery, RGA right gastric artery, LGA left gastric artery, SMA superior mesenteric artery, MCV middle colic vein, MCA middle colic artery
Drain Placement
The abdominal cavity was irrigated with distilled water and physiological saline. An 8-mm soft pleated drain (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) was inserted from the right side of the navel and placed in the left subphrenic space through the pancreatic stump. The mobilized colon was then placed back behind the stomach.
Postoperative Management
The amylase concentration in the drain fluid was measured every day until drain removal. In patients with pancreatic fistulas, drain replacement was routinely performed once a week under contrast radiography. The liver arterial flow was confirmed using Doppler imaging. During postoperative days 7 to 10, all patients underwent contrast-enhanced dynamic CT to confirm the liver and gastric arterial supply and screen for postoperative complications. Prophylactic anticoagulation was not administered.
Results
Table 1 shows the characteristics and preoperative course of 10 patients in this series. Five patients had specific symptoms. Three were initially diagnosed with unresectable PDAC. Two patients had locally advanced unresectable PDAC, and after systemic chemotherapy, the diagnosis was converted to borderline resectable PDAC. The remaining patient had a solitary liver metastasis that was resected and confirmed by initial exploration. The resection was abandoned and the patient underwent nine courses of systemic chemotherapy (gemcitabine + nab-paclitaxel). The main tumor shrunk and no new metastases were detected; thus, surgical resection was indicated again. The other seven patients had borderline resectable PDAC, and five patients received neoadjuvant chemotherapy.
Table 2 shows the surgical outcomes. Severe complications of CD grade ≥III occurred in three patients. In case 3, a pancreatic fistula developed into an abdominal abscess requiring reoperation under general anesthesia (CD IIIb). Case 8 also developed an abdominal abscess, which was drained under local anesthesia (CD IIIa). Case 9 developed atrioventricular conduction block and required temporary transcutaneous pacing (CD IIIa). A grade B/C pancreatic fistula occurred in seven patients, and grade B/C DGE developed in four patients. Eight patients developed postoperative diarrhea, and seven patients required opium tincture to control their hyperperistalsis. All patients underwent postoperative contrast-enhanced dynamic CT, and the patency of the reconstructed LGA was 90% (9/10). We assessed postoperative liver dysfunction by elevations in the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations. The maximum AST and ALT concentrations were 637 and 836 IU/L, respectively. The peak value was observed on postoperative day 1 or 2. In most cases, the elevated AST/ALT normalized within 10 days without any specific treatment.
Table 3 shows the histopathological findings. The mean tumor diameter was 4.5 ± 2.2 cm (median, 3.95 cm; range, 1.4–8.5 cm). Nine patients had lymph node metastasis. Three patients had para-aortic lymph node metastasis, and they underwent para-aortic lymph node dissection. The R0 resection rate was 70%. Nine patients received adjuvant chemotherapy. Seven patients were able to start it within 90 days. During the median follow-up period of 12.3 months, recurrence was detected in seven patients (ascites in one, remnant pancreas in one, lung in three, liver in one, and local recurrence in one). Among the nine patients with confirmed patency of the reconstructed LGA, no patients showed delayed obstruction of the reconstructed artery on the follow-up CT scans after 3 to 6 months.
Discussion
This is the first technical report to focus on the reconstruction method of the LGA in DP-CAR. We have described a novel technique involving three methods of LGA reconstruction using the MCA. No patient developed ischemic gastric or colonic complications. DP-CAR is a highly aggressive approach for treatment of advanced PDAC and has been reported to be associated with high morbidity and mortality. In our series, major complications (CD grade ≥3) were observed in only 30% of patients without mortality. Although the rate of delayed gastric emptying remained substantial, no patients developed any symptoms potentially associated with ischemic gastropathy. The reconstructed LGAs were highly patent (90%), and there were no technical complications associated with arterial anastomosis such as bleeding. Thus, LGA reconstruction is a feasible and reasonable option to enhance safety for patients undergoing DP-CAR.
To achieve R0 resection, a part of the stomach and liver blood flow is sacrificed during the performance of standard DP-CAR, which is associated with delayed postoperative recovery and initiation of adjuvant chemotherapy. Recent meta-analyses and systematic reviews of DP-CAR were published by Gong et al.6 and Klompmaker et al.15 In their reports, the overall morbidity rate was 49.4%, and the major morbidity rate was 27.0%. The incidence of ischemic gastric complications was 12.9 to 18.8% and that of ischemic liver complications was 4.2 to 5.1%. The incidence of clinically relevant DGE was 30.1%, and the overall postoperative mortality rate was 3.5 to 6.7%. In our series of patients who underwent LGA reconstruction, no patients developed ischemic liver or gastric complications, and the mortality rate was 0.0%. Although no standard test has been established to quantify the tissue perfusion in the stomach intraoperatively, we usually use intraoperative angiography with indocyanine green fluorescence imaging to ensure adequate tissue perfusion in the stomach.
In previous reports, the arterial reconstruction rate in DP-CAR was 11.5%. However, all of these studies focused on the CHA flow and reconstruction of the CHA, not the LGA. We found no reports describing reconstruction of the LGA. Although preoperative arterial embolization was introduced in 35.5% of the patients, the morbidity rate showed no significant difference. Preoperative CHA embolization would reinforce the proper hepatic arterial flow via the gastroduodenal artery, but there is no theoretical or clinical evidence that this embolization enhances the gastric arterial flow. The LGA is considered to be the main feeder of the stomach.8 , 16 Therefore, to preserve the gastric arterial flow, the LGA should be reconstructed directly, which seems to be the most simple and reasonable technique.
We used the MCA in the reconstruction for three reasons: an interposition technique is unnecessary, the SMA supplies the blood flow, and the MCA can be sacrificed. When sacrificing the MCA, we must be aware of the risk of colonic ischemia. However, the feasibility of MCA diversion has been shown in previous reports. The mesenteric approach is one of the most representative techniques for treatment of advanced PDAC; it was reported as a part of isolated pancreatectomy by Nakao et al.,17 and in their procedure, the MCA was sacrificed at the beginning. Arterial reconstruction using the MCA has also been reported. Kondo et al.18 reported MCA–gastroepiploic arterial bypass in two patients with accidental injury of the inferior pancreaticoduodenal artery during DP-CAR, and they used the left branch of the MCA for the reconstruction. Okochi et al.19 used the right branch of the MCA to reconstruct the right gastroepiploic artery during pancreaticoduodenectomy because the patient had undergone esophagectomy with gastric tube reconstruction.
While this procedure is technically feasible and has good short-term outcomes, its operative indications must be carefully determined because patients for whom DP-CAR is necessary for complete cancer clearance are always at much higher risk of early local/distant recurrence than are patients with resectable cancer without concomitant arterial resection. Most of our patients developed recurrence within 12 months after resection despite neoadjuvant chemotherapy. Thus, we recommend this procedure only for very select patients based on strict criteria, such as prolonged neoadjuvant therapy with a good response.
Conclusions
We experienced a small number of cases of DP-CAR with LGA reconstruction and have herein described the technical details of this procedure. Further studies are necessary to define the optimal oncologic criteria for selection of patients who can benefit from this complex procedure.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:7-30.
Appleby LH. The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer. 1953;6:704-707.
Ozaki H, Kinoshita T, Kosuge T, Yamamoto J, Shimada K, Inoue K, Koyama Y, Mukai K. An aggressive therapeutic approach to carcinoma of the body and tail of the pancreas. Cancer. 1996;77:2240-2245.
Kimura W, Han I, Furukawa Y, Sunami E, Futakawa N, Inoue T, Shinkai H, Zhao B, Muto T, Makuuchi M, Komatsu H. Appleby operation for carcinoma of the body and tail of the pancreas. Hepato-Gastroenterology. 1997;44:387-393.
Mayumi T, Nimura Y, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Hamaguchi K, Hayakawa N. Distal pancreatectomy with en bloc resection of the celiac artery for carcinoma of the body and tail of the pancreas. International Journal of Pancreatology. 1997;22:15-21.
Gong H, Ma R, Gong J, Cai C, Song Z, Xu B. Distal Pancreatectomy With En Bloc Celiac Axis Resection for Locally Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2016;95:e3061.
Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H. Preservation of the left gastric artery on the basis of anatomical features in patients undergoing distal pancreatectomy with celiac axis en-bloc resection (DP-CAR). World Journal of Surgery. 2014;38:2980-2985.
Kimura A, Yamamoto J, Aosasa S, Hatsuse K, Nishikawa M, Nishiyama K, Tsujimoto H, Moriya T, Hase K, Shinmoto H, Kaji T. Importance of maintaining left gastric arterial flow at Appleby operation preserving whole stomach for central pancreatic cancer. Hepato-Gastroenterology. 2012;59:2650-2652.
Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H. Surgical strategy for patients with pancreatic body/tail carcinoma: who should undergo distal pancreatectomy with en-bloc celiac axis resection? Surgery. 2013;153:365-372.
Sato T, Saiura A, Inoue Y, Takahashi Y, Arita J, Takemura N. Distal Pancreatectomy with En Bloc Resection of the Celiac Axis with Preservation or Reconstruction of the Left Gastric Artery in Patients with Pancreatic Body Cancer. World Journal of Surgery. 2016;40:2245-2253.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery. 2004;240:205-213.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula D. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13.
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Buchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768.
Konishi M, Kinoshita T, Nakagori T, Inoue K, Oda T, Kimata T, Kikuchi H, Ryu M. Distal pancreatectomy with resection of the celiac axis and reconstruction of the hepatic artery for carcinoma of the body and tail of the pancreas. Journal of Hepato-Biliary-Pancreatic Surgery. 2000;7:183-187.
Klompmaker S, de Rooij T, Korteweg JJ, van Dieren S, van Lienden KP, van Gulik TM, Busch OR, Besselink MG. Systematic review of outcomes after distal pancreatectomy with coeliac axis resection for locally advanced pancreatic cancer. British Journal of Surgery. 2016;103:941-949.
El-Eishi HI, Ayoub SF, El-Khalek MA. The arterial supply of the human stomach. Acta Anatomica. 1973;86:565-580.
Nakao A, Takagi H. Isolated pancreatectomy for pancreatic head carcinoma using catheter bypass of the portal vein. Hepato-Gastroenterology. 1993;40:426-429.
Kondo S, Ambo Y, Katoh H, Hirano S, Tanaka E, Okushiba S, Morikawa T, Igawa H, Yamamoto Y, Sugihara T. Middle colic artery-gastroepiploic artery bypass for compromised collateral flow in distal pancreatectomy with celiac artery resection. Hepato-Gastroenterology. 2003;50:305-307.
Okochi M, Ueda K, Sakaba T, Kenjo A, Gotoh M. Right gastro-omental artery reconstruction after pancreaticoduodenectomy for subtotal esophagectomy and gastric pull-up. International Journal of Surgery Case Reports. 2015;15:42-45.
Acknowledgements
Authors’ Contributions
Study conception and design: Sato, Inoue, Takahashi, Ishizawa, Mise, Saiura
Acquisition of data: Sato, Inoue
Analysis and interpretation of data: Sato, Inoue, Ito, Saiura
Drafting of manuscript: Sato, Inoue, Saiura
Critical revision: Takahashi, Ishizawa, Mise, Ito
Final approval: Sato, Inoue, Takahashi, Ishizawa, Mise, Ito, Saiura
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
None of the authors received any grant support.
Rights and permissions
About this article
Cite this article
Sato, T., Inoue, Y., Takahashi, Y. et al. Distal Pancreatectomy with Celiac Axis Resection Combined with Reconstruction of the Left Gastric Artery. J Gastrointest Surg 21, 910–917 (2017). https://doi.org/10.1007/s11605-017-3366-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3366-5