Abstract
Familial partial lipodystrophy type 2 (FPLD2) is a rare disorder associated with LMNA gene mutations. It is usually marked by loss of subcutaneous fat on the limbs and trunk and severe insulin resistance. Scattered reports have indicated that Roux-en-Y bypass helps to control the diabetes mellitus in these patients. We present here a very unusual patient with FPLD2 who had life-threatening retroperitoneal and renal fat accumulation accompanied by bilateral renal cancers. Following cryotherapy of one renal cancer and a contralateral nephrectomy with debulking of the retroperitoneal fat, Roux-en-Y gastric bypass (RYGB) has successfully controlled the disease for 3 years. The clinical presentations and causes of FPLD are reviewed and the role of RYGB is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial lipodystrophies are a heterogeneous group of genetic disorders associated with abnormal subcutaneous and visceral fat distribution accompanied by severe insulin resistance, diabetes, hyperlipidemia, early cardiovascular disease, and a variety of other manifestations such as pancreatitis, hepatic steatosis, acanthosis nigricans, and hypertension. Lipodystrophy can be generalized with a total loss of subcutaneous fat, or partial, affecting only some regions of the body. At present, six types of familial partial lipodystrophy (FPLD) have been defined (Table 1).1,2 The phenotypic abnormalities are more easily diagnosed and the metabolic effects appear more severe in women than in men. The most common type, familial partial lipodystrophy type 2 (FPLD2), was described by Dunnigan in 1974 in four women with absent subcutaneous fat in the limbs and trunk, normal or excessive adipose tissue on the face and neck, hyperlipoproteinemia, and diabetes mellitus.3 In 1975, Köbberling reported a similar syndrome in three women with limb involvement, but normal facial and truncal fat.4 These two syndromes were later called Types 1 and 2 “Köbberling-Dunnigan syndrome;” however, Köbberling and Dunnigan cautioned that the two syndromes were probably genetically separate.5 Indeed, Köbberling’s syndrome is now called FPLD1, and its genetic basis still has not been elucidated. FPLD2 results from missense mutations in the lamin A/C gene (LMNA).1,6 Symptoms usually start in late childhood or at puberty with gradual loss of subcutaneous fat in the extremities, anterior chest, and abdomen; accumulation of fat in the face, chin, neck, and vulva; and muscular hypertrophy of the calves. A variety of metabolic derangements related to severe insulin resistance can be seen, including diabetes, hypertriglyceridemia, pancreatitis, steatosis, and premature atherosclerotic cardiovascular disease.7 Female patients may exhibit increased skeletal muscle volume, hirsutism, menstrual abnormalities, and polycystic ovary syndrome.8 Therapeutic approaches include cosmetic removal of excess fat, dietary management, and hypoglycemic and lipid-lowing agents. Thiazolidinedione therapy has been tried, but does not reverse fat loss.9 Leptin replacement improves insulin resistance and triglyceride levels, but does not improve glycosylated hemoglobin levels.10
Case Report
A 60-year-old woman with a 30-year history of poorly controlled diabetes despite several oral hypoglycemic agents and multiple daily insulin injections (total daily dose ∼100 units) was referred for early satiety, abdominal pain, and an abdominal mass. She had been diagnosed with Dunnigan’s syndrome at age 50 and had hypertriglyceridemia, hypertension, and microalbuminuria. In addition, she had recently undergone cryoablation of a 2.5-cm left renal carcinoma. On renal flow scan, the right kidney was non-functional, and her left renal function was normal. Her past surgical history included bilateral oophorectomy for cysts and multiple procedures to remove fatty deposits around the neck, hips, and vulva. Her family history was significant for abnormal fat distribution in her paternal grandfather and father, a paternal aunt, one brother, and one sister. Her daughter had type 2 diabetes mellitus with severe insulin resistance, polycystic ovaries, and multiple hepatic adenomas (possibly due to oral contraceptives).
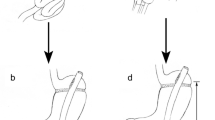
On examination, she was noted to have acromegaloid features in the face and digits; accumulation of fat around the neck, vulva, lateral thighs, and buttocks; a paucity of subcutaneous fat on the chest and lower extremities, prominent leg veins, and well-defined lower extremity musculature (Fig. 1a); and an enormously protuberant abdomen (Fig. 1b). An abdominal CT scan showed extensive retroperitoneal fat displacing the liver and infiltrating both kidneys, abnormal fat distribution in the pelvic girdle, cholelithiasis, atherosclerotic changes in the aorta, and severe stretching of her mesenteric vessels (Fig. 2a, b). Given her symptoms and the risk of mesenteric occlusion/thrombosis from external compression, we recommended resection of the mass, right nephrectomy, and cholecystectomy.
At operation, a normal-appearing liver, ascending colon, and small bowel were displaced to the left of the midline by a 12-kg retroperitoneal lipomatous mass measuring at least 30 × 25 × 15 cm. The mass was not encapsulated and was inseparable from the right kidney. After a cholecystectomy was performed, the mass was dissected away from the overlying bowel and removed together with the right kidney and adrenal gland. A similar mass in the left retroperitoneum was not resected since this would have necessitated sacrificing her remaining renal function. She had an uneventful recovery and was discharged home. Final pathology revealed mature adipose tissue with infiltration into the right kidney. The right kidney also contained an 8-mm papillary renal cell carcinoma and multiple cortical papillary adenomas. There was no amplification of the MDM2 gene, ruling out well-differentiated liposarcoma.
Genetic testing revealed a heterozygous R482W LMNA mutation in exon 8 defined as c.1444C→T, predicted to result in the amino acid substitution of arginine (CGG) to tryptophan (TGG). This mutation has been previously described in FPLD2.7 In addition, she had two other heterozygous mutations thought to be non-pathogenic, an intronic c.514-32 C→T in exon 3, and c.1698 C→T predicted to result in the amino acid substitution p.His566His. The latter mutation has been found in individuals without PFLD. PTEN gene testing was negative.
Eight months later, an abdominal MRI showed continued enlargement of the left retroperitoneal mass. Encouraged by a few case reports of short-term improvement in metabolic parameters after RYGB for lipodystrophy patients (Table 2), she was referred for laparoscopic RYGB which was successfully performed. A liver biopsy showed no evidence of hepatic steatosis or fibrosis. Surveillance MRI 6 months after RYGB showed an interval decrease in retroperitoneal fat proliferation, and subsequent imaging at 9, 12, 15, and 24 months showed no further growth. The subcutaneous deposits around her hips were noticeably smaller. Her blood pressure has declined from 165/75 to 124/73. Her weight declined from 79.5 to 60.3 kg at 24 months, and her BMI improved from 30.0 to 24.3. At 36 months, her BMI is 24.5. She remains off subcutaneous insulin therapy, and her diabetes is maintained with glimepiride 0.5 mg daily and metformin 1 g twice daily. Her hemoglobin A1C was 10.3 % prior to her bypass, declined to 6.9 % 3 months after her bypass, and was 7.7 % at 36 months. She recently underwent a successful coronary artery bypass graft procedure.
Discussion
The lamin A/C gene codes for several spliced proteins, including lamin A and lamin C, that polymerize with type B lamins to form the nuclear lamina beneath the inner nuclear membrane.14 Lamin A is derived from its precursor, prelamin A. Efficient processing makes prelamin A almost undetectable in normal cells, but it accumulates under conditions of stress, aging, and differentiation of some cell types.14 The nuclear lamina provides structural scaffolding for the cell nucleus and is important in the control of chromatin conformational changes, connection between the nucleus and cytoplasm, gene transcription, and mitosis.7,14 The exact mechanisms by which LMNA mutations cause PFLD2 are unclear, but they probably relate to accumulation of prelamin A and disorders in nuclear function producing apoptosis, cellular toxicity, premature adipocyte aging, and cell death.15 Mutated lamin A has been shown to down-regulate adipocyte gene expression in fatty tissue from the thigh but not the abdomen in patients with FPLD2,16 suggesting that the precursor adipocytes in these region differ from one another. Not surprisingly, mutations affecting lamins are associated with a wide variety of other diseases, including muscular dystrophies, cardiomyopathy, neuropathies, and progeroid syndromes.14 Other PFLD syndromes (Table 1) are associated with abnormalities in adipocyte differentiation (PFLD3), lipid droplet formation (PFLD4), downstream insulin signaling (PFLD4), conversion of prelamin A to lamin A (PFLD5), and hormone-sensitive lipase (PFLD6).
To our knowledge, this is the first report of a patient with PFLD2 with such profound retroperitoneal and renal adipose tissue deposition. Haque et al. reported significant retroperitoneal and omental fat at autopsy of a woman with an R482Q mutation (CGG→CAG) resulting in substitution of arginine by glutamine.17 As in our patient, the lower limbs lacked subcutaneous fat and there was excess fat in the face, neck, and axillae, but she did not have the fat accumulation around the pelvic girdle or such significant renal involvement. Hepatic steatosis was absent. The association of PFLD2 with renal cancer has not been previously emphasized, but it should be noted that one of Dunnigan’s four patients died of renal cancer3 and our patient harbored bilateral renal cancers.
The differential diagnoses of such a fatty mass include benign and malignant fatty tumors such as lipomas and liposarcomas which may arise from the retroperitoneum or the small bowel mesentery. Another possible source of confusion is sclerosing mesenteritis, sometimes called lipodystrophy, which is characterized by a mass at the root of the mesentery with a hazy appearance on CT. These conditions are not associated with insulin-resistant diabetes or abnormal fat distribution elsewhere in the body.
The medical treatment of the metabolic abnormalities of FPLD includes diet modification, exercise, and medications to lower lipid and blood glucose levels. Response may differ according to the genetic mutation, but insulin resistance remains a hallmark. There have been three prior reports of improvement in insulin resistance in patients with FPLD treated by Roux-en-Y gastric bypass (RYGB) surgery. McGrath et al. reported a woman with clinical signs of FPLD, but negative genetic testing.11 Eighteen months after surgery, her BMI decreased from 29 to 24.7 and her HbA1c fell from 11.7 to 7.6 % off all therapies. RYGB also helped lower her blood cholesterol and triglyceride levels and restored normal menstrual cycles. Similarly, Utzschneider et al. reported a female patient clinically diagnosed with FPLD who underwent laparoscopic RYGB for severe gastroesophageal reflux and gastroparesis.12 The patient experienced a 30 % weight loss, improvement of blood pressure, and marked improvement of HbA1c, fasting insulin, and blood triglyceride levels while off all medications at 16 months after surgery. Ciudin et al. reported a female patient with an LMNA gene mutation (R482W), who underwent RYGB due to progressive metabolic effects and premature heart disease.13 Fasting insulin, HDL cholesterol, triglycerides, and free fatty acids normalized and the patient was off all medications 3 months after surgery. DEXA scan showed a reversal of fat distribution from an android to a gynoid pattern with reduction of total and truncal body fat.
Our case is the fourth report of RYGB successfully improving the metabolic effects of PFLD. Three years after gastric bypass surgery, our patient remains off insulin, and her diabetes is well controlled with metformin and glimepiride. She also achieved a 25 % weight loss and 16 % reduction in BMI. We have yet to find another report of such dramatic, life-threatening renal and retroperitoneal fat deposition. The adipose tissue burden was successfully debulked with surgery, and further progression of the disease was ameliorated with RYGB. We are encouraged that the retroperitoneal fat has stabilized and that the peripheral fat deposits around the pelvic girdle appear to have decreased. She is cancer free 42 months after her left renal cancer was ablated. These four cases suggest that RYGB is an effective treatment for PFLD with insulin-resistant diabetes and is superior to medical treatment alone. A thorough review of the mechanisms by which RYGB may improve diabetic control is beyond the scope of this article. The current theories attribute the improvement to (1) duodenal exclusion, (2) the effects of rapid entry and absorption of nutrients in the distal small bowel with increase in the hormone GLP-1, and (3) the role of increased bile acids on the regulation of hepatic glucose metabolism by the nuclear receptor farnesoid X receptor and its effect on the intestinal biota.18 The latter is particularly interesting since farnesylation is necessary for production of mature lamin A. We hope that the improvement seen in our patient will persist. Reports of lasting beneficial effects of gastric bypass for morbid obesity are encouraging in this regard.19
Conclusion
This is the first report of a case of type 2 familial partial lipodystrophy with severe hypertrophy of retroperitoneal and renal fat requiring surgical debulking. This is also the second report of Dunnigan syndrome and renal carcinoma, although this association has to be confirmed. RYGB was previously shown to have short-term success in treating insulin resistance in FPLD. Our case shows that RYGB not only markedly improves the control of diabetes but it also appears to prevent progression of disease with prolonged beneficial metabolic effects.
References
Garg A. Clinical review: Lipodystrophies: Genetic and acquired body fat disorders. Journal of Clinical Endocrinology & Metabolism 2011;96:3313–25.
Farhan SM, Robinson JF, McIntyre AD, Marrosu MG, Ticca AF, Loddo S, Carboni N, Brancati F, Hegele RA. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Can J Cardiol 2014;30:1649–54.
Dunnigan M, Cochrane M, Kelly A, Scott J. Familial lipoatrophic diabetes with dominant transmission: A new syndrome. Q J Med 1974;43:33–48.
Köbberling J, Willms B, Kattermann R, Creutzfeldt W. Lipodystrophy of the extremities. A dominantly inherited syndrome associated with lipatrophic diabetes. Humangenetik 1975;29:111–20.
Köbberling J, Dunnigan M. Familial partial lipodystrophy: Two types of an X linked dominant syndrome, lethal in the hemizygous state. J Med Genet 1986;23:120–7.
Caron M, Auclair M, Donadille B, Bereziat V, Guerci B, Laville M, Narbonne H, Bodemer C, Lascols O, Capeau J, Vigouroux C. Human lipodystrophies linked to mutations in A-type lamins and to HIV protease inhibitor therapy are both associated with prelamin A accumulation, oxidative stress and premature cellular senescence. Cell Death & Differentiation 2007;14:1759–67.
Mory PB, Crispim F, Freire MB, Salles JE, Valerio CM, Godoy-Matos AF, Dib SA, Moises RS. Phenotypic diversity in patients with lipodystrophy associated with LMNA mutations. European Journal of Endocrinology 2012;167:423–31.
Ji H, Weatherall P, Adams-Huet B, Garg A. Increased skeletal muscle volume in women with familial partial lipodystrophy, Dunnigan variety. Journal of Clinical Endocrinology & Metabolism 2013;98:E1410–3.
Simha V, Rao S, Garg A. Prolonged thiazolidinedione therapy does not reverse fat loss in patients with familial partial lipodystrophy, Dunnigan variety. Diabetes Obes Metab 2008;10:1275–6.
Simha V, Subramanyam L, Szczepaniak L, Quittner C, Adams-Huet B, Snell P, Garg A. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. Journal of Clinical Endocrinology & Metabolism 2012;97:785–92.
McGrath NM, Krishna G. Gastric bypass for insulin resistance due to lipodystrophy. Obesity Surg 2006;16:1542–4.
Utzschneider KM, Trence DL. Effectiveness of gastric bypass surgery in a patient with familial partial lipodystrophy. Diabetes Care 2006;29:1380–2.
Ciudin A, Baena-Fustegueras JA, Fort JM, Encabo G, Mesa J, Lecube A. Successful treatment for the Dunnigan-type familial partial lipodystrophy with Roux-en-Y gastric bypass. Clin Endocrinol (Oxf) 2011;75:403–4.
Camozzi D, Capanni C, Cenni V, Mattioli E, Columbaro M, Squarzoni S, Lattanzi G. Diverse lamin-dependent mechanisms interact to control chromatin dynamics. Focus on laminopathies. Nucleus (Calc) 2014;5:427–40.
Guenantin AC, Briand N, Bidault G, Afonso P, Bereziat V, Vatier C, Lascols O, Caron-Debarle M, Capeau J, Vigouroux C. Nuclear envelope-related lipodystrophies. Semin Cell Dev Biol 2014;29:148–57.
Araujo-Vilar D, Lattanzi G, Gonzalez-Mendez B, Costa-Freitas AT, Prieto D, Columbaro M, Mattioli E, Victoria B, Martinez-Sanchez N, Ramazanova A, Fraga M, Beiras A, Forteza J, Dominguez-Gerpe L, Calvo C, Lado-Abeal J. Site-dependent differences in both prelamin A and adipogenic genes in subcutaneous adipose tissue of patients with type 2 familial partial lipodystrophy. J Med Genet 2009;46:40–8.
Haque WA, Vuitch F, Garg A. Post-mortem findings in familial partial lipodystrophy, Dunnigan variety. Diabetic Med 2002;19:1022–5.
Corcelles R, Daigle CR, Schauer PR. Management of endocrine disease: Metabolic effects of bariatric surgery. European Journal of Endocrinology 2016;174:R19–28.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ESH, Nissen SE, Kashyap SR, STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014;370:2002–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors listed above meet the criteria of the ICMJE. They have made substantial contributions to the conception or design of the work or the acquisition, analysis, and interpretation of data for the work, and/or drafting or revising it critically for important intellectual content. All authors have given final approval of the version to be published and are accountable for all aspects of it.
Rights and permissions
About this article
Cite this article
Grundfest-Broniatowski, S., Yan, J., Kroh, M. et al. Successful Treatment of an Unusual Case of FPLD2: The Role of Roux-en-Y Gastric Bypass—Case Report and Literature Review. J Gastrointest Surg 21, 739–743 (2017). https://doi.org/10.1007/s11605-016-3300-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3300-2