Abstract
Introduction
Patients with metastatic pancreatic adenocarcinoma have traditionally been offered palliative chemotherapy alone, and the role of surgery in these patients remains unknown.
Methods
A bi-institutional retrospective review was performed for patients with metastatic pancreatic adenocarcinoma who underwent resection of the primary tumor from 2008 to 2013. The primary outcome measured was postoperative overall survival. Secondary outcomes included postoperative disease-free survival and overall survival from the time of diagnosis.
Results
Twenty-three patients were identified who met the study criteria with a median follow-up of 30 months. Metastatic sites included the liver (n = 16), the lung (n = 6), and the peritoneum (n = 2). Chemotherapy included FOLFIRINOX (n = 14) and gemcitabine-based regimens (n = 9), with a median of 9 cycles (range 2–31) prior to surgical treatment. Median time from diagnosis to surgery was 9.7 months (IQR 5.8–12.8). Median overall survival (OS) from surgery, disease-free survival, and OS from diagnosis were 18.2 months (95 % CI 11.8–35.5), 8.6 months (95 % CI 5.2–16.8), and 34.1 months (95 % CI 22.5–46.2), respectively. The 1- and 3-year OS from surgery were 72.7 % (95 % CI 49.1–86.7) and 21.5 % (95 % CI 4.3–47.2), respectively.
Conclusion
Resection of the primary tumor in patients with metastatic pancreatic adenocarcinoma may be considered in highly selected patients with favorable imaging and CA 19-9 response following chemotherapy at high-volume centers providing multidisciplinary care. These patients should be enrolled in prospective clinical trials or institutional registries to better quantify the potential benefits of such a strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer has climbed to the third leading cause of cancer-related death while remaining just the 12th most common cancer in incidence.1 Only in 2010 did the national 1-year overall survival from diagnosis eclipse 30 %. The poor prognosis is secondary to a high rate of locally advanced and distant metastatic disease at the time of diagnosis and characteristic rapid progression. For those with distant metastases from pancreatic ductal adenocarcinoma (PDAC) at diagnosis, the median survival remains less than 1 year and survival beyond even 2 years is rare.
Within the past 5 years, two large-scale randomized controlled trials have demonstrated a survival benefit with the use of combination systemic chemotherapy regimens over gemcitabine monotherapy in metastatic PDAC.2 , 3 In the face of the dismal prognosis associated with PDAC, these regimens have provided new optimism for patients who exhibit a favorable biologic and radiologic response to treatment.
Improvement in systemic therapy combined with metastasectomy has improved survival in other advanced diseases such as colorectal cancer. Patients presenting with stage IV disease in the form of resectable liver metastases now have a 5-year overall survival (OS) of greater than 50 % in the era of multimodality therapy.4 Even in the face of unresectable distant metastases, resection of the primary tumor has demonstrated a small survival benefit in colorectal cancer.5 , 6 However, to date surgical resection of metastatic disease has not been applied to patients with PDAC largely due to the lack of effective systemic chemotherapy and the high morbidity rates associated with removal of the primary tumor.
We sought to investigate the outcomes of patients with stage IV PDAC who underwent primary tumor resection with or without metastasectomy following a favorable response to systemic chemotherapy.
Materials and Methods
The study was designed as a retrospective review at two high-volume pancreatic surgery centers, the University of Pittsburgh Medical Center and the Johns Hopkins Hospital. Both institutions utilize a multidisciplinary tumor board. Criteria for patient selection for review included stage IV biopsy-proven PDAC at presentation with synchronous metastases detected on preoperative imaging. All patients included received an initial course of systemic therapy at the discretion of the treating medical oncologist without specified protocol. The site and number of metastases was recorded for each patient. Performance status was determined according to the Eastern Cooperative Oncology Group (ECOG) status.
Cancer antigen 19-9 (CA19-9) levels at diagnosis and following systemic therapy were reviewed and stratified by >50 % reduction and >90 % reduction. Radiologic response was measured by the modified RECIST criteria.7 Surgical outcomes of interest included R0 resection rate and short-term morbidity which was graded by the Clavien-Dindo classification.8
The primary outcome measure was OS from the time of surgery. Secondary outcomes included DFS and OS from the time of diagnosis. Kaplan-Meier analyses were performed. Survival between groups was compared by chemotherapy regimen and CA19-9 response with cutoff of 90 % decrease using the log-rank test. A p value of <0.05 was considered significant.
Results
Patient Population
During the study period, there were 1147 patients with stage IV PDAC treated at the two institutions. Of these patients, 23 (2.0 %) underwent surgical resection of the primary tumor with or without metastasectomy after favorable response to chemotherapy. Patient demographics are listed in Table 1. The ECOG performance status was 0 in 78.2 % and 1 in the remainder of the cohort. The obesity rate was 21.7 %, and mean preoperative albumin was 3.9 ± 0.4.
Disease Presentation
The characteristics of disease at diagnosis are listed in Table 1. Tumors were predominantly located in the head of the pancreas and were resectable. Twelve of fifteen patients (80 %) with pancreatic head lesions received pre-treatment biliary stents. The sites of metastases were the liver (65.2 %), the lung (21.7 %), the peritoneum (8.7 %), and both the liver and lung in one patient (4.3 %). Thirteen (56.5 %) patients had two or fewer metastatic lesions, four (17.4 %) had 3–4 lesions, and six (26.1 %) had ≥5 lesions. The median CA19-9 at diagnosis was 464 U/mL (IQR 133–2498). CA19-9 was elevated in 18/20 (90 %) patients who had a baseline level available from the time of diagnosis.
Systemic Treatment and Response
The FOLFIRINOX regimen was used for induction therapy in 14 (60.9 %), and gemcitabine-based regimens were used in 9 (39.1 %). Six of the nine patients (66.9 %) received gemcitabine as monotherapy initially. Four patients (28.6 %) converted from FOLFIRINOX to gemcitabine-based regimens, and two (22.2 %) converted from gemcitabine-based regimens to FOLFIRINOX. The median number of cycles of preoperative chemotherapy received for all patients was 9 (IQR 6–12). The median number of cycles in the FOLFIRINOX group was 6 (range 3–25) with the four converters receiving an additional 4–12 cycles of gemcitabine-based regimens. The median number of cycles in the gemcitabine monotherapy group was 5 (range 2–14) with two converters receiving an additional 8 and 18 cycles of FOLFIRINOX, respectively. The three patients on gemcitabine dual-therapy regimens received 6, 12, and 14 preoperative cycles.
The CA19-9 level decreased by >50 % in 17/18 (94.4 %) and by >90 % in 11/18 (61.1 %). The median percentage decrease of CA19-9 level was 92.1 % (IQR 70.5–98.6 %). The overall radiologic response included complete response in 1 (4.3 %), partial response in 10 (43.5 %), and stable disease in 12 (52.2 %) according to the modified RECIST criteria. For metastatic lesions only, complete response was observed in 8 patients (34.8 %) including 2 patients with peritoneal metastases. For patients with lung metastases, 4/6 (66.7 %) demonstrated partial response and 2/6 (33.3 %) had stable disease.
Surgical Treatment
The median time from diagnosis to surgical treatment was 9.7 months (IQR 5.8–12.8). Operative procedure included pancreaticoduodenectomy in the majority (65.2 %) and distal pancreatectomy (34.8 %) in the remaining. Seven patients in this series had a robotic-assisted procedure with no conversions to open. Procedures for metastatic lesions were performed in 11 (47.8 %) that included metastasectomy of liver (n = 9) and lung (n = 2) lesions and radiofrequency ablation (n = 1). Mean operative duration was 424 ± 147 min. Venous resection was performed in 3/23 (13.0 %). The rate of R0 resection was 21/23 (91.3 %). The median length of stay was 8 days (IQR 7–10). Major complications occurred in 3/23 (13.0 %) with no perioperative mortalities.
Final pathologic T stage included T1 = 7/23 (30.4 %), T2 = 3/23 (13.0 %), T3 = 12/23 (52.2 %), and T4 = 1/23 (4.4 %). Mean nodal yield was 19 ± 8, and nodal metastases were identified in 9/23 (39.1 %). Four patients had variants from classic ductal adenocarcinoma which included two with focal squamous differentiation, one with mucinous features, and one arising within an intraductal papillary mucinous neoplasm. All metastatic lesions were concordant with the primary tumor pathology.
Survival
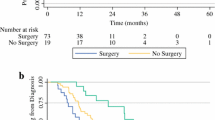
The median DFS was 8.6 months (95 % CI 5.2–16.8) from the date of surgery (Fig. 1). Six patients (26.1 %) have neither expired nor experienced recurrent disease since surgery with a median follow-up of 22.5 months. Conversely, 7/23 (30.4 %) experienced an early disease recurrence <6 months from surgery. Sites of initial recurrence/progression included the liver (n = 11), the lung (n = 4), and the peritoneum (n = 5) among the 17 patients with recurrence. The site of recurrence was the same as the initial metastatic disease in 13/17 (76.5 %) and a new site in 4/17 (23.5 %). Palliative chemotherapy was utilized in 12 patients (52.2 %) for a median of 3 cycles (range 1–6).
The median OS from the time of surgery was 18.2 months (95 % CI 11.8–35.5) (Fig. 2). The 1- and 3-year OS rates were 72.7 and 21.5 %, respectively. OS did not differ based on chemotherapeutic regimen or CA19-9 response (p = NS). Median OS from the time of diagnosis was 34.1 months (95 % CI 22.5–46.2) with 3-year OS of 43.1 %.
Discussion
The surgical treatment of PDAC has been traditionally restricted to patients with resectable or borderline resectable tumors and absence of distant metastasis. The premise of this philosophy originated from the aggressive and systemic nature of PDAC and abbreviated survival in the setting of metastatic disease with available chemotherapeutic regimens.
Two recent trials of systemic chemotherapy in metastatic PDAC have shown promise. The PRODIGE trial utilized the FOLFIRINOX regimen and demonstrated an absolute survival benefit of 4.3 months when compared to monotherapy with gemcitabine.2 The addition of nab-paclitaxel to gemcitabine in the MPACT trial also demonstrated a survival benefit of 1.8 months compared with gemcitabine alone.3 Despite these improvements, median survival of metastatic pancreas cancer remains less than 1 year.
Prior to the current era of improved systemic therapy, experience with surgical treatment of metastatic PDAC in the literature was limited. Shrikhande et al. examined 29 patients with M1 disease who underwent pancreatic resection and found a median survival of 13.8 months.9 However, only one of these patients underwent neoadjuvant therapy, and 9 of 29 only had microscopic disease in aortocaval lymph nodes diagnosed at the time of postoperative histologic examination. Poor survival has been described in other series, but these also predominantly consist of disease diagnosed at the time of operation and without neoadjuvant treatment.10 , 11 The outcomes of pancreatic resection in the setting of metastatic disease treated with modern chemotherapeutic regimens are largely unknown and have only been described in isolated case series.12 , 13
In this study, we report our cumulative experience with primary tumor resection with or without metastasectomy following systemic therapy for metastatic PDAC at two high-volume pancreatic surgery centers. All patients in the study had synchronous metastases and demonstrated favorable response to systemic chemotherapy. The median OS from surgery and DFS were 18.2 and 8.6 months, respectively. These are relatively comparable to the survival seen in patients who have undergone attempted curative resection with receipt of adjuvant gemcitabine in the absence of known distant metastases.14
Changes in CA19-9 level and RECIST criteria were used to evaluate response to systemic therapy and appeared to be the primary determining factors in patient selection in addition to high performance status. The CA19-9 level decreased by >50 % in all but one patient, and the median decrease was 92 %. The rate of radiologic response was 47.8 % as judged by partial or complete response while the remainder had stable disease. Complete radiologic response of the metastatic lesions was seen in 34.8 %. In the two patients without CA19-9 elevation at diagnosis, a partial radiologic response was demonstrated after chemotherapy. These factors were associated with improved survival in the PRODIGE trial and have been a favorable prognostic factor in borderline and resectable disease.15 However, CA19-9 response >90 % was not associated with improved DFS or OS in this cohort, likely related to the small sample size and favorable response overall in this highly selected cohort. Obtaining high-quality cross-sectional imaging and baseline CA19-9 level at diagnosis are of utmost importance in guiding treatment. Surgical treatment outside of excellent response by these measures would be inadvisable in the setting of metastatic disease.
The basic tenants of oncologic surgery are to cure or prolong life. Local therapy of the primary tumor with or without metastasectomy is unlikely to achieve cure. Only six patients in our series have not experienced recurrent disease to date. While the prolongation of life is a worthwhile pursuit, this can be difficult to quantify from a highly selected cohort. One potential benefit of offering resection is to provide an extended time period for the patient to be off systemic chemotherapy treatment and potentially enhancing quality of life. Aside from the general toxicities seen with chemotherapy, the FOLFIRINOX regimen can be difficult to tolerate and is often limited by the degree of peripheral neuropathy. Once patients experience this toxicity, it is typically unable to be used again in the future. Additionally, the efficacy of second-line chemotherapy is poor, as evidenced by the 4-month survival seen in the PRODIGE trial for both study groups once cross-over was initiated.2
The OS from diagnosis for this cohort was 34 months. In an update of the MPACT trial, survival beyond 3 years with nab-paclitaxel plus gemcitabine was seen in 3 %.16 These data are not as clear from the PRODIGE trial as the number at risk on the KM curves drops off steeply after 24 months, but there were also a small percentage with survival beyond 3 years. In our cohort, six patients survived beyond 3 years with the longest at 62 months with no evidence of disease recurrence. As the natural history of these excellent responders is otherwise unknown, it is difficult to estimate the true impact of surgery due to lack of an adequate control group.
As perioperative care has improved, the surgical risk of mortality to patients undergoing pancreatic resections has decreased to <3 % at high-volume centers.17 While the morbidity remains significant, this must be balanced with the availability of additional therapies available to the patient (i.e., radiation, second-line chemotherapy) and potential, but unknown benefit of prolonging survival in select cases. Though conflicting data exist, surgical complications have been associated with poor short- and long-term oncologic outcomes in a number of cancers. Early disease progression (<6 months) was seen in sevent patients in our study: four within the liver, one within the lung, one within both the liver and lung, and one with peritoneal disease. While there was no link to complications evident as major morbidity only occurred in the two patients, these patients likely received minimal benefit from undergoing resection of their primary tumor. Also, the postoperative recovery may result in an overall negative impact on quality of life compared with continued observation and non-operative management. Both a minimally invasive approach and the development of enhanced recovery after surgery pathways may reduce this burden. The risk-benefit discussion must be had openly with patients if consideration is being given to primary tumor resection with or without metastasectomy in select patients with metastatic PDAC. In this cohort of patients, informed consent was obtained with full explanation of the risks of surgery and that any potential benefit of surgical resection in their situation is unknown and without supporting data.
The limitations of our study are primarily a small sample size and the lack of a control group. These patients represent outliers to the usual progression of metastatic PDAC, even with the new combination chemotherapy regimens. Furthermore, these were highly selected patients in a multidisciplinary setting only after a somewhat durable response was observed as is evident from the greater than 9 month interval from the time of diagnosis to surgery. Both centers are high-volume pancreatic surgery centers which may limit the reproducibility of these results.
When challenging the standard of care, the ideal form of investigation is a prospective clinical trial to limit or better define selection bias. There is such a prospective trial ongoing at Johns Hopkins Hospital where surgical resection of oligometastatic pancreas cancer (≤3 liver metastases) is offered following 6 months of systemic therapy if there is lack of disease progression.
Conclusion
We investigated patients who presented with synchronous distant metastases from PDAC who demonstrated excellent response to systemic chemotherapy and underwent resection of the primary tumor with or without metastasectomy. The median postoperative survival outcomes were similar to patients who undergo upfront resection and adjuvant chemotherapy for resectable disease. Excellent long-term survival was demonstrated in a select number of patients. As the agents and application of systemic therapy improves in PDAC, further study of the optimal treatment of this patient subgroup is warranted.
References
Howlander N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER cancer statistics review, 1975-2013, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission. Accessed May 25, 2016.
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Kehmissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montot-Grillot C, Ducreux M for the Groupe Tumeurs Digestives of Unicancer and the PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825.
Von Hoff DD, Ervin T, Arena F, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Custem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703.
Leal JN, Bressan AK, Vachharajani N, Gonen M, Kingham TP, D’Angelica MI, Allen PJ, DeMatteo RP, Doyle MB, Bathe OF, Greig PD, Wei A, Chapman WC, Dixon E, Jarnagin WR. Time-to-surgery and survival outcomes in resectable colorectal liver metastases: a multi-institutional evaluation. J Am Coll Surg. 2016;222:766-779.
Tarantino I, Warschkow R, Worni M, Cerny T, Ulrich A, Schmied BM, Guller U. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: a population-based, propensity score-adjusted trend analysis. Ann Surg. 2015;262:112-120.
Clancy C, Burke JP, Barry M, Kalady MF, Coffey JC. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol. 2014;21:3900-3908.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuch S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1.). Eur J Cancer. 2009;45:228-247.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213.
Shrikhande SV, Kleef J, Reiser C, Weitz J, Hinz U, Esposito I, Schmidt J, Friess H, Buchler MW. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2007;14:118-127.
Seelig SK, Burkert B, Chromik AM, Tannapfel A, Uhl W, Seelig MH. Pancreatic resections for advanced M1-pancreatic carcinoma: the value of synchronous metastasectomy. HPB Surg. 2010;2010:579672.
Klein F, Puhl G, Guckelberger O, Pelzer U, Pullankavumkal JR, Guel S, Neuhaus P, Bahra M. The impact of simultaneous liver resection for occult liver metastases of pancreatic adenocarcinoma. Gastroenterol Res Pract. 2012;2012:939350.
Schneitler S, Kropil P, Riemer J, Antoch G, Knoefel WT, Haussinger D, Graf D. Metastasized pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection. World J Gasteroenterol. 2015;21:6384-6390.
Neofytou K, Giakoustidis A, Smyth EC, Cunningham D, Mudan S. A case of metastatic pancreatic adenocarcinoma with prolonged survival after combination of neoadjuvant FOLFIRINOX therapy and synchronous distal pancreatectomy and hepatectomy. J Surg Oncol. 2015;111:768-770.
Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zulke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481.
Boone BA, Steve J, Zenati MS, Hogg ME, Singhi AD, Bartlett DL, Zureikat AH, Bahary N, Zeh III HJ. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21:4351-4358.
Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem JL, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD. J Natl Cancer Inst. 2015;107:1-10.
McPhee J, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, Anderson FA, Tseng JF. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246-253.
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors meet authorship criteria according to the ICMJE definition.
Rights and permissions
About this article
Cite this article
Wright, G.P., Poruk, K.E., Zenati, M.S. et al. Primary Tumor Resection Following Favorable Response to Systemic Chemotherapy in Stage IV Pancreatic Adenocarcinoma with Synchronous Metastases: a Bi-institutional Analysis. J Gastrointest Surg 20, 1830–1835 (2016). https://doi.org/10.1007/s11605-016-3256-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3256-2