Abstract
Despite recent advances in surgical techniques including staple closure and ultrasonic devices, the reported incidence of postoperative pancreatic fistula (POPF) after distal pancreatectomy (DP) remains high. Therefore, we devised a new strategy in which the pancreatic stump is enveloped with the elevated jejunum (EJ) by a modified Blumgart anastomotic technique. Eighty-one patients who underwent open DP with splenectomy from January 2008 to December 2014 were enrolled. Comparisons were made between 42 patients who underwent placement of an EJ patch using the modified Blumgart method after scalpel transection and 39 patients who underwent scalpel transection alone, using unmatched and propensity score-matched analysis. After 25 patients from each group were selected by propensity score matching, the EJ patch technique was significantly associated with a lower incidence of clinically relevant POPF (P = 0.036). Multivariate analysis showed that the EJ patch was an independent predictor of a lower incidence of POPF (odds ratio, 0.16; 95 % confidence interval, 0.01–0.48; P = 0.017) as was the estimated remnant pancreatic volume. Addition of the EJ patch improves postoperative outcomes in patients who undergo open DP with splenectomy by scalpel transection and hand-sewn closure of the pancreatic remnant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatectomy may be associated with serious and potentially lethal complications. Despite the substantial decline in operative mortality over the past two decades, even high-volume centers with extensive experience in pancreatic surgery report major complication rates of 20 to 40 % after pancreatectomy.1–3 Most of this morbidity results from the development of postoperative pancreatic fistula (POPF) and intra-abdominal hemorrhage from ruptured aneurysms.2 Distal pancreatectomy (DP) is a standard procedure for benign and malignant neoplasms of the distal pancreas. Despite recent advances in surgical techniques and perioperative management, the reported incidence of POPF after DP ranged from 10 to 40 %.4–7 The main reported risk factors for POPF are a high body mass index, history of diabetes, large-volume pancreatic remnant, extended lymphadenectomy, longer operative time, pancreatic texture, diameter of the main pancreatic duct (MPD), and thick pancreatic stump after staple closure.7–12
In an effort to avoid this complication, numerous techniques and tools including staple closure, suture ligation with mattress stitches, pancreas banding, pancreaticoenteric anastomosis, surface active mesh, fibrin glue, and ultrasonic devices have been proposed and investigated.6,7,13–22 However, there is currently no universally accepted effective technique. A meta-analysis by Knaebel et al.5 revealed a trend favoring the stapling technique for pancreatic stump closure. In a large randomized controlled trial, however, Diener et al.6 demonstrated in the DISPACT study that staple closure was not associated with a lower incidence of POPF than was hand-sewn closure. Although staple closure is known to be simple technique, this trial implied that staple closure does not improve outcomes in spite of the high material cost.
POPF can occur after DP unless sufficient time and adequate conditions are provided for the process of healing and closing of the peripheral pancreatic ducts; this situation differs from that in patients who undergo pancreatoduodenectomy. Therefore, various methods for reinforcement of the pancreatic stump have been reported. We recently reported that modified Blumgart anastomosis during pancreaticojejunostomy dramatically reduced the incidence of POPF from 35.8 to 2.5 % compared with the conventional suturing technique in patients who underwent pancreatoduodenectomy.23 We applied this useful suturing technique in DP to envelope the pancreatic stump with the elevated jejunum (EJ). Surgical procedures highlighted and compared in most articles reporting on DP, including randomized controlled trials, are inconsistent mixtures of various pairs of alternatives such as staple closure versus hand-sewn closure, resection of the spleen versus preservation, and the open versus laparoscopic approach.6,7,24–26 It seems inappropriate to simultaneously evaluate all of these factors together in one comparison. In the present study, therefore, we only included patients who underwent open DP combined with splenectomy in which the pancreas was manually transected with a scalpel. The aim of this retrospective study was to evaluate the effect of adding an EJ patch using the modified Blumgart anastomotic technique. Propensity score-matched analysis was used to reduce the impact of background-related bias.
Methods
Patients
A prospectively maintained pancreatic resection database was queried to identify all cases of DP. From January 2008 to December 2014, 104 patients underwent DP at the Department of Gastroenterological Surgery (Surgery II), Nagoya University Graduate School of Medicine, Nagoya, Japan. The pancreas was transected using a scalpel in all cases, and the EJ patch was added in all cases exclusively after August 2011. Of all 104 patients, 17 who underwent laparoscopic DP and 9 who underwent spleen-preserving DP (these techniques overlapped in 3 patients) were excluded because the principal purpose of the present study was to compare the two abovementioned methods in patients who underwent open DP with splenectomy. Consequently, 39 preceding patients who underwent scalpel transection alone (no EJ patch group) and 42 patients who underwent EJ patch placement after scalpel transection (EJ patch group) were enrolled in this study. Written informed consent for inclusion in the current analysis was obtained from all patients as required by the Institutional Review Board of Nagoya University.
Distal Pancreatectomy
All operative procedures other than closure of the pancreatic stump were identical in the two groups, as previously reported.27,28 The pancreas was transected with a scalpel, and only the MPD on the cut surface was closed by a continuous suture of 5-0 polypropylene. Bleeding from the pancreatic parenchyma was controlled with a combination of cautery and suture ligation. Staplers were not used for transection of the pancreas even in the recent cases, reflecting the results of the DISPACT study,6 nor were energy devices or sealing devices used. Neither mattress sutures nor the fish mouth technique was used on the transected face. A silastic flexible drain (Blake®; Ethicon, Inc., Somerville, NJ, USA) was routinely placed adjacent to the pancreatic remnant and connected to a continuous-suction device (J-Vac Suction Reservoir; Johnson & Johnson, Tokyo, Japan). All operations were performed by the same surgical team, invariably including either or both of the two experienced surgical operators (T.F. and A.N.). All operative procedures were performed in the same manner throughout the 6 years of the study.
Elevated Jejunum Patch
EJ patch placement was added after August 2011. After scalpel transection of the pancreas and extraction of the specimen, the upper jejunum was transected with a stapler at approximately 20 cm aboral to the ligament of Treitz. The jejunum was elevated to the upper abdomen through the retrocolic route in a Roux-en-Y fashion, and the length of the Roux limb was about 15 cm.
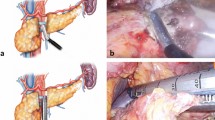
The cut surface of the remnant pancreas was covered by EJ using a modified Blumgart anastomosis technique. Briefly, we used a double-armed 4-0 polypropylene suture to place a U-suture with both arms through the pancreatic stump and a 10- to 15-mm longitudinal suture through the seromuscular layer of the EJ (Fig. 1a). Two such sutures were placed, and one of them, an upper suture in many cases, crossed the MPD (Fig. 1b). Sutures were then placed through the seromuscular layer of the jejunum 5 to 7 mm lateral to the previous sutures (Fig. 1b). These sutures were then tied to approximate the pancreas and jejunum at the ventral wall of the jejunum rather than on the surface of the pancreas to avoid laceration of the pancreas (Fig. 1c). This procedure completely covered the pancreatic stump with the jejunal serosa. After completion of the EJ patch, end-to-side anastomosis of the jejunum was performed.
Elevated jejunum (EJ) patch placement using the modified Blumgart technique. a Two U-sutures were placed with both arms through the pancreatic stump and a 10- to 15-mm longitudinal suture through the seromuscular layer of the EJ. b Sutures were then placed through the seromuscular layer of the jejunum 5 to 7 mm lateral to the previous sutures. c These sutures were then tied to approximate the pancreas and jejunum. This procedure completely covered the pancreatic stump with the jejunal serosa. After completion of the EJ patch, end-to-side anastomosis of the jejunum was performed. d Preoperative CT examination of the pancreas was performed to measure the estimated volume of the pancreatic remnant after distal pancreatectomy. The borders of the estimated pancreatic parenchymal remnant and the estimated transection line were outlined on every CT slice (gray area), and the corresponding volume was calculated as the product of the pancreatic parenchymal area times the slice thickness
Postoperative Management
No patients underwent preoperative radiotherapy or received postoperative somatostatin analogues. Second-generation cephem antibiotics were administered immediately before surgery and every 3 h during surgery.29 In all patients, administration of antibiotics and H2 blockers was routinely continued for 3 days after surgery. Oral intake was routinely started around postoperative day (POD) 4 unless postoperative complications such as delayed gastric emptying occurred.
The amylase concentration in the drainage fluid was measured on POD 1, 3, and 5. POPF was diagnosed and graded in accordance with the International Study Group on Pancreatic Fistula classification. POPF was diagnosed when the amylase concentration in the drainage fluid on or after POD 3 was more than three times the upper limit of the normal serum level.30 A grade B fistula (fistula requiring any therapeutic intervention) or higher was regarded as clinically significant. Abdominal drains were removed on POD 4 in patients without POPF. Overall postoperative complications were classified according to the Clavien–Dindo criteria.31
Evaluated Factors
Factors potentially associated with the formation of POPF were analyzed in the present study: age, sex, histologic diagnosis, lesion location, comorbidities, preoperative body mass index,32 blood test results (serum albumin level, total lymphocyte count, hemoglobin concentration, platelet count, cholinesterase level, and serum level of nutritional factors including prealbumin, transferrin, and retinol-binding protein), texture of the remnant pancreas, MPD diameter, operative time, blood loss volume, and intraoperative blood transfusion.
Measurement of Estimated Pancreatic Parenchymal Remnant Volume by Preoperative Computed Tomography
All patients underwent preoperative multiphasic computed tomography (CT) using a 64-channel multidetector CT scanner (Aquilion; Toshiba Medical Systems Corporation, Tokyo, Japan) following administration of intravenous contrast material. The estimated pancreatic parenchymal remnant volume was measured on transverse sections by two independent physicians who were blinded to the postoperative outcomes, as previously reported.10,33 Briefly, serial transverse CT images were obtained at 2.0-mm intervals, the borders of the estimated pancreatic parenchymal remnant and the estimated transection line were outlined on every CT slice, and the corresponding volume was calculated as the product of the pancreatic parenchymal area times the slice thickness (Fig. 1d). In patients with an MPD diameter of ≥2 mm, the MPD volume was also calculated. The estimated pancreatic parenchymal remnant volume was calculated by subtracting the estimated remnant MPD volume from the estimated whole pancreatic remnant volume.
Statistical Analysis
Before matching, baseline characteristics and surgical outcomes were compared between the no EJ patch and EJ patch groups. Differences in the numerical data between the two groups were examined using the chi-squared test or Fisher’s exact test when n < 5. Differences in quantitative variables between the two groups were evaluated using Student’s t test or the Mann–Whitney U test if the distribution was abnormal.
To reduce the impact of treatment selection bias and potential confounding in an observational study, matching between the no EJ patch and EJ patch groups was performed by estimating a propensity score for each patient and matching the patients from each group in a 1:1 ratio. A logistic regression model using eight covariates was performed to estimate the propensity score. The selected eight variables were clinicopathologic factors that could affect the postoperative outcome and treatment selection.34,35 These included five continuous variables (age [years], preoperative body mass index [kg/m2], operative time [min], intraoperative blood loss [mL], and the estimated pancreatic parenchymal remnant volume [cm3]) and three categorical variables (sex [male or female], history of preoperative diabetes mellitus, and texture of pancreatic remnant [soft or hard]). Each patient in the EJ patch group was matched to a patient in the no EJ patch group using greedy nearest neighbor matching at a ratio of 1:1 within a specified caliper width.36,37 This matching was performed without replacement using a caliper width of 0.2 standard deviations of the logit of the estimated propensity score.38
After propensity score matching, multivariable linear regressions for the length of the postoperative hospital stay, length of drain placement, and drainage fluid amylase level on POD 1, 3, and 5 were performed with adjustment for propensity scores. Logistic regressions for clinically relevant POPF (grade B/C), intra-abdominal abscess formation, and postoperative ileus were also conducted with adjustment for propensity scores. Predictive factors for clinically relevant POPF were evaluated using binomial logistic regression analysis. Statistical analysis was performed using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC, USA). A P value of <0.05 was considered statistically significant.
Results
Characteristics and Preoperative Status of all Unmatched Patients
The characteristics, preoperative status, and preoperative blood test results of all patients (n = 81) are summarized in Table 1. There were no significant differences in median age or sex between the no EJ patch group and EJ patch group. The two groups did not differ significantly in terms of preoperative comorbidities including a history of diabetes mellitus. The preoperative albumin level, total lymphocyte count, hemoglobin concentration, platelet count, cholinesterase level, and levels of rapid-turnover proteins including prealbumin, transferrin, and retinol-binding protein were also similar in both groups. No significant difference was found in the estimated pancreatic parenchymal volume.
Perioperative Details and Postoperative Complications in Unmatched Patients
Table 2 shows the perioperative status and postoperative complications of the two groups. The operative time tended to be longer in the EJ patch group, although there was no significant difference. The performance of intraoperative blood transfusions, diameter of the MPD, and incidence of combined resection of other organs were comparable between the two groups; however, no significant differences were found in the blood loss volume or texture of the remnant pancreas.
The overall postoperative complication rate tended to be lower in the EJ patch group than in the no EJ patch group (55 vs 67 %, respectively; P = 0.274). The rate of clinically relevant POPF (grade B/C) formation was significantly lower (10 vs 35 %, respectively; P = 0.006) and the median duration of drain placement was significantly shorter (9 vs 12 days, respectively; P = 0.030) in the EJ patch group. POPF-related intra-abdominal hemorrhage was found in one patient in each group. The incidence of postoperative ileus and infectious complications including intra-abdominal abscess formation, bacteremia, and wound infection were also statistically similar between the two groups. There was no case of 30-day mortality in either group, and in-hospital death occurred in one patient in the no EJ patch group. The median length of the postoperative hospital stay was significantly shorter in the EJ patch group (11 vs 17 days, respectively; P = 0.006).
Analysis of Propensity Score-Matched Patients
To reduce the impact of selection bias, propensity score matching was performed using eight selected baseline characteristics. After 25 patients in each group were matched, the statistical differences between the two matched groups in terms of the blood loss volume and texture of the remnant pancreas disappeared (P = 0.367 and P = 0.480, respectively) (Table 3).
Figure 2 shows comparisons of drainage fluid amylase concentrations in matched patients. The median amylase concentration was significantly lower in the EJ patch group than in the no EJ patch group on POD 1 (2466 vs 4070 IU/L, respectively; P = 0.025) and POD 3 (577 vs 1437 IU/L, respectively; P = 0.047). Table 4 shows the results of the multivariate linear regressions. The drainage fluid amylase levels on POD 1 and 3 were 2607.57 and 1185.64 IU/L less in the EJ patch group than in the no EJ patch group (95 % confidence interval, –4569.78 to –645.36; P = 0.010 and 95 % confidence interval, –2030.45 to –340.83; P = 0.007, respectively). Table 5 shows the results of the multivariate logistic regressions in which the incidence of clinically relevant POPF (grade B/C) was significantly lower in the EJ patch group (odds ratio, 0.18; 95 % confidence interval, 0.03–0.97; P = 0.036) and was not associated with the presence of overall postoperative complications, intra-abdominal abscess formation, or postoperative ileus.
Comparison of amylase concentration in the drainage fluid in matched patients. The median amylase concentration was significantly lower in the elevated jejunum (EJ) patch group than in the no EJ patch group on postoperative day (POD) 1 (2466 vs 4070 IU/L, respectively; P = 0.025) and POD 3 (577 vs 1437 IU/L, respectively; P = 0.047)
Factors Predicting Clinically Relevant POPF
The factors predicting POPF in propensity score-matched patients are shown in Table 6. Univariate analyses showed that a lower preoperative total lymphocyte count and a larger estimated remnant pancreatic volume were significantly associated with POPF formation, and that POPF formation was significantly less frequent in the EJ patch group than in the no EJ patch group. Multivariate analysis showed that the EJ patch technique was an independent predictor of a lower rate of clinically relevant POPF formation (odds ratio, 0.16; 95 % confidence interval, 0.01–0.48; P = 0.017) as was the estimated remnant pancreatic volume (odds ratio, 4.23; 95 % confidence interval, 2.74–12.64; P = 0.011)
Discussion
Pancreatic surgeons worldwide have attempted to develop various techniques to reduce the incidence of POPF formation after DP, including staple closure and transection by energy devices; however, all attempts have failed to show clinical benefits. Recently, new surgical stapler devices including the Endo-GIA reloads with Tri-staple Technology (Covidien, New Haven, CT, USA) or ECHELON FLEX Powered ENDOPATH Stapler (Ethicon Endo-Surgery, Cincinnati, OH, USA) have been developed. In a recent single-institution randomized controlled trial of staple closure with mesh reinforcement, the incidence of POPF at 1.9 % was outstandingly low when compared with previous reports of staple closure.39 However, this contradicts the fact that staple closure was associated with a significantly higher incidence of POPF in the largest consecutive series.7
With respect to the methods used for reinforcement of the pancreatic stump, some small-cohort studies showed that the addition of a falciform ligament patch reduced the rate of POPF; however, a recent randomized controlled trial denied the efficacy of this technique.24 The efficacy of seromuscular patches of the digestive tract has not yet been fully addressed. Moriura et al.19 proposed a unique but complicated method involving a pedicled seromuscular flap of the stomach or jejunum, but did not report the surgical outcome. Kluger et al.20 described four cases of staple closure with a gastric wall patch. Oláh et al.26 compared staple closure alone and staple closure with a seromuscular jejunal patch in a randomized controlled trial and reported that the incidence of clinically relevant POPF was comparable between the two techniques. The techniques described in these studies involved simple suturing between the pancreatic stump and the wall of the digestive tract, including the stomach or small intestine. Some recent reports of pancreaticojejunostomy in patients undergoing pancreatoduodenectomy described high efficacy and credibility of Blumgart anastomosis,40,41 and we have herein reported a simplified version of this technique with even better operative results.23 In the present study, a modified Blumgart suturing method was applied to DP, and the efficacy of the addition of an EJ patch using this modified Blumgart technique was compared with that of scalpel transection alone by propensity score-matched analysis to reduce treatment selection bias. We chose the jejunum, not the stomach, to reinforce the pancreatic stump because delayed gastric emptying reportedly occurs at a high rate (13.9 %) among patients who undergo seromuscular patch placement using the stomach wall.7 Addition of the EJ patch significantly reduced the median amylase concentration in the drainage fluid and the incidence of clinically relevant POPF, and shortened both the length of drain placement and postoperative hospital stay. This technique may have some disadvantages associated with bowel transection; however, there were no significant differences in the operative blood loss volume or postoperative complications, including ileus. The operative time tended to be longer in the EJ patch group, but not significantly so presumably because of the short time necessary for the EJ patch placement. Our multivariate analysis showed that the estimated remnant pancreatic parenchymal volume and addition of the EJ patch were independent predictors of POPF formation. This result is compatible with previous reports.10,33 The estimated remnant pancreatic volume was confirmed to be significantly associated with pancreatic juice secretion and the drain amylase content, supporting our result that patients with a large remnant pancreatic parenchyma volume have a high capacity for secretion of pancreatic juice and thus a higher rate of POPF formation, although most surgeons attempt to conserve the pancreatic parenchyma to prevent endocrine dysfunction.
The limitation of this study is its nonrandomized design. Despite efforts to control for baseline factors by matching, this was not a prospective, randomized trial, and the two groups of patients were not the same. Although there may be concern that the incidence of POPF formation (32 %) was rather high among the matched patients in the no EJ patch group, the incidence of POPF was 28 % in the hand-sewn group of the DISPACT study6; thus, there appears to be no considerable difference. The length of postoperative stay in the present study was slightly longer than that in previous reports. This probably reflects the differences in medical insurance systems, including lower hospitalization fees, and the fact that the postoperative stay in Japan generally tends to be much longer than that in other developed countries, as previously described.42,43
Conclusions
In conclusion, addition of the EJ patch is safe and simple and improves postoperative outcomes in patients undergoing open DP with scalpel transection and hand-sewn closure of the pancreatic remnant combined with splenectomy. We suggest that the EJ patch is suitable for use as a standard method of reinforcement after DP and could be more beneficial if added to staple closure with mesh reinforcement or laparoscopic pancreatectomy.
References
DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, Clavien PA. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 2006;244:931-937.
Balcom JHT, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg 2001;136:391-398.
Nagai S, Fujii T, Kodera Y, Kanda M, Sahin TT, Kanzaki A, Yamada S, Sugimoto H, Nomoto S, Takeda S, Morita S, Nakao A. Impact of operative blood loss on survival in invasive ductal adenocarcinoma of the pancreas. Pancreas 2011;40:3-9.
Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg 1999;229:693-698.
Knaebel HP, Diener MK, Wente MN, Büchler MW, Seiler CM. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg 2005;92:539-546.
Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, Tomazic A, Bruns CJ, Busch OR, Farkas S, Belyaev O, Neoptolemos JP, Halloran C, Keck T, Niedergethmann M, Gellert K, Witzigmann H, Kollmar O, Langer P, Steger U, Neudecker J, Berrevoet F, Ganzera S, Heiss MM, Luntz SP, Bruckner T, Kieser M, Büchler MW. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377:1514-1522.
Kleeff J, Diener MK, Z'graggen K, Hinz U, Wagner M, Bachmann J, Zehetner J, Müller MW, Friess H, Büchler MW. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg 2007;245:573-582.
Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 2011;98:268-274.
Seeliger H, Christians S, Angele MK, Kleespies A, Eichhorn ME, Ischenko I, Boeck S, Heinemann V, Jauch KW, Bruns CJ. Risk factors for surgical complications in distal pancreatectomy. Am J Surg 2010;200:311-317.
Frozanpor F, Albiin N, Linder S, Segersvärd R, Lundell L, Arnelo U. Impact of pancreatic gland volume on fistula formation after pancreatic tail resection. JOP 2010;11:439-443.
Ferrone CR, Warshaw AL, Rattner DW, Berger D, Zheng H, Rawal B, Rodriguez R, Thayer SP, Fernandez-del Castillo C. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg 2008;12:1691-1697.
Yoshioka R, Saiura A, Koga R, Seki M, Kishi Y, Morimura R, Yamamoto J, Yamaguchi T. Risk factors for clinical pancreatic fistula after distal pancreatectomy: analysis of consecutive 100 patients. World J Surg 2010;34:121-125.
Harris LJ, Abdollahi H, Newhook T, Sauter PK, Crawford AG, Chojnacki KA, Rosato EL, Kennedy EP, Yeo CJ, Berger AC. Optimal technical management of stump closure following distal pancreatectomy: a retrospective review of 215 cases. J Gastrointest Surg 2010;14:998-1005.
Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PW. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg 2003;90:190-196.
Farkas G, Leindler L, Farkas Jr G Safe closure technique for distal pancreatic resection. Langenbecks Arch Surg 2005;390:29-31.
Sadek S, Holdsworth R, Cuschieri A. Experience with pancreatic banding: results of a simple technique for dealing with the pancreatic remnant after distal partial pancreatectomy. Br J Surg 1988;75:486-487.
Wagner M, Gloor B, Ambühl M, Worni M, Lutz JA, Angst E, Candinas D. Roux-en-Y drainage of the pancreatic stump decreases pancreatic fistula after distal pancreatic resection. J Gastrointest Surg 2007;11:303-308.
Suzuki Y, Fujino Y, Tanioka Y, Hori Y, Ueda T, Takeyama Y, Tominaga M, Ku Y, Yamamoto YM, Kuroda Y. Randomized clinical trial of ultrasonic dissector or conventional division in distal pancreatectomy for non-fibrotic pancreas. Br J Surg 1999;86:608-611.
Moriura S, Kimura A, Ikeda S, Iwatsuka Y, Ikezawa T, Naiki K. Closure of the distal pancreatic stump with a seromuscular flap. Surg Today 1995;25:992-994.
Kluger Y, Alfici R, Abbley B, Soffer D, Aladgem D. Gastric serosal patch in distal pancreatectomy for injury: a neglected technique. Injury 1997;28:127-129.
Ohwada S, Ogawa T, Tanahashi Y, Nakamura S, Takeyoshi I, Ohya T, Ikeya T, Kawashima K, Kawashima Y, Morishita Y. Fibrin glue sandwich prevents pancreatic fistula following distal pancreatectomy. World J Surg 1998;22:494-498.
Thaker RI, Matthews BD, Linehan DC, Strasberg SM, Eagon JC, Hawkins WG. Absorbable mesh reinforcement of a stapled pancreatic transection line reduces the leak rate with distal pancreatectomy. J Gastrointest Surg 2007;11:59-65.
Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, Hattori M, Inokawa Y, Nomoto S, Fujiwara M, Kodera Y. Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg 2014;18:1108-1115.
Carter TI, Fong ZV, Hyslop T, Lavu H, Tan WP, Hardacre J, Sauter PK, Kennedy EP, Yeo CJ, Rosato EL. A dual-institution randomized controlled trial of remnant closure after distal pancreatectomy: does the addition of a falciform patch and fibrin glue improve outcomes? J Gastrointest Surg 2013;17:102-109.
Montorsi M, Zerbi A, Bassi C, Capussotti L, Coppola R, Sacchi M; Italian Tachosil Study Group. Efficacy of an absorbable fibrin sealant patch (TachoSil) after distal pancreatectomy: a multicenter, randomized, controlled trial. Ann Surg 2012;256:853-859.
Oláh A, Issekutz A, Belágyi T, Hajdú N, Romics L Jr. Randomized clinical trial of techniques for closure of the pancreatic remnant following distal pancreatectomy. Br J Surg 2009;96:602-607.
Kanda M, Fujii T, Sahin TT, Kanzaki A, Nagai S, Yamada S, Sugimoto H, Nomoto S, Takeda S, Kodera Y, Morita S, Nakao A. Invasion of the splenic artery is a crucial prognostic factor in carcinoma of the body and tail of the pancreas. Ann Surg 2010;251:483-487.
Sahin TT, Fujii T, Kanda M, Nagai S, Kodera Y, Kanzaki A, Yamamura K, Sugimoto H, Kasuya H, Nomoto S, Takeda S, Morita S, Nakao A. Prognostic implications of lymph node metastases in carcinoma of the body and tail of the pancreas. Pancreas 2011;40:1029-1033.
Fujii T, Yamada S, Suenaga M, Kanda M, Takami H, Sugimoto H, Nomoto S, Nakao A, Kodera Y.Preoperative internal biliary drainage increases the risk of bile juice infection and pancreatic fistula after pancreatoduodenectomy: a prospective observational study. Pancreas 2015;44:465-470.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205-213.
Fujii T, Kanda M, Nagai S, Suenaga M, Takami H, Yamada S, Sugimoto H, Nomoto S, Nakao A, Kodera Y. Excess Weight Adversely Influences Treatment Length of Postoperative Pancreatic Fistula: A Retrospective Study of 900 Patients. Pancreas 2015;44:971-976.
Kanda M, Fujii T, Takami H, Suenaga M, Inokawa Y, Yamada S, Kobayashi D, Tanaka C, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Kodera Y. Novel diagnostics for aggravating pancreatic fistulas at the acute phase after pancreatectomy. World J Gastroenterol 2014;20:8535-8544.
Bonjer HJ, Hop WC, Nelson H, Sargent DJ, Lacy AM, Castells A, Guillou PJ, Thorpe H, Brown J, Delgado S, Kuhrij E, Haglind E, Påhlman L; Transatlantic Laparoscopically Assisted vs Open Colectomy Trials Study Group. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg 2007;142:298-303.
Thorpe H, Jayne DG, Guillou PJ, Quirke P, Copeland J, Brown JM; Medical Research Council Conventional versus Laparoscopic-Assisted Surgery In Colorectal Cancer Trial Group. Patient factors influencing conversion from laparoscopically assisted to open surgery for colorectal cancer. Br J Surg 2008;95:199-205.
Reeve BB, Smith AW, Arora NK, Hays RD. Reducing bias in cancer research: application of propensity score matching. Health Care Financ Rev. 2008;29:69-80.
D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-2281.
Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J 2009;51:171-184.
Hamilton NA, Porembka MR, Johnston FM, Gao F, Strasberg SM, Linehan DC, Hawkins WG. Mesh reinforcement of pancreatic transection decreases incidence of pancreatic occlusion failure for left pancreatectomy: a single-blinded, randomized controlled trial. Ann Surg 2012;255:1037-1042.
Kleespies A, Rentsch M, Seeliger H, Albertsmeier M, Jauch KW, Bruns CJ. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg 2009;96:741-750.
Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg 2010;210:54-59.
Tokunaga J, Imanaka Y. Influence of length of stay on patient satisfaction with hospital care in Japan. Int J Qual Health Care 2002;14:493-502.
Kondo A, Zierler BK, Isokawa Y, Hagino H, Ito Y. Comparison of outcomes and costs after hip fracture surgery in three hospitals that have different care systems in Japan. Health Policy 2009;91:204-210.
Acknowledgments
The authors are grateful to Leon Sakuma, BA, of the Kawasaki University of Medical Welfare, for providing the illustrations.
Grant support
No grant support was provided for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujii, T., Yamada, S., Murotani, K. et al. Modified Blumgart Suturing Technique for Remnant Closure After Distal Pancreatectomy: a Propensity Score-Matched Analysis. J Gastrointest Surg 20, 374–384 (2016). https://doi.org/10.1007/s11605-015-2980-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2980-3