Abstract
Background
Gastric cardia cancer is currently treated with several operations. The purpose of the current study was to compare outcomes associated with three common operative approaches.
Methods
The ACS-NSQIP Participant Use File was searched to identify all patients with gastric cardia malignancy who underwent total gastrectomy (TG), transhiatal esophagectomy (THE), or thoraco-abdominal esophagectomy (TAE) between 2005 and 2012. Demographic, perioperative risk factors, and outcomes were analyzed.
Results
Overall, there were 982 patients identified in the database who met inclusion criteria. The median age was 65 years (range 20–88) and 807 (82.2 %) were male. The number of patients allocated to each approach was 204 TGs (20.8 %), 271 THE (27.6 %), and 507 TAE (51.6 %). All approaches had similar major morbidity, cardiopulmonary morbidity, and 30-day mortality, however, TAE was associated with the highest overall morbidity (TAE 49.9 % vs. TG 40.7 % and THE 43.5 %, p = 0.048). The independent risk factors predicting mortality were age greater than 65 years, history of myocardial infarction, and postoperative cardiopulmonary morbidity.
Conclusions
For patients with proximal gastric cancer, the three most common operative approaches were associated with clinically-significant rates of overall and major morbidity. Approach-associated morbidity should be considered along with tumor location and extent when choosing a technique for resection of gastric cardia malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Tumors of the gastroesophageal junction and gastric cardia present many challenges to the clinician, the first of which is accurate classification. Gastric cardia malignancies are considered separate entities from both esophageal and distal gastric cancers.1 The methods of characterizing proximal gastric malignancies include the American Join Committee on Cancer (AJCC) and Siewert classifications which both emphasize commonalities with esophageal cancer.2–4 Although gastric cancer incidence and mortality have decreased over the previous several decades, gastric cancer remains the fourth most common cancer and second leading cause of cancer-related death worldwide.5 In the USA, gastric cancer is the seventh most common cause of cancer-related death.5 In addition, the incidences of proximal gastric adenocarcinoma and esophageal adenocarcinoma have been observed to increase in recent years despite a decrease in the incidence of all gastric adenocarcinomas.6–8
Several different operative approaches are commonly employed for resection of proximal gastric cancers, including abdominal, transthoracic, and thoraco-abdominal. For tumors judged to be more distal and sharing characteristics with other gastric adenocarcinomas, total gastrectomy is often employed as first-line surgical therapy. A recent American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) analysis showed that total gastrectomy had increased rates of major morbidity and mortality when compared to partial gastrectomy; however, this analysis looked at all gastric malignancies and did not differentiate based on disease site.9 Likewise, another recent ACS-NSQIP analysis compared gastrectomy to esophagectomy for proximal gastric cancer, but did not parse the surgical approach.10
More proximal gastroesophageal cancers are typically treated in a manner similar to distal esophageal malignancies requiring gastroesophageal resection for adequate treatment. A recent meta-analysis of transthoracic approaches to esophageal resection shows increased postoperative morbidity but equivalent short-term mortality when compared to abdominal approaches.11 Approach-associated morbidity rates are important, as complications from surgical resection of proximal gastric cancer can lead to delays in adjuvant therapy, which adversely affect oncologic outcomes.12–15
The aim of this study was to characterize the short-term morbidity and mortality associated with various surgical approaches utilized to resect malignancies of the gastric cardia. Additionally, this study sought to characterize risk factors for morbidity and mortality that are common to patients undergoing resection of a gastric cardia malignancy.
Methods
The ACS-NSQIP is a multi-center, prospectively gathered, and risk-adjusted database. The ACS-NSQIP database includes more than 150 perioperative risk factor and outcomes variables. Details regarding ACS-NSQIP data collection, variables, and analysis have been previously published.16
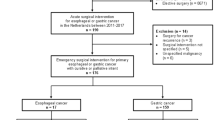
The ACS-NSQIP Participant Use File (PUF) was interrogated to identify patients who underwent an operation for malignant neoplasm predominantly located at the gastric cardia (ICD-9 code 151.0 only) at participating NSQIP hospitals from 2005 to 2012. Current Procedure Terminology (CPT) codes were used to group patients by surgical approach. Patients who underwent total gastrectomy (TG) (CPT code 43620, 43621, and 43622), transhiatal esophagogastrectomy (THE) (CPT code 43107), or thoraco-abdominal esophagogastrectomy (TAE) (CPT code 43112 and 43117) were identified. Ancillary PUF fields were used to exclude esophagectomies associated with an intestinal conduit and emergency operations from analysis. There were 204 patients who underwent TG, 271 patients underwent THE, and 507 patients underwent TAE. These patients’ clinical characteristics are detailed in Table 1. The surgical approach was also recoded into a binary categorical variable with the two abdominal (non-thoracotomy) approaches (TG + THE) combined and compared to TAE.
Major morbidity was defined as the occurrence of at least one of the following complications: organ space infection, pneumonia, unplanned intubation, pulmonary embolism, ventilator requirement greater than 48 h, progressive renal insufficiency, acute renal failure, cerebrovascular accident, coma, cardiac arrest, myocardial infarction, deep venous thrombosis, sepsis, septic shock, and return to the operating room.17 Cardiopulmonary morbidity was defined as the occurrence of at least one of the following complications: pneumonia, unplanned intubation, pulmonary embolism, ventilator requirement greater than 48 h, cardiac arrest, or myocardial infarction. Mortality was defined as death from any cause within 30 days of surgery or death occurring over 30 days from surgery if the operation and mortality were within a single prolonged hospitalization.
Statistics
All continuous variables are reported as median value with interquartile range (IQR) and/or range, or mean value ± standard deviation. Continuous variables were compared using the Kruskal-Wallis test or the Mann–Whitney U test. Categorical variables were compared using the chi-squared test or Fisher’s exact test. A 2-tailed p value less than 0.05 was considered significant. All appropriate risk factors found to have a univariate p value less than 0.10 were included in multivariate analysis. Multivariate analysis was done using binary logistic regression and the backwards stepwise method. All statistical analysis was performed using SPSS Version 22.0.0.0 (SPSS, Inc., Chicago, IL, USA).
Results
Overall, there were 982 patients identified in the database who met inclusion criteria. The median age was 65 years (range 20–88) and 807 (82.2 %) were male. The number of patients allocated to each approach was 204 TGs (20.8 %), 271 THE (27.6 %), and 507 TAE (51.6 %). Patients undergoing TG were older (p < 0.001), had lower ASA scores (p = 0.030), and were less likely to be male (p < 0.001) than those undergoing THE or TAE. Patients in all three groups had similar rates of functional independence, body mass index (BMI), smoking, and alcohol use (Table 1).
The most frequently present preoperative comorbidity for all three approaches was hypertension. There were few differences in preoperative comorbidity rates; however, patients with CHF or renal failure were found exclusively in the TG group, all be it at low levels. Patients undergoing a THE and TAE resection were more likely to receive preoperative radiotherapy than those undergoing gastric only resection (TG 9.8 %, THE 24.7 %, and TAE 23.9 %, p < 0.001). Patients undergoing TG were also more likely to have had an operation in the previous 30 days (p = 0.016) (Table 2).
Regarding perioperative outcomes, operative times were increased for TAE (391.1 ± 121.2 min) vs. TG (321.1 ± 130.3 min) or THE (294.1 ± 101.1 min) (p < 0.001). Hospital length of stay was near 14 days for all patients in this cohort, with no differences based on surgical approach. Morbidity (TG 40.7 %, THE 43.5 %, and TAE 49.9 %, p = 0.048) and major morbidity (TG 32.8 %, THE 32.1 %, and TAE 36.7 %, p = 0.368) were considerable across all three approaches with TAE being the most morbid procedure (Fig. 1). Although cardiopulmonary morbidity rates were equivalent across approaches, postoperative myocardial infarction was more common after TAE (2.0 %) compared to TG (0 %) and THE (0.4 %) (p = 0.035). There was no difference in 30-day mortality by operative approach (Table 3). Additionally, TAE had higher rates of perioperative transfusion (p = 0.035) when compared to abdominal approaches (Table 4).
For all patients with proximal gastric cancer undergoing surgery, the factors associated with major morbidity by univariate analysis included smoking (p = 0.042), alcohol use (p = 0.013), dyspnea (p = 0.031), diabetes (p = 0.033), COPD (p = <0.001), history of myocardial infarction (p = 0.003), hypertension treated with medication (p = 0.011), perioperative transfusion (p = 0.034), prior cardiothoracic surgery (p = 0.003), and preoperative chemotherapy (p = 0.016). On multivariate analysis, alcohol use (p = 0.023, OR = 2.30), COPD (p = 0.001, OR = 2.69), history of myocardial infarction (p = 0.041, OR = 9.26), hypertension treated with medication (p = 0.034, OR = 1.35), perioperative transfusion (p = 0.034, OR = 1.45), prior cardiothoracic surgery (p = 0.050, OR = 2.54), and operation in the previous 30 days (p = 0.049, OR = 2.94) were independent factors associated with major morbidity. The only independent factor protective for major morbidity was preoperative chemotherapy (p = 0.005, OR = 0.50).
The factors which were associated with mortality included age greater than 65 years old (p = 0.003), dyspnea (p = 0.009), diabetes (p = 0.022), COPD (p = 0.019), history of myocardial infarction (p = 0.015), hypertension (p = 0.031), renal failure (p = 0.048), operation within the previous 30 days (p = 0.044), any morbidity (p < 0.001), major morbidity (p < 0.001), and major morbidity (p < 0.001). On multivariate analysis, the independent predictors of 30-day mortality were age greater than 65 years (p = 0.003, OR = 8.34), history of myocardial infarction (p = 0.004, OR = 21.89), and cardiopulmonary morbidity (p < 0.001, OR = 27.03) (Table 5).
Discussion
Analysis of the ACS NSQIP data shows that there is significant morbidity associated with all surgical approaches to resection of proximal gastric cancer. TAE proved to be the most morbid of procedures likely because of the additional thoracic field. It is important to note that the study population was contained to patients diagnosed with proximal gastric cancer, excluding patients coded as esophageal cancer. Although the utilization of TAE could indicate appropriate surgical management of a more aggressive gastroesophageal junction lesion that may engender more complications, in this dataset, there were identical rates of neoadjuvant chemoradiotherapy between the THE and TAE groups, suggesting that choice of surgical technique was more surgeon specific than patient or cancer specific. These data indicate that when oncologic equipoise is present, the least morbid approach should be considered.
The ACS-NSQIP dataset does not collect information regarding the use of laparoscopy prior to 2010 and therefore was not analyzed in this study. One possible explanation for the differences in morbidity, besides an additional operative field, is the difference in laparoscopy utilization and diffusion to practice for each procedure. The first laparoscopic partial gastrectomy for cancer was reported in the early 1990s and laparoscopic total gastrectomy in the early 2000s. Laparoscopic gastrectomy for malignancy has proved to be a safe alternative to open surgery in a randomized controlled trial published in 2005 with at least equivalent long-term outcomes.18 The first minimally invasive esophagectomy was performed much later with the first randomized controlled trial showing equivalent improved short-term outcomes published in 2012.19 In the future, increased utilization of minimally invasive esophagectomy may help to decrease the gap in morbidity and length of stay as compared to open abdominal approaches.
In selecting an appropriate operative intervention for gastric cardia malignancy, there are still many variables to consider. The ability to perform a complete oncologic resection remains the cornerstone of operative resection; however, additional factors including patient age, comorbidity status, extent of gastroesophageal involvement, histologic type, and the risks inherent to each operation should also the factors into surgical decision making.20 Multi-modality treatment of proximal gastric cancer has been shown to improve outcomes but delays from surgical complications have been shown to lead to delays in adjuvant therapy as well as increased rates of early recurrence.12,13,21 In patients with significant comorbidities who are more likely to experience surgical complications, selection of the least morbid approach that will achieve an R0 resection is paramount to improving outcomes.
This analysis also demonstrates the importance of considering patient comorbidities when deciding to operate for proximal gastric cancer. A history of advanced age combined with prior myocardial infarction, renal failure, or a recent operation was associated with the highest risk and should trigger a specific informed discussion with the patient prior to proceeding with resection. The analysis did show modifiable risk factors associated with major morbidity. Patient’s alcohol consumption of greater than two drinks a day within 2 weeks of operation was independently associated with major morbidity. This indicates that patients should be counseled regarding alcohol cessation in the preoperative period. Additionally, having a history of another operation performed within 30 days was associated with major morbidity. While it’s not always possible to delay definitive cancer treatments due to a previous operation, clinicians should reconsider scheduling of multiple invasive procedures in temporal proximity.
This study has several limitations. In order to investigate proximal gastric cancer, gastric cardia malignancy was used as a surrogate diagnosis code. Gastric cardia malignancy is not a perfect surrogate for proximal gastric cancer and represents a weakness in the current ICD-9 code classification that is a component of the ACS-NSQIP database. In future iterations of diagnostic coding, it may be beneficial to redefine terminology used to report tumor location. The ACS NSQIP database does not collect cancer-specific variables including cancer staging, histologic grading, margin status, and resection status. The database also does not collect oncologic outcomes. Unfortunately, this limits the ability of the analysis to account for stage-specific differences in the utilization of neoadjuvant therapy and/or radical surgical resection that may be more risky, but simultaneously more likely provide optimal long-term oncologic outcomes. Finally, ACS-NSQIP data does not supply information regarding hospital or operative surgeon procedural volumes and specialty training that is likely to influence patient outcomes.22–24
Conclusion
In proximal gastric cancer, all three operative approaches were associated with clinically significant rates of major morbidity. Abdominal approaches are utilized with less frequency but are associated with significantly decreased morbidity. The ability to perform a complete oncologic resection, morbidity by approach, tumor location, margin status, clinical staging, and other patient risk factors all need to be considered in concert when selecting an operative approach for gastric cardia cancer.
References
Husemann B. Cardia carcinoma considered as a distinct clinical entity. Br J Surg. 1989;76(2):136–139.
Edge S BD, Compton Cc, Fritz Ag, Green Fl, Trotti A. AJCC Cancer Staging Manual. 2010.
Rice TW, Blackstone EH and Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–1724.
Siewert JR, Holscher AH, Becker K and Gossner W. [Cardia cancer: attempt at a therapeutically relevant classification]. Chirurg. 1987;58(1):25–32.
Jemal A, Bray F, Center MM, Ferlay J, Ward E and Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Blot WJ, Devesa SS, Kneller RW and Fraumeni JF, Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287–1289.
Devesa SS, Blot WJ and Fraumeni JF, Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–2053.
Vial M, Grande L and Pera M. Epidemiology of adenocarcinoma of the esophagus, gastric cardia, and upper gastric third. Recent Results Cancer Res. 2010;182:1–17.
Papenfuss WA, Kukar M, Oxenberg J, Attwood K, Nurkin S, Malhotra U and Wilkinson NW. Morbidity and Mortality Associated with Gastrectomy for Gastric Cancer. Ann Surg Oncol. 2014.
Martin JT, Mahan A, Zwischenberger JB, Mcgrath PC and Tzeng CW. Should Gastric Cardia Cancers Be Treated with Esophagectomy or Total Gastrectomy? A Comprehensive Analysis of 4,996 NSQIP/SEER Patients. J Am Coll Surg. 2015;220(4):510–520.
Yang K, Chen HN, Chen XZ, Lu QC, Pan L, Liu J, Dai B, Zhang B, Chen ZX, Chen JP and Hu JK. Transthoracic resection versus non-transthoracic resection for gastroesophageal junction cancer: a meta-analysis. PLoS One. 2012;7(6):e37698.
Ahmed S, Iqbal N, Yadav S, Zaidi A, Ahmed O, Alvi R, Gardner D and Haider K. Time to adjuvant therapy and other variables in localized gastric and gastroesophageal junction (GEJ) cancer (IJGC-D-13-00162). J Gastrointest Cancer. 2014;45(3):284–290.
Lerut T, Moons J, Coosemans W, Van Raemdonck D, De Leyn P, Decaluwe H, Decker G and Nafteux P. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg. 2009;250(5):798–807.
Xia BT, Rosato EL, Chojnacki KA, Crawford AG, Weksler B and Berger AC. Major perioperative morbidity does not affect long-term survival in patients undergoing esophagectomy for cancer of the esophagus or gastroesophageal junction. World J Surg. 2013;37(2):408–415.
Aloia TA, Zimmitti G, Conrad C, Gottumukalla V, Kopetz S and Vauthey JN. Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol. 2014;110(2):107–114.
Acs. User Guide for the 2012 ACS NSQIP. 2012.
Tzeng CW, Cooper AB, Vauthey JN, Curley SA and Aloia TA. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford). 2014;16(5):459–468.
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A and Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241(2):232–237.
Biere SS, Van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, De Lange ES, Bonjer HJ, Van Der Peet DL and Cuesta MA. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887–1892.
Ito H, Clancy TE, Osteen RT, Swanson RS, Bueno R, Sugarbaker DJ, Ashley SW, Zinner MJ and Whang EE. Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg. 2004;199(6):880–886.
Lerut T, Moons J, Coosemans W, Decaluwe H, Decker G, De Leyn P, Nafteux P and Van Raemdonck D. Multidisciplinary treatment of advanced cancer of the esophagus and gastroesophageal junction: a European center's approach. Surg Oncol Clin N Am. 2008;17(3):485–502, vii-viii.
Meyer HJ. The influence of case load and the extent of resection on the quality of treatment outcome in gastric cancer. Eur J Surg Oncol. 2005;31(6):595–604.
Coupland VH, Lagergren J, Luchtenborg M, Jack RH, Allum W, Holmberg L, Hanna GB, Pearce N and Moller H. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004–2008. Gut. 2013;62(7):961–966.
Anderson O, Ni Z, Moller H, Coupland VH, Davies EA, Allum WH and Hanna GB. Hospital volume and survival in oesophagectomy and gastrectomy for cancer. Eur J Cancer. 2011;47(16):2408–2414.
Author information
Authors and Affiliations
Corresponding author
Additional information
Primary Discussant
Kyle A. Perry, M.D. (Columbus, OH)
Thank you for sharing this nicely presented study examining the surgical approaches utilized to manage proximal gastric cancers. Proximal gastric cancer presents a challenging clinical dilemma, and consideration of oncologic outcome, the rate of return to normal functional status postoperatively, and perioperative risk all play an important role in planning the operative approach. This study aimed to identify predictive factors that predispose patients to major morbidity following esophagectomy or extended total gastrectomy in order to guide clinical decision making. In light of this, why was the decision made to combine the total gastrectomy and transhiatal esophagectomy groups in the multivariate analysis? These operations are associated with different risks and consequences, and it seems that analyzing them separately may identify different predictors for postoperative complications. Also, you noted significant differences between the patient populations undergoing esophagectomy and gastrectomy. Is it possible that a subset of the total gastrectomy patients was undergoing palliative surgery for inability to tolerate oral intake rather than a curative operation? If so, how might this influence the interpretation of the results. Congratulations again on an excellent presentation, and thank for the opportunity to discuss it.
Closing Discussant
Dr. Day
We thank the Dr. Perry for these insightful comments and questions.
Regarding the grouping of cases, initially each of the approaches was separately analyzed. However, there was recognition that the combined abdomino-thoracic approach may have a different complication profile compared to the two non-thoracotomy approaches (total gastrectomy and transhiatal esophagogastrectomy). The separate analysis yielded similar results to the combined analysis, but due to the small number of some comorbidities, the 95 % confidence intervals were very large. Since both groups had similar outcomes for the same disease, the decision was made to combine the analysis for increased power in the face of small numbers, allowing the potential to specifically comment on the morbidity of thoracotomy.
Regarding the use of total gastrectomy as a palliative maneuver, it is possible that some individuals underwent surgery without curative intent. Unfortunately, oncologic intent and stage of disease cannot be obtained from the NSQIP PUF. This having been said, we believe that most patients undergoing palliation for gastric cardia cancer would not have a total gastrectomy as the operation of choice in that scenario. Given the contemporary cohort, stenting, partial gastrectomy, bypass, or palliative feeding tube placement, which were excluded from the analysis, seem to be more likely surgical palliation options for this disease.
NSQIP Disclaimer
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in it represent the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or for the conclusions derived by the authors.
This manuscript was presented in a plenary session at the Annual Meeting of the SSAT on May 18th, 2015.
Rights and permissions
About this article
Cite this article
Day, R.W., Badgwell, B.D., Fournier, K.F. et al. Defining the Impact of Surgical Approach on Perioperative Outcomes for Patients with Gastric Cardia Malignancy. J Gastrointest Surg 20, 146–153 (2016). https://doi.org/10.1007/s11605-015-2949-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2949-2