Abstract
Background and Objectives
Indications for the resection of liver metastases from gastric cancers (GLM) remain controversial, and few previous studies have reported subsequent surgical outcomes. Thus, the present retrospective study was designed to clarify the benefits of surgical treatment and identify prognostic factors.
Methods
Outcomes of 47 patients with or without hepatectomy for GLM were retrospectively compared.
Results
A total of 22 patients received surgical treatment for GLM, and overall 1-, 3-, and 5-year survival rates were 86, 26, and 26 %, respectively, and the median survival time (MST) was 22 months. Among 25 patients who did not receive hepatic surgical treatment, the overall survival rates were 24, 8.0, and 4.0 % at 1-, 3-, and 5-years, respectively, with an MST of 7 months. A significant difference was observed between patients with and without the liver surgical treatment (P < 0.001). Univariate and multivariate analyses of recipients of surgery, only the number of liver metastases (solitary or multiple) was significantly predictive of survival (HR = 0.26, P = 0.029) following hepatic resection for GLM.
Conclusions
Surgical treatment of GLM should be considered when complete excision including the primary tumor appears to be possible, particularly in cases of solitary hepatic metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth most common cancer and the second most common cause of cancer-related death globally.1 In Japan, gastric cancer is second only to lung cancer as a cause of cancer death. Early tumor detection, curative surgical resection including extended lymph node dissection (D2), and appropriate adjuvant therapy have led to the improved survival of patients with primary gastric cancer. In a previous study, the resection rate in patients with primary gastric cancers was 95.4 %, and the 5-year survival rate of resected patients was 70.7 %.2 However, prognoses for patients with advanced or recurrent gastric cancer remain poor, with median survival time (MST) of approximately 1 year. The liver is one of the most common sites of advanced gastric cancer metastasis, and liver metastases from gastric cancer (GLM) are found in 4–14 % of patients with primary gastric cancer. Moreover, after the curative resection of primary gastric adenocarcinomas, 3.5–14 % of the patients experience intrahepatic recurrence.3–7 GLM are often diagnosed as multiple intrahepatic nodules occupying both lobes and coexist with extrahepatic disease, including peritoneal carcinomatosis, lymph node metastases, and direct tumor invasions of other organs. Although systemic chemotherapy with or without new molecular targeting agents is the standard treatment modality for GLM, the 5-year survival of patients with GLM without surgical treatment is <10 %.8 However, because outcomes in patients with noncurative resections for gastric cancer are extremely poor,9,10 the benefits of surgery for GLM remain debatable.
Although several authors have reported 5-year survival rates of 0–39 % among selected surgically resected patients with GLM,5,6,11–17 its significance is still controversial. Thus, given the severity of gastric cancer, surgical indications for GLM require careful investigation18 to identify patients with GLM who are most likely to receive benefits from surgical treatment. In the present retrospective study, the benefits of surgical treatment were assessed among patients with and without hepatectomy for GLM, and prognostic factors were identified.
Materials and Methods
Patients
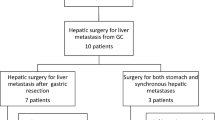
From 1995 to 2010, 857 patients with primary gastric cancer (adenocarcinoma) received surgery at the Department of Surgery, Hokkaido Cancer Center, Sapporo, Japan. Among these, a total of 47 patients (5.5 %) developed liver metastases, including 38 (4.4 %) with synchronous liver metastases and nine with metachronous liver metastases after resection of the primary gastric cancer. Liver resections were performed only in cases with curative potential. All possible alternative treatments were explained to the patients prior to surgery, and informed consent was obtained. Patients were excluded from analyses if they had received synchronous en bloc resections of gastric cancers that were directly invading the liver or no surgical treatment for metachronous liver metastases.
Thirteen of 38 patients with synchronous liver metastases received gastrectomy with concomitant hepatic resection or intraoperative radiofrequency ablation (RFA). The other 25 patients received laparotomy without surgical treatment for GLM. All nine patients with metachronous liver metastases received hepatic resection after curative primary tumor resection. A total of 22 patients with liver metastases received liver resection or RFA. Among these, three were noncurative, and two patients with metachronous liver metastases received repeat hepatectomy. Preoperative chemotherapy was performed in 14 cases (29.8 %), and eight patients received oral S1, including four patients who received S1 plus cisplatin and docetaxel (DCS), five patients received intravenous 5-fluorourcil (5-FU), and one patient received hepatic arterial infusions of 5-FU. Outcomes in these 47 patients with liver metastases were retrospectively reviewed, and patients were followed until death or until January 2014.
Study Design
A total of 47 patients were classified into groups according to surgical treatment for GLM. The following clinicopathological factors were retrospectively analyzed, and patients were divided to subgroups according to age, gender, status of serosa invasion (T4), lymph node metastases, and histological differentiation in the primary gastric cancer, status of extrahepatic metastasis, time between primary disease and liver metastasis (synchronous or metachronous), tumor number, size and location of liver metastases, types of liver surgical procedures, and receipt of systemic chemotherapy (preoperative and postoperative chemotherapy).
The depth of tumor penetration into the gastric wall (T parameter) and the number of metastatic regional lymph nodes involved (N parameter) were classified according to the 7th edition of the Union for International Cancer Control (UICC) at the time of gastric resection. Synchronous liver metastases were defined by detection before or during surgery or within 3 months of primary tumor resection. Operative death was defined as that occurring within 30 days of surgery. Morbidity included any type of complication, including surgical and nonsurgical events. The overall survival time was measured from the date of hepatic resection or other surgical operation in patients without hepatectomy until the date of death.
Statistical Analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan, 2012), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria version 2.13.0). This modified version of R commander (version 1.6-3) was designed to add statistical functions that are frequently used in biostatistics. Univariate analyses of categorical data were performed using cross-linked tables and Fisher’s exact test. Differences were considered significant when P < 0.05. Survival analyses were performed using the Kaplan–Meier method. Prognostic factors were identified using univariate and multivariate analyses with log-rank tests and Cox’s proportional hazard models.
Results
Patients and Tumor Characteristics
The baseline characteristics of eligible patients are presented in Table 1. The study group comprised 38 men and nine women with an average age of 66.7 years (range 29–81). A total of 22 patients received surgery for GLM. At the time of diagnosis, 13 patients had synchronous metastases and nine had metachronous liver metastases. Surgical procedures for GLM included anatomic resection in six patients (27 %), limited resection in 12 patients (56 %), and RFA in four patients (18 %). The other 25 patients had synchronous liver metastases and did not receive hepatic surgery. The distribution of surgical procedures included primary gastric resection in 21 patients (84 %) and exploratory laparotomy in four patients only (16 %).
Six of 14 patients who received preoperative chemotherapy also received surgical treatment for liver metastases. Gastrectomy was performed in eight patients. Three patients (DCS, n = 2; S1 plus various drugs, n = 1) achieved complete clinical responses for liver tumors before surgery. One patient received gastrectomy with partial liver resection and survived for >5 years without further recurrence. Two patients who received gastrectomy without liver resection were recorded as curable and were included in the no hepatic surgery group. One of these patients survived for >5 years, and the other died of systemic recurrence at 39 months after surgery.
Characteristics of primary gastric cancers, such as the status of T4 factor, lymph node metastasis, and extrahepatic metastasis, significantly differed between patients who did and did not receive surgery for GML. The states of liver metastases also significantly differed, with differing distributions of metastatic nodules. However, no differences in numbers or sizes of lesions were observed.
Operative Outcomes in All Patients
Among the 22 patients who received surgical treatment for liver metastases, the overall 5-year survival rate was 26 % and MST was 22 months (range 15–35). Among the 25 patients without aggressive treatment, the overall survival rate was 4.0 % at 5 years with an MST of 7 months (range 5–9). A significant difference was observed between patients with and without the liver surgical treatment (P < 0.001, Fig. 1). Three patients in the treatment group and one in the no treatment group were still alive at the end of the cutoff date.
Overall survival of patients with and without hepatic surgical treatment. For the 22 patients in the surgical treatment for liver metastases (solid line), the overall 1-, 3-, and 5-year survival rates were 86, 26, and 26 %, respectively. The median survival time (MST) was 22 months (range 15–35). In the 25 patients without hepatic surgical treatment (dotted line), the overall survival rate was 24, 8.0, and 4.0 % at 1-, 3-, and 5-years, respectively, with the MST of 7 months (range 5–9). A significant difference was observed between patients with and without the liver surgical treatment (P < 0.001)
Univariate analyses of all 47 patients revealed that the curability of gastric cancer (hazard ratio = 0.113), surgical treatment for GLM (0.273), T4 factor (0.3), degree of histological differentiation in the primary gastric cancer (0.512), distribution of liver metastases (0.362), solitary liver metastasis (0.388), and status of extrahepatic metastases (0.45) significantly influenced prognoses for GLM (Table 2). Multivariate analyses of all 47 patients revealed that surgical treatment for liver metastasis (0.239), solitary liver metastasis (0.423), and the degree of histological differentiation (0.486) were significant prognostic factors for GLM (Table 3).
Operative Results of Hepatic Surgical Treatment
Among the 19 patients who achieved curative resection (R0), the 1-, 3-, and 5-year survival rates were 83.3, 31.7, and 31.7 %, respectively, with an MST of 27 months (range 15–35). The 1-, 3-, and 5-year recurrence-free survival (RFS) rates were 42.0, 26.0, and 26.0 %, respectively (Fig. 2). Factors for poor prognosis were identified in univariate and multivariate analyses and are shown in Tables 4 and 5. The number of liver metastasis (solitary or multiple) was the only significant prognostic factor for survival in patients with hepatic resection for GLM. There was not a significant difference between patients with liver resection and those who received RFA treatment for GLM. Systemic chemotherapy did not influence prognoses.
Overall survival (OS, solid line) and recurrence-free survival (RFS, dotted line) in the 19 patients in whom curative resection (R0) was achieved. The 1-, 3-, and 5-year OS rates were 83.3, 31.7, and 31.7 %, with MST of 27 months. The 1-, 3-, and 5-year RFS rates were 42.0, 26.0, and 26.0 %, respectively
Morbidity and Mortality
At 30 days, postoperative mortality was 0 in the hepatic surgery group, and due to the progression of cancer in three patients, it was 12 % in the no hepatic surgery group. The overall morbidity rate was 19 % (four patients) in the hepatic surgery group and included intestinal leakage, bile leakage, pulmonary infarction, bleeding, and intra-abdominal abscess. The overall morbidity was 28 % (seven patients) in the no surgery group and included liver failure, liver dysfunction, gastric emptying disorder, urinary tract infection, and wound infection.
Discussion
The surgical resection of GLM is rarely indicated because prognoses for patients with noncurative resection for advanced gastric cancers remain poor,9,10 and most patients with GLM remain incurable, even after extended surgery. Among these patients, incurable features include bilobar multinodular liver tumor spread, gross peritoneal dissemination, extensive lymph node metastasis, distant metastasis, and unresectable primary tumor spread. In the present study, we retrospectively reviewed outcomes in 47 patients and compared patients who received surgical treatment for GLM with those who did not. Patients who were inoperable at the time of diagnosis of GLM were excluded. Nonetheless, the serous invasions, extensive lymph node metastases, and extrahepatic metastases significantly disturbed surgical liver resections in the present patients. As a result, the TNM stage of primary gastric cancers considerably influenced the indication for surgical resection of GLM. In addition, the number and distribution of liver metastases significantly differed between the two treatment groups. Especially, bilobar tumor spread within the liver appears more frequently in gastric cancers than in other gastrointestinal malignancies.15 These results showed poorer biologic nature in gastric cancer. Thus, even in cases of potentially resectable gastric liver metastases, the surgical procedure for GLM may be avoided.
Patients receiving curative surgical resection for GLM are expected to have better prognosis. Among 19 patients with curative surgical treatment for GLM in the present study, MST was 27 months, and the 5-year survival rate was 31.7 %. Moreover, the present univariate analyses of patients with GLM identified status of serosa invasion and degree of histological differentiation in the primary gastric cancer, distribution of liver metastases, solitary liver metastasis, and status of extrahepatic metastases as good prognostic factors. Similarly, according to univariate and multivariate analyses of patients who received hepatic resection for GLM, solitary nodule of liver metastases was the only factor with a significant impact on the survival.
Among previous studies of GLM, the 5-year survival rates ranged from 0 to 39 %, and MST ranged from 9 to 48 months.5,6,11–17 In earlier studies, the cumulative survival rates were generally poor and reflected generalized disease, with a 5-year survival in <20 % of the cases after hepatic resection.4,5 In contrast, more recent studies show the 5-year survival rates of 11–39 %, with eight trials exceeding 30 %.6,11–17 Kerkar et al.19 performed meta-analyses of 19 studies of survival following liver resection for GLM and showed a median survival for all 436 patients of 17 months, with a 5-year survival of 26.5 % of the cases. Similar studies have compared surgical outcomes between patients with and without hepatic resection.6,13,15,20 In agreement with the present date, these studies suggest that hepatic resection should always be considered as an option for patients with GLM when complete excision appears to be possible.
Multiple factors are associated with outcomes following liver resection for GLM and can hamper the establishment of indications for surgery. However, indications for liver resection could be based on analyses of prognostic factors. The first study of GLM prognostic factors was performed by Ochiai et al.4, who described serosal perforation by the primary tumor, lymphangiosis, and venangiosisas negative prognostic factors. Several subsequent studies have described novel prognostic factors, including the size of the primary gastric tumor,12,13 D2 lymphadenectomy,13 serosal invasion,4,7,12 lymph node metastasis,5,19 histological type,6 number of GLM,6,7,13,16,17,20 maximum size of GLM,12,18 distribution12,20 and timing of hepatectomy,6,12 surgical margins,14 and absence of peritoneal dissemination.16,19 In addition, the number of liver metastases is often described as an important prognostic factor, and solitary liver metastases are a favorable prognostic factor according to previous univariate and multivariate analyses.13,16,17 Consequently, the present and previous data indicate that the number of liver metastases (solitary or multiple) may be the most significant prognostic factor for survival after initial hepatic resection for GLM.
RFA is widely used to treat primary and secondary liver tumors.21 However, few studies have evaluated outcomes following RFA for GLM. Kim et al.22 treated 20 patients with synchronous GLM using RFA and gastrectomy and achieved an MST of 30.7 months and a median progression-free survival time of 6.8 months. This procedure was performed in four of the present patients, and the resulting MST of 27 months indicates at least the same efficacy as that of liver resection. However, high recurrence rates are commonly associated with RFA.
Systemic chemotherapy is a standard treatment approach for most patients with GLM. However, the specification of appropriate regimens remains controversial.23 Moreover, the efficacy of preoperative and postoperative chemotherapy in patients with GLM after liver resection has not been fully evaluated.12,13,20,23 Nonetheless, systemic chemotherapy before and after liver resection may provide significant benefits to future patients.
Conclusions
Although curative liver resection is rarely achieved in patients with GLM, this surgical option may produce survival benefits. Therefore, the present data suggest that liver resection should be considered when complete excision including the primary tumor appears to be possible, particularly in cases of solitary hepatic metastases.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Canc J Clin 2011;61:69-90.
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, Ono H, Tanabe S, Kaminishi M.Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer 2013;16:1-27.
Koga S, Kawaguchi H, Kishimoto H, Tanaka K, Miyano Y, Kimura O, Takeda R, Nishidoi H.Therapeutic significance of noncurative gastrectomy for gastric cancer with liver metastasis. Am J Surg 1980;140:356–359
Ochiai T, Sasako M, Mizuno S, Kinoshita T, Takayama T, Kosuge T, Yamazaki S, Maruyama K.Hepatic resection for metastatic tumours from gastric cancer: analysis of prognostic factors. Br J Surg 1994;81:1175–1178
Saiura A, Umekita N, Inoue S, Maeshiro T, Miyamoto S, Matsui Y, Asakage M, Kitamura M.Clinicopathological features and outcome of hepatic resection for liver metastasis from gastric cancer. Hepatogastroenterology 2002;49:1062-5.
Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, Usuki H, Maeta H.Hepatic resection for metastatic tumors from gastric cancer. Ann Surg 2002;235:86–91.
Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, Yamaguchi T.Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J ClinOncol. 2007;37:836-42.
Yagi Y, Seshimo A, Kameoka S.Prognostic factors in stage IV gastric cancer: univariate and multivariate analyses. Gastric Cancer 2000;3:71-80.
Baba H, Okuyama T, Hiroyuki O,Anai H, Korenaga D, Maehara Y.Prognostic factors for non-curative gastric cancer: univariate and multivariate analysis. J SurgOncol. 1992;51:104–108.
Li C, Yan M, Chen J, Xiang M, Zhu ZG, Yin HR, Lin YZ.Survival benefit of non-curative gastrectomy for gastric cancer patients with synchronous distant metastasis. J Gastrointest Surg 2010;14: 282-88.
Roh HR, Suh KS, Lee HJ, Yang HK, Choe KJ, Lee KU.Outcome of hepatic resection for metastatic gastric cance. Am Surg. 2005;71:95-9
Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, Katai H, Kosuge T, Sasako M.Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J SurgOncol 2007;95:534–539.
Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, Ahn JB, Roh JK, Noh SH, Chung HC.Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146-53.
Thelen A, Jonas S, Benckert C, Lopez-Hänninen E, Neumann U, Rudolph B, Schumacher G, Neuhaus P.Liver resection for metastatic gastric cancer. Eur J Surg Oncol 2008;34:1328-34.
Dittmar Y, Altendorf-Hofmann A, Rauchfuss F, Götz M, Scheuerlein H, Jandt K, Settmacher U.Resection of liver metastases is beneficial in patients with gastric cancer: report on 15 cases and review of literature. Gastric Cancer 2012;15:131-6.
Wang YN, Shen KT, Ling JQ, Gao XD, Hou YY, Wang XF, Qin J, Sun YH, Qin XY.Prognostic analysis of combined curative resection of the stomach and liver lesions in 30 gastric cancer patients with synchronous liver metastases. BMC Surg. 2012;12:20.
Qiu JL, Deng MG, Li W, Zou RH, Li BK, Zheng Y, Lao XM, Zhou K, Yuan YF.Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol. 2013;39:694-700.
Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today. 2010;40:287-94.
Kerkar SP, Kemp CD, Avital I. Liver resections in metastatic gastric cancer. HPB (Oxford). 2010;12:589-96.
Ueda K, Iwahashi M, Nakamori M, Nakamura M, Naka T, Ishida K, Ojima T, Yamaue H.Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Arch Surg. 2009;394: 647–653
Decadt B, SiriwardenaAK.Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol 2004;5:550-560
Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, Chung HC, Noh SH, Rha SY.Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma.Int J Hyperthermia 2010;26:305-15.
Chen L, Song MQ, Lin HZ, Hao LH, Jiang XJ, Li ZY, Chen YX.Chemotherapy and resection for gastric cancer with synchronous liver metastases. World J Gastroenterol. 2013;19:2097-103.
Conflict of Interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinohara, T., Maeda, Y., Hamada, T. et al. Survival Benefit of Surgical Treatment for Liver Metastases from Gastric Cancer. J Gastrointest Surg 19, 1043–1051 (2015). https://doi.org/10.1007/s11605-015-2775-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2775-6