Abstract
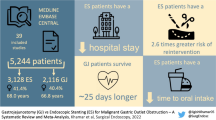
Whether nasogastric or nasojejunal decompression (ND) prevents anastomotic leakage, hastens the return of bowel function, and shortens hospital stay after gastrectomy for gastric cancer has long been controversial. We evaluated the necessity of routine ND after radical gastrectomy for gastric cancer with a systematic review and meta-analysis. We searched literature published prior to January 2014 in PubMed, Embase, Cochrane Library, Web of Science, and BIOSIS Previews for relevant randomized controlled trials (RCTs). Only prospective RCTs comparing individuals with and without ND after gastrectomy for gastric cancer were included. Outcome measures included time to first flatus, time to starting oral diet, anastomotic leakage, pulmonary complications, wound dehiscence, length of hospital stay, morbidity, and mortality. Cochrane Collaboration RevMan 5.2 software was used for the meta-analysis. Eight RCT studies fulfilled our inclusion criteria. Of the 1,141 patients in those RCTs, 570 received nasogastric or nasojejunal decompression and 571 did not. Anastomotic leakage, pulmonary complications, wound dehiscence, morbidity, and mortality were comparable between the groups. Stratified by the type of gastrectomy or gastrojejunostomy, no significant differences in above mentioned outcomes were observed in subgroup analyses. The no ND group displayed a significantly shorter time to oral diet (weighted mean difference [WMD] = 0.45, 95 % confidence interval [CI] = 0.29 to 0.61, p < 0.001) and a marginally shorter end of hospital stay (WMD = 0.48, 95 % CI = −0.01 to 0.98, p = 0.05). The ND group significantly shortened time to first flatus (WMD = −0.7, 95 % CI = −1.13 to −0.27, p = 0.001), especially with Roux-en-Y reconstruction (WMD = −1.0, 95 % CI = −1.52 to −0.48, p = 0.0002) and prolonged time to starting oral diet (WMD = 0.52, 95 % CI = 0.13 to 0.90, p = 0.009) in the patients with subtotal gastrectomy. Routine ND appears to be unnecessary after gastrectomy for gastric cancer, irrespective of the extent of resection, and the type of digestive reconstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative nasogastric or nasojejunal decompression (ND) after gastrectomy for gastric cancer has been used extensively until relatively recently. Most surgeons still believe that such decompression might decrease postoperative ileus (nausea, vomiting, and gastric distension), wound and respiratory complications, and the incidence of anastomotic leaks, or leakage from the duodenal stump, thereby hastening the return of bowel function and shortening postoperative hospital stays.1 The necessity of this practice has been increasingly questioned, however, over the last several years. Several new randomized controlled studies (RCTs), some of which were multicentric with large sample sizes, assessing the efficacy of nasojejunal decompression after gastrectomy for gastric cancer have been reported since the most recent meta-analysis of this issue, which included only five RCTs.1–4 However, the results of these studies are conflicting rather than conclusive, similar to those included in the previous meta-analyses, which lacked sufficient power because of the relatively small sample size in each of the published studies.2,5 The controversy with regard to the significance of nasogastric or nasojejunal tube insertion after gastric surgery has not been established to date. Given the amount of recently accumulated data, an updated systematic review and meta-analysis of RCTs is now appropriate to determine whether ND is necessary after gastrectomy for gastric cancer.
Materials and Methods
Search Strategy
PubMed, Embase, Cochrane Library, Web of Science, and BIOSIS Previews were searched for literature published prior to January 2014 that compared the outcomes following gastrectomy for gastric cancer between patients who had postoperative ND and those who did not. The following terms and their combinations were used: nasogastric decompression, nasojejunal decompression, nasogastric tube insertion, gastrectomy, and gastric cancer surgery. The summary, methods, and references of the retrieved articles were browsed to broaden the search range manually. There was no language restriction. Two investigators (D W and TT L) independently reviewed the titles and abstracts, and assessed the full texts to establish the eligibility of the studies for inclusion in our meta-analysis, thereby identifying all relevant RCTs.
Outcome Measures
The primary outcomes included anastomotic leakage, pulmonary complications, wound dehiscence, morbidity, and mortality. The secondary outcomes included time to first flatus, time to starting oral diet, and length of hospital stay.
Inclusion and Exclusion Criteria with Quality Assessment of the Literature
For the study selection, citations identified by the initial search were subsequently screened for eligibility. The inclusion criteria were (1) studies that compared outcomes following ND with those following no ND, (2) studies of patients who had gastrectomy for gastric cancer, (3) RCT studies, and (4) any sample size. The exclusion criteria were (1) studies including benign gastric diseases, unless the data were presented separately, (2) studies in which fewer than three interested indices were reported or those in which the indices were difficult to calculate from the results, and (3) studies with overlapping data. The selected trials were reviewed and appraised for methodological quality using the Cochrane Collaboration’s tool for assessing risk of bias, which addressed seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, freedom from selective reporting, and freedom from other bias.6
Data Extraction
Two independent reviewers (D W and TT L) extracted and critically appraised the data and decided upon the controversial issues through discussion. We extracted the following variables using a structured pro forma: author, geographical region, study period, number of patients, age, gender, operative time, type of operation, time to first flatus, time to starting oral diet, anastomotic leakage, pulmonary complications, wound dehiscence, length of hospital stay, morbidity, and mortality. If necessary and possible, the primary authors were contacted to retrieve further information.
Statistical Analysis
Statistical analysis was performed using RevMan software, version 5.2 (Cochrane Collaboration, Oxford, UK). The meta-analysis was performed in accordance with the recommendations of the PRISMA statement. Continuous variables were assessed using the weighted mean difference (WMD) with 95 % confidence interval (CI), and the dichotomous variables were analysed using the odds ratio (OR) with 95 % CI. A fixed-effect model or a random-effect model was used according to heterogeneity. A sensitivity analysis was applied to identify significant sources of heterogeneity by removing individual studies from the data set and analysing the effect of the removal on the overall results.7 Subgroup analyses were performed to explore the potential sources of heterogeneity. Inspection of funnel plots was used to screen for risk of publication bias. A p value < 0.05 was considered statistically significant.
Results
Search Results and Methodological Quality
After the titles, abstracts, full texts, or combinations thereof identified by electronic search were screened, eight RCTs1,3,4,8–12 comparing nasogastric or nasojejunal decompression with no ND after gastrectomy for gastric cancer were included in the meta-analysis (Fig. 1). All the articles were published between 1992 and 2013. The risk-of-bias assessment of the included studies is shown in Fig. 2. The main study limitations pertained to the justification of the sample size, allocation concealment, double blinding, and the subjectivity of reporting the return of gastrointestinal function. For example, no study described the allocation concealment specifically, and only two indicated that physicians who were not associated with the surgical teams monitored the postoperative courses of the patients.
Of the 1,141 patients included in the meta-analysis, 570 received postoperative ND and 571 did not. The basic and postoperative characteristics of the studies are listed in Table 1. Postoperative oral intake was restricted for all patients until the passage of flatus. None of the studies routinely used feeding jejunostomies or other enteral or parenteral nutrition for the patients unless a complication occurred portending a prolonged restriction of oral intake.
Primary Outcomes
Anastomotic Leakage
All of the included studies1,3,4,8–12 reported on anastomotic leakage. When the extent of gastric resection was considered as a whole, there was no difference between the patients who received decompression and those who did not (OR = 1.25, 95 % CI = 0.68 to 2.27, p = 0.47; test of heterogeneity: p = 1.00, I2 = 0 %; Fig. 3a).
Meta-analysis comparing with or without nasogastric or nasojejunal decompression. a Effect of nasogastric or nasojejunal decompression on anastomotic leak. b Effect of nasogastric or nasojejunal decompression on pulmonary complications. c Effect of nasogastric or nasojejunal decompression on wound dehiscence. d Effect of nasogastric or nasojejunal decompression on morbidity rates. e Effect of nasogastric or nasojejunal decompression on mortality rates. f Effect of nasogastric or nasojejunal decompression on time to first flatus. g Effect of nasogastric or nasojejunal decompression on time to starting oral diet. h Effect of nasogastric or nasojejunal decompression on length of hospital stay
Pulmonary Complications
Seven studies1,3,8–12 reported the incidence of postoperative pulmonary complications (pneumonia, atelectasis, and pleural effusion). The meta-analysis showed no difference in pulmonary complication risk (OR = 1.31, 95 % CI = 0.90 to 1.89, p = 0.16; test of heterogeneity: p = 0.96, I2 = 0 %; Fig. 3b).
Wound Dehiscence
Six studies1,3,8,9,11,12 reported the incidence of wound dehiscence. The meta-analysis showed no significant difference between the patients who received decompression and those who did not (OR = 0.82, 95 % CI = 0.33 to 2.04, p = 0.67; test of heterogeneity: p = 0.79, I2 = 0 %; Fig. 3c).
Morbidity Rates
Six studies1,3,8,10–12 reported on morbidity rates. No significant difference was observed between the patients who received decompression and those who did not (OR = 1.13, 95 % CI = 0.83 to 1.55, p = 0.44; test of heterogeneity: p = 0.94, I2 = 0 %; Fig. 3d).
Mortality Rates
In all the selected studies,1,3,4,8–12 mortality rates were reported. The meta-analysis indicated that they were similar between the patients who received decompression and those who did not (OR = 1.27, 95 % CI = 0.34 to 4.78, p = 0.72; test of heterogeneity: p = 0.90, I2 = 0 %; Fig. 3e). This result must be interpreted with caution, however, owing to the low number of events for this outcome.
Secondary Outcomes
Time to First Flatus
Six studies1,3,4,10–12 reported the mean time to first flatus with precise standard deviations. Yoo et al.9 reported the median time to first flatus, and Wu et al.8 did not report the time to first flatus. The meta-analysis showed no significant difference between the two groups of patients (WMD = 0.03, 95 % CI = −0.22 to 0.28, p = 0.83; test of heterogeneity: p = 0.01, I2 = 66 %; Fig. 3f).
Time to Starting Oral Diet
Six studies1,3,4,10–12 reported mean time to starting oral diet with precise standard deviations; the others reported the median time. A pooled analysis showed that the time to starting oral diet was significantly shorter for the patients who did not receive decompression (WMD = 0.45, 95 % CI = 0.29 to 0.61, p < 0.001; test of heterogeneity: p = 0.56, I2 = 0 %; Fig. 3g).
Postoperative Hospital Stay
Six studies1,3,4,10–12 reported the mean length of hospital stay with precise standard deviations; the other studies reported median values. The meta-analysis showed no significant difference between the patients who received decompression and those who did not. The average length of hospital stay was 0.48 days shorter among the patients who did not receive decompression compared with that among the patients who received decompression (WMD = 0.48, 95 % CI = −0.01 to 0.98, p = 0.05; test of heterogeneity: p = 0.93, I2 = 0 %; Fig. 3h).
Adverse Events
None of the studies reported any adverse events related specifically to tube insertion, such as intracranial insertion or pneumothorax and esophageal perforation.
Sensitivity Analysis
Removing individual studies from the data set did not substantially change the Peto OR or the level of significance for the five most important clinical outcomes (anastomotic leakage, pulmonary complications, wound dehiscence, morbidity, and mortality).
Subgroup Analysis
When the patients were stratified by the extent of gastrectomy and the type of digestive reconstruction (partial and total gastrectomy or Roux-en-Y gastrojejunostomy for total gastrectomy, Roux-en-Y gastrojejunostomy for subtotal gastrectomy, and Billroth I + Billroth II gastrojejunostomy for subtotal gastrectomy), the results of subgroup analyses were shown in Tables 2 and 3. ND could significantly shorten time to flatus (WMD = −0.7, 95 % CI = −1.13 to −0.27, p = 0.001), but prolong time to starting oral diet (WMD = 0.52, 95 % CI = 0.13 to 0.90, p = 0.009) in the patients with subtotal gastrectomy. But more specifically, only in Roux-en-Y reconstruction for subtotal gastrectomy subgroup, the difference reached a significant level in term of time to flatus (WMD = −1.0, 95 % CI = −1.52 to −0.48, p = 0.0002). However, no significant differences were found in stratified subgroups with respect to other major outcomes such as anastomotic leakage, pulmonary complications, wound dehiscence, morbidity, and mortality.
Test for Publication Bias
One funnel plot of the anastomotic leakage outcome demonstrated symmetry, indicating no serious publication bias (Fig. 4).
Discussion
Routine decompression following gastrectomy has been widely practiced and is considered to differ from decompression for other abdominal reasons. The proximal anastomoses (esophagojejunal, gastrojejunal, or gastroduodenal) and the duodenal stump pose a potential risk for early postoperative fistula formation. In addition, most radical gastrectomies with lymph node dissection (especially D2 and D3) for gastric cancers are extensively destructive procedures, which may impact on gut motility after the operation.13 For these reasons, ND remains a routine part of perioperative care after radical gastrectomy in many centers.
Our meta-analysis demonstrated that the statistical differences for the outcomes of every major clinical complication (anastomotic leakage, pulmonary complications, wound dehiscence, morbidity, and mortality) coincided with those in the individual studies and all the subgroups by different stratifications, suggesting that the power calculations with respect to the major complications in the eligible RCTs were realistic. These findings supported that it turned out to be safe and feasible even without ND for the patients who received radical gastrectomy for gastric cancer.
Anastomotic leakage is the most crucial complication after gastrectomy, increasing morbidity, length of hospital stay, and mortality. Our meta-analyses of gastric resections, either as a whole or stratified into partial and total gastrectomies or Roux-en-Y and Billroth I + Billroth II gastrojejunostomies, showed that anastomotic leakage rates were not affected by decompression, suggesting that decompression does not decrease the risk of anastomotic leakage. The avoidance of tension or impaired vascularisation of the conduit and meticulous suture technique has been shown to be more important in this respect.14
Pulmonary and wound complications are common after gastrectomy for gastric cancer. Our review demonstrated that decompression did not significantly reduce such complications. In contrast, a recent multivariate analysis found that preoperative ND was independently associated with an increased risk of pulmonary complications, especially aspiration pneumonia, after elective non-thoracic surgery.15 This might also help to prolong the duration of hospital stay.
Our analysis suggests that the time to flatus was shorter for the no ND group, but the result was not statistically significant and came with significant heterogeneity. Only the study by Fabio et al.1 found that the time to flatus was significantly longer among patients without ND after partial distal gastrectomy. The result was only significant following Roux-en-Y reconstruction, however, and was not statistically significant following Billroth II reconstruction. The other five studies consistently showed that the time to first flatus was shorter without decompression.3,4,10–12 To find out the potential sources of heterogeneity, subgroup analysis by stratification according to the type of gastrectomy and gastrojejunostomy was performed. The results indicated that the return of flatus passage was significantly accelerated only in the patients who underwent distal gastrectomy with Roux-en-Y reconstruction. Another aspect of gut motility after the operation, the time to starting oral diet, was significantly shorter among the patients who did not receive decompression, even when the study by Fabio et al. was included. Fabio et al.1 explained the paradoxical difference in their study with respect to the time to first flatus and the time to first oral intake as the result of ND related complications and discomfort that prolonged the time that decompression was maintained and delayed the time to starting oral diet.1 And this might be one of the possible reasons for that the mean postoperative stay was 0.48 days shorter among the patients who did not receive decompression. In any case, our results suggest that decompression does not help hasten the return of bowel function on the whole.
Mattei et al.16 suggested that decompression may not prevent, but instead may actually facilitate postoperative ileus, probably due to the interruption of normal gastrointestinal reflexes triggered by orogastric secretions. Ileus does obviously have several contributing factors including inflammatory response, anaesthetic and opioid administration, autonomic nervous system dysfunction, and gastrointestinal hormone disruption.14 Moreover, the nasogastric or nasojejunal tube may induce vomiting and other complications such as nasopharyngeal soreness and intestinal fluid loss. In addition, the discomfort caused by a nasogastric or nasojejunal tube is one of the most unpleasant aspects of the postoperative course. Considering the shorter hospital stay as well as the reduced requirements for nursing and pharmaceutical services, the omission of a nasogastric tube can be a cost-effective treatment for patients with gastric cancer.
Several reports17,18 indicated that compared with traditional feeding after the passage of flatus, early oral feeding within 2 days after operation in patients undergoing gastrectomy did not reduce postoperative complications but did reduce the postoperative time to flatus and the length of hospital stay. The main limitations of these studies were monocentric with small sample sizes.
There are limitations to any meta-analysis, especially with respect to the quality of the data in the original trials. The most important point is that we have no individual patients’ data that has become essential for the present-day meta-analysis. We made every attempt to increase the clinical homogeneity among the trials. For instance, we included only patients undergoing gastrectomy for gastric cancer, and we excluded studies that included benign gastric disease. But still, none of the eligible studies received a score greater than five using the Cochrane Collaboration’s tool for assessing risk of bias, indicating, to some extent, that the methodological quality of the studies was a limitation. In particular, the studies rarely included allocation concealment and blinding assessment of the outcomes, probably because of ethical concerns or practical difficulties. It was reported, however, that individual quality measures, such as blinding and allocation concealment, are not reliably associated with the strength of the treatment effects in meta-analyses of RCTs.19 Therefore, the outcomes in our meta-analysis were slightly impaired. However, with increasing accumulated data, it permitted that the necessity of ND following gastrectomy for gastric cancer could be explored under more specific backgrounds.
Conclusion
Our results do not support the routine use of ND following gastrectomy for gastric cancer, irrespective of the extent of gastric resection and the type of digestive reconstruction, because there is no convincing evidence that postoperative decompression is associated with reduced anastomotic leakage, decreased pulmonary complications, fewer wound problems, earlier recovery of bowel function, or shorter hospital stay,.
References
Pacelli F, Rosa F, Marrelli D, Morgagni P, Framarini M, Cristadoro L, Pedrazzani C, Casadei R, Cozzaglio L, Covino M, Donini A, Roviello F, de Manzoni G, Doglietto GB. Naso-gastric or naso-jejunal decompression after partial distal gastrectomy for gastric cancer. Final results of a multicenter prospective randomized trial. Gastric Cancer : Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 2013 [doi: 10.1007/s10120-013-0319-x]
Yang Z, Zheng Q, Wang Z. Meta-analysis of the need for nasogastric or nasojejunal decompression after gastrectomy for gastric cancer. The British Journal of Surgery 2008; 95(7): 809–816 [doi: 10.1002/bjs.6198]
Li C, Mei JW, Yan M, Chen MM, Yao XX, Yang QM, Zhou R, Zhu ZG. Nasogastric decompression for radical gastrectomy for gastric cancer: a prospective randomized controlled study. Digestive Surgery 2011; 28(3): 167–172 [doi: 10.1159/000323744]
Tavassoli A, Rajabi MT, Abdollahi A, Bagheri R, Noorshafiee S. Efficacy and necessity of nasojejunal tube after gastrectomy. International Journal of Surgery 2011; 9(3): 233–236 [doi: 10.1016/j.ijsu.2010.11.017]
Cheatham ML, Chapman WC, Key SP, Sawyers JL. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg 1995; 221(5): 469–476; discussion 476–468.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011; 343: d5928 [doi: 10.1136/bmj.d5928]
Sutton AJ, Abrams KR, Jones DR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research: J. Wiley, 2000
Wu CC, Hwang C, Liu T. There is no need for nasogastric decompression after partial gastrectomy with extensive lymphadenectomy. Eur J Surg 1994; 160(6–7): 369–373.
Hak YC, Ho SB, Kon HW, Kil PW. Nasogastric decompression is not necessary in operations for gastric cancer prospective randomised trial. Eur J Surg 2002; 168(7): 379–383
Lee JH, Hyung WJ, Noh SH. Comparison of gastric cancer surgery with versus without nasogastric decompression. Yonsei Med J 2002; 43(4): 451–456
Doglietto GB, Papa V, Tortorelli AP, Bossola M, Covino M, Pacelli F, Group ITGS. Nasojejunal tube placement after total gastrectomy a multicenter prospective randomized trial. Arch Surg 2004; 139(12): 1309–1313; discussion 1313
Hsu SD, Yu JC, Chen TW, Chou SJ, Hsieh HF, Chan DC. Role of Nasogastric Tube Insertion after Gastrectomy. Chirurgische Gastroenterologie 2007; 23(3): 303–306 [doi: 10.1159/000105624]
Katai H. Function-preserving surgery for gastric cancer. International Journal of Clinical Oncology 2006; 11(5): 357–366
Hölscher AH, Vallböhmer D, Brabender J. The prevention and management of perioperative complications. Best Practice & Research Clinical Gastroenterology 2006; 20(5): 907–923
McAlister FA, Bertsch K, Man J, Bradley J, Jacka M. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. American Journal of Respiratory and Critical Care Medicine 2005; 171(5): 514–517
Mattei P, Rombeau JL. Review of the pathophysiology and management of postoperative ileus. World Journal of Surgery 2006; 30(8): 1382–1391
Suehiro T, Matsumata T, Shikada Y, Sugimachi K. Accelerated rehabilitation with early postoperative oral feeding following gastrectomy. Hepato-Gastroenterology 2003; 51(60): 1852–1855
Hur H, Kim SG, Shim JH, Song KY, Kim W, Park CH, Jeon HM. Effect of early oral feeding after gastric cancer surgery: a result of randomized clinical trial. Surgery 2011; 149(4): 561–568
Balk EM, Bonis PA, Moskowitz H, Schmid CH, Ioannidis JP, Wang C, Lau J. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. Jama 2002; 287(22): 2973–2982
Acknowledgments
This work was supported by The National Key Technology R&D Program (no.2013BAI05B00), the Major Program of Science and Technology Program of Guangzhou (no.201300000087), Research Fund of Public welfare in Health Industry of Health (No.201402015), Ministry of Health of PR China, and the program of the national key clinical medical specialty
Conflict of Interest
None declared.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Da Wang and Tingting Li contributed equally to this work.
Hao Liu and Guoxin Li also contributed equally and should be considered as co-corresponding author.
Rights and permissions
About this article
Cite this article
Wang, D., Li, T., Yu, J. et al. Is Nasogastric or Nasojejunal Decompression Necessary Following Gastrectomy for Gastric Cancer? A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J Gastrointest Surg 19, 195–204 (2015). https://doi.org/10.1007/s11605-014-2648-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2648-4