Abstract
Introduction
The impact of neoadjuvant therapy on postpancreatectomy complications is inadequately described.
Methods
Data from the NSQIP Pancreatectomy Demonstration Project (11/2011 to 12/2012) was used to identify patients with pancreatic adenocarcinoma who did and did not receive neoadjuvant therapy. Neoadjuvant therapy was classified as chemotherapy alone or radiation ± chemotherapy. Outcomes in the neoadjuvant vs. surgery first groups were compared.
Results
Of 1,562 patients identified at 43 hospitals, 199 (12.7 %) received neoadjuvant therapy (99 chemotherapy alone and 100 radiation ± chemotherapy). Preoperative biliary stenting (57.9 vs. 44.7 %, p = 0.0005), vascular resection (41.5 vs. 17.3 %, p < 0.0001), and open resections (94.0 vs. 91.4 %, p = 0.008) were more common in the neoadjuvant group. Thirty-day mortality (2.0 vs. 1.5 %, p = 0.56) and postoperative morbidity rates (56.3 vs. 52.8 %, p = 0.35) were similar between groups. Neoadjuvant therapy patients had fewer organ space infections (3.0 vs. 10.3 %, p = 0.001), and neoadjuvant radiation patients had fewer pancreatic fistulas (7.3 vs. 15.4 %, p = 0.03).
Conclusions
Despite evidence for more extensive disease, patients receiving neoadjuvant therapy did not experience more complications. Neoadjuvant radiation was associated with lower pancreatic fistula rates. These data provide evidence against higher postoperative complication rates in patients with pancreatic cancer who are treated with neoadjuvant therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The National Comprehensive Cancer Network guidelines recommend the use of neoadjuvant therapy for patients with borderline resectable pancreatic adenocarcinoma and allow for consideration of neoadjuvant therapy for patients with resectable pancreatic cancer in the context of a clinical trial.1 Although the use of neoadjuvant therapy was initially limited to a small number of specialized institutions, in recent years, acceptance has grown for the use of this strategy. Neoadjuvant therapy is the preferred strategy in borderline resectable disease and, while controversial, is being used with increasing frequency in patients with anatomically resectable pancreatic cancer.2 As the use of neoadjuvant chemotherapy and/or chemoradiation becomes more widespread, an understanding of its potential impact on postoperative complications is of growing importance.
Initial reports of neoadjuvant chemoradiation for locally advanced pancreatic adenocarcinoma appeared in the 1990s.3–5 Since that time, multiple studies have established the feasibility of treatment with neoadjuvant chemotherapy and/or neoadjuvant chemoradiation in patients with locally advanced, borderline resectable and potentially resectable pancreatic cancer.6–10 However, national rates of neoadjuvant therapy use remain low,2 and outcomes comparing rates of postoperative complications for patients treated with neoadjuvant therapy to those of patients treated with an initial surgery strategy for pancreatic cancer have not been reported outside of highly specialized centers. The aim of this study was to compare the rates of postoperative morbidity and mortality after pancreatectomy for patients with pancreatic adenocarcinoma treated with initial surgery or neoadjuvant therapy followed by surgery using data that was preexisting and deidentified but originally prospectively defined and collected in the ACS-NSQIP Pancreatectomy Demonstration Project, which was a feasibility project within the ACS NSQIP.
Materials and Methods
Data Source
The American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP) is a standardized multicenter national database that prospectively collects multiple preoperative demographic, laboratory, and comorbidity variables to provide robust risk-adjusted rates of overall postoperative morbidity and mortality rates.11–13 The ACS-NSQIP Pancreatectomy Demonstration Project was piloted in 2011; its goal was to evaluate the feasibility of prospective collection of variables relevant for short-term outcomes following pancreatectomy. Forty-three institutions, both academic and community, participated in this endeavor. Prospective data collection was standardized to allow comparison of data from the broad spectrum of participating hospitals.
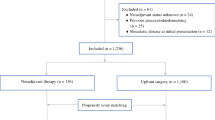
We identified all patients from ACS-NSQIP Pancreatectomy Demonstration Project from November 2011 to December 2012 (N = 2,805). We only included those patients with a diagnosis of adenocarcinoma (N = 1,605). Patients with missing information on preoperative chemotherapy or radiation were excluded (N = 38). Finally, patients who underwent enucleation were excluded (N = 5). Our final cohort had 1,562 patients.
Variables
The ACS-NSQIP Pancreatectomy Demonstration data set contains 240 Health Insurance Portability and Accountability Act (HIPAA) compliant variables on preoperative patient characteristics, intraoperative variables, and postoperative outcomes. In addition to the standard variables available from the ACS-NSQIP, the 43 participating institutions submitted data on 24 additional variables specific to pancreatic surgery. The additional pancreas-specific variables were also included for this study: jaundice, biliary stent, chemotherapy in 90 days before surgery, radiation in 90 days before surgery, pancreatic duct size, pancreatic gland texture, vascular resection, pancreatic reconstruction, gastrojejunostomy vs. duodenojejunostomy, drain placement, and drain amylase on postoperative day 1, last day of drain removal, pancreatic fistula, delayed gastric emptying, percutaneous drainage, and pathology. Pancreatic fistulas were considered to be clinically significant if they required percutaneous drainage or reoperation. Detailed definitions of these variables and database have been published elsewhere.13,14
Within the Pancreatectomy Demonstration Project, patients were coded as having received preoperative chemotherapy if they received any chemotherapy within 90 days prior to the date of operation. Similarly, patients were coded as having received preoperative radiation if they received any radiation within 90 days prior to the date of operation. Patients coded as having received either preoperative chemotherapy and/or radiation were included within the neoadjuvant therapy group, whereas patients with neither of these codes were assigned to the initial surgery group. Patients who received radiation either with or without chemotherapy were included within the radiation subgroup, and those who received only chemotherapy without any radiation were categorized within the chemotherapy subgroup.
Hospital volume was measured as quartile of volume for the participating institutions. The majority of the participating 43 institutions (N = 38) were categorized as high volume by Leapfrog criteria. Postoperative complications were defined as any of the following: delayed gastric emptying, pancreatic fistula, reoperation, any surgical site infection, acute renal failure, pneumonia, pulmonary embolism, need for cardiopulmonary resuscitation, myocardial infarction, postoperative bleeding, sepsis, or septic shock.14
Statistical Analyses
Bivariate analyses were performed to compare patient characteristics and outcomes by receipt of neoadjuvant therapy. Chi-square tests were used for categorical variables and t tests were used for continuous variables. Multivariable logistic regression models were used to determine factors independently associated with postoperative complications and deep organ space infection. Variables that were significantly associated with postoperative complications in bivariate analyses were included in these models. Additional variables that were felt to be clinically associated with complications but not statistically significant in unadjusted analyses were forced into the model. Statistical significance was accepted at the p < 0.05 level. All analyses were performed using SAS 9.2 (SAS Inc., Cary, NC).
Results
Incidence and Demographics
We identified 1,562 patients with pancreatic adenocarcinoma within the Pancreatectomy Demonstration Project. Of these patients, 199 (12.7 %) received neoadjuvant therapy, 99 (6.3 %) patients were treated with chemotherapy alone, and 100 (6.4 %) patients with radiation with or without chemotherapy. The 199 neoadjuvant cases were performed at 31 (72.1 %) of the 43 centers that participated in the Pancreatectomy Demonstration Project. Only eight patients received radiation without documented chemotherapy. For the entire cohort of pancreatic cancer patients, the mean age was 65.4 ± 11.2, 46.2 % of patients were female, 88.1 % of patients were white, and 6.9 % of patients were African-American. Demographic data (Table 1) were similar for patients in the neoadjuvant therapy vs. initial surgery groups; however, patients in the neoadjuvant therapy group were significantly younger and more likely to have a body mass index in the normal range. Comorbidities were also similar between patients treated with neoadjuvant therapy and those treated with initial surgery (Table 2). Preoperative albumin levels did not differ significantly between the neoadjuvant and initial surgery groups; however, the neoadjuvant group did have a significantly lower mean preoperative bilirubin level.
Perioperative and Operative Variables
In the neoadjuvant therapy group, 13.6 % of patients underwent a distal pancreatectomy, 80.9 % of patients underwent a pancreaticoduodenectomy, and 5.5 % of patients underwent a total pancreatectomy. By comparison, in the initial surgery group, 19.9 % of patients required a distal pancreatectomy, 78.1 % of patients required a pancreaticoduodenectomy, and 2.0 % of patients required a total pancreatectomy (p = 0.002, Table 3). Patients who received neoadjuvant therapy were significantly more likely to have undergone preoperative biliary stenting than those who were treated with initial surgery. Neoadjuvant therapy patients were also significantly less likely to undergo laparoscopic resection and were significantly more likely to require a vascular resection than initial surgery patients. In the 989 patients who had pancreatic texture data reported, firm pancreatic texture was more common in patients who received neoadjuvant therapy than those who underwent surgery first (65.3 vs. 51.1 %, p = 0.017, Table 3).
Postoperative Outcomes
The 30-day morbidity rate was similar for the initial surgery and the neoadjuvant therapy groups (52.8 vs. 56.3 %, respectively, p = 0.35) (Fig. 1a). The 30-day mortality rate was also similar between the initial surgery and the neoadjuvant therapy groups (1.5 vs. 2.0 %, respectively, p = 0.56) (Fig. 1b). Mean length of stay at the time of pancreatic resection was 10.4 days (range 4–43 days) for the neoadjuvant therapy group and 11.1 days (range 0–107 days, p = 0.32) for the initial surgery group. The rates of specific postoperative complications did not differ significantly between the initial surgery and the neoadjuvant therapy groups with the exception of the rates of organ space infections, which were significantly lower in the neoadjuvant therapy group (3.0 vs. 10.3 %, p = 0.001) (Table 4, Fig. 2a). In a multivariable model, initial surgery remained an independent predictor of organ space infections, along with male gender and soft or unknown pancreatic gland texture (Table 5). In addition, the rates of pancreatic fistula were lower in patients who received neoadjuvant radiation (7.3 %) compared to patients who either underwent initial surgery or were treated with neoadjuvant chemotherapy only (15.4 %, p = 0.03) (Fig. 2b).
Independent Predictors of Postoperative Complications
Multivariable modeling identified several factors that were independently associated with higher rates of postoperative complications (Table 6). The use of neoadjuvant therapy (chemotherapy alone, chemoradiation, or radiation alone) was not independently associated with postoperative complications. Factors associated with increased complications included increasing age, decreasing albumin, nonwhite race, and obesity (compared to normal weight), chronic obstructive pulmonary disease, preoperative biliary stent placement, open resection (compared to laparoscopic), need for vascular resection, soft or unknown pancreatic gland texture (compared to firm gland texture), and total pancreatectomy (compared to Whipple).
Discussion
This study is the first analyzing prospectively collected multi-institutional data of postoperative complications in pancreatic cancer patients treated with and without neoadjuvant therapy. The small overall percentage of patients who received neoadjuvant therapy and the large number of hospitals at which it was administered suggest that neoadjuvant therapy is not the preferred approach across a large number of high- and medium-volume specialized centers. In cases where neoadjuvant therapy was performed, patients who underwent neoadjuvant therapy were not at increased risk for postoperative morbidity and mortality, despite evidence for more advanced disease. In addition, our data suggest that these patients may be at reduced risk for pancreatic fistulae and organ space infections.
The results of this study are compelling for several reasons. First, the lack of significantly higher postoperative complication rates for patients treated with neoadjuvant therapy is somewhat surprising as these patients had more extensive disease (as evidenced by more frequent need for vascular resection) than patients not treated with neoadjuvant therapy. Secondly, patients treated with neoadjuvant therapy more frequently had biliary stents placed, which is another known risk factor for postoperative complications.15–17 Additionally, patients treated with neoadjuvant therapy were significantly less likely than initial surgery patients to undergo laparoscopic resection, an independent predictor of lower postoperative complication rates in the multivariate model.
Several previous studies have compared postoperative complication rates for patients undergoing pancreatectomy after neoadjuvant therapy and those undergoing primary pancreatic resection. In a single-institution case-matched analysis, patients with initial borderline or locally advanced pancreatic cancers who underwent pancreatectomy after neoadjuvant chemoradiation were compared to initially resected patients.18 Similar to the current study, this study found that rates of postoperative complications and postoperative mortality (at 90 days) were not significantly different between the two groups. In contrast to the current study, however, this study did not find a significant difference in the rates of clinically significant pancreatic fistulae with or without the use of neoadjuvant chemoradiation.18 Another study matched patients with locally advanced pancreatic adenocarcinoma who had been treated with preoperative chemotherapy with control patients who underwent operation as their primary treatment modality.19 Similarly, this study also found no significant difference in postoperative morbidity or mortality rates or in rates of pancreatic fistulae.19 A recent meta-analysis also assessed postoperative outcomes in patients treated with neoadjuvant chemoradiation for pancreatic cancer.20 Compared to patients treated with primary surgical resection, this study found that patients receiving neoadjuvant chemoradiation had similar rates of overall morbidity and pancreatic fistula but had higher rates of perioperative mortality.20 Finally, one retrospective study has reported significantly lower rates of pancreatic fistulae in pancreatic adenocarcinoma patients undergoing distal pancreatectomy following neoadjuvant chemoradiation compared to treatment-naïve patients undergoing distal pancreatectomy.21 The current study expands on these previous findings by utilizing data prospectively collected from multiple institutions.
While the major strength of this study is its use of standardized, rigorously collected prospective data from multiple institutions, the limitations of this study should also be addressed. First, a significant majority of institutions contributing data to the Pancreatectomy Demonstration Project are considered high-volume institutions for pancreatectomy by the Leapfrog criteria (>11 pancreatectomies per year), and so the results presented here may not be generalizable to lower volume hospitals, which typically have higher complication rates.22,23 It should be noted, however, that some of the hospitals which have traditionally been known for frequent use of neoadjuvant therapy in the treatment of patients with pancreatic cancer were not participants in the Pancreatectomy Demonstration Project and it is likely that a significant proportion of the patients in the neoadjuvant therapy group of this study received their neoadjuvant therapy at hospitals with more limited experience with the delivery of neoadjuvant therapy. In addition, the overall sample size for the neoadjuvant chemotherapy and radiation ± chemotherapy groups is fairly small. While, if anything, this makes the significant results in this study more robust, it also makes it possible that the nonsignificant difference in complication rates between the neoadjuvant and initial surgery groups is a false negative due to inadequate power to detect a small difference between groups. The small sample size also limits the ability of this study to identify other patient factors that may have been different between the two groups and could potentially influence outcomes. Another limitation of this study is that data was missing for certain variables in a significant number of patients. The most notable of these was pancreatic duct size, which was missing in almost 25 % of patients and is known to be associated with pancreatic fistula rates.24 The absence of this data called into question any multivariate model for pancreatic fistula rates, and so it is possible that the lower pancreatic fistula rates seen in patients treated with neoadjuvant radiation are more attributable to differences in baseline characteristics of the gland rather than to the radiation itself.
Conclusion
The results of this study corroborate the lack of a negative impact of neoadjuvant therapy on overall postoperative complication rates in patients undergoing pancreatic resection for adenocarcinoma reported by earlier studies. Taken together, these data contribute to growing evidence that fear of increasing postoperative complications should not represent a barrier to the use of neoadjuvant therapy in patients with pancreatic cancer. The data also suggest that rates of organ space infections may be lower in patients treated with neoadjuvant therapy. The data in this study suggesting that preoperative radiation may decrease pancreatic fistula rates is less conclusive, and the heterogeneity among studies suggests that this point is unlikely to be conclusively answered outside of a randomized controlled trial.
References
Tempero, MA, Arnoletti, JP, Behrman, SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10: 703-713.
Parmar AD, VG, Tamirisa NP, Sheffield KM, Riall TS. Trajectory of Care and Use of Multimodality Therapy in Locoregional Pancreatic Adenocarcinoma. Surgery.2014;156:280-9.
Weese, JL, Nussbaum, ML, Paul, AR, et al. Increased resectability of locally advanced pancreatic and periampullary carcinoma with neoadjuvant chemoradiotherapy. Int J Pancreatol. 1990;7: 177-185.
Jessup, JM, Steele, G, Jr., Mayer, RJ, et al. Neoadjuvant therapy for unresectable pancreatic adenocarcinoma. Arch Surg. 1993;128: 559-564.
Yeung, RS, Weese, JL, Hoffman, JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer. 1993;72: 2124-2133.
Evans, DB, Varadhachary, GR, Crane, CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26: 3496-3502.
Varadhachary, GR, Wolff, RA, Crane, CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26: 3487-3495.
Cetin, V, Piperdi, B, Bathini, V, et al. A Phase II Trial of Cetuximab, Gemcitabine, 5-Fluorouracil, and Radiation Therapy in Locally Advanced Nonmetastatic Pancreatic Adenocarcinoma. Gastrointest Cancer Res. 2013;6: S2-9.
Lee, JL, Kim, SC, Kim, JH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery. 2012;152: 851-862.
Landry, J, Catalano, PJ, Staley, C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol. 2010;101: 587-592.
Pitt, HA, Kilbane, M, Strasberg, SM, et al. ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB (Oxford). 2009;11: 405-413.
Parikh, P, Shiloach, M, Cohen, ME, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB (Oxford). 2010;12: 488-497.
Parmar, AD, Sheffield, KM, Vargas, GM, et al. Factors associated with delayed gastric emptying after pancreaticoduodenectomy. HPB (Oxford). 2013;15: 763-772.
User Guide for the 2010 Participant Use Data File. [American College of Surgeons National Surgical Quality Improvement Program]. Available from URL: http://site.acsnsqip.org/wp-content/uploads/2012/03/ACS-NSQIP-Participant-User-Data-File-User-Guide_06.pdf. [accessed January, 2013.
Fang, Y, Gurusamy, KS, Wang, Q, et al. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2012;9: CD005444.
Sewnath, ME, Karsten, TM, Prins, MH, et al. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236: 17-27.
van der Gaag, NA, Rauws, EA, van Eijck, CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362: 129-137.
Araujo, RL, Gaujoux, S, Huguet, F, et al. Does pre-operative chemoradiation for initially unresectable or borderline resectable pancreatic adenocarcinoma increase post-operative morbidity? A case-matched analysis. HPB (Oxford). 2013;15: 574-580.
Pecorelli, N, Braga, M, Doglioni, C, et al. Preoperative chemotherapy does not adversely affect pancreatic structure and short-term outcome after pancreatectomy. J Gastrointest Surg. 2013;17: 488-493.
Laurence, JM, Tran, PD, Morarji, K, et al. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15: 2059-2069.
Takahashi, H, Ogawa, H, Ohigashi, H, et al. Preoperative chemoradiation reduces the risk of pancreatic fistula after distal pancreatectomy for pancreatic adenocarcinoma. Surgery. 2011;150: 547-556.
Birkmeyer, JD, Dimick, JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135: 569-575.
Allareddy, V, Ward, MM, Allareddy, V, Konety, BR. Effect of meeting Leapfrog volume thresholds on complication rates following complex surgical procedures. Ann Surg. 2010;251: 377-383.
Roberts, KJ, Storey, R, Hodson, J, et al. Pre-operative prediction of pancreatic fistula: is it possible? Pancreatology. 2013;13: 423-428.
Acknowledgements
The authors would like to acknowledge the Surgical Clinical Reviewers, Surgeon Champions, and pancreatic surgeons who participated in the Pancreatectomy Demonstration Project at the institutions listed below. We also wish to thank the leadership of the American College of Surgeons and of ACS-NSQIP for the opportunity to conduct the demonstration project.
• Albany Medical Center
• Baptist Memphis
• Baylor University
• Baystate Medical Center
• Beth Israel Deaconess
• Boston Medical Center
• Brigham & Women’s Hospital
• California Pacific Medical Center
• Cleveland Clinic
• Emory University
• Hospital University Pennsylvania
• Intermountain
• Indiana University University
• Indiana University Methodist
• Johns Hopkins Hospital
• Kaiser Permanente SF
• Kaiser Walnut
• Lehigh Valley
• Massachusetts General Hospital
• Mayo- Methodist
• Mayo-St Mary’s
• Northwestern University
• Ohio State University
• Oregon Health Sciences Center
• Penn State University
• Providence Portland
• Sacred Heart
• Stanford University
• Tampa General Hospital
• Thomas Jefferson University
• University Alabama
• University of California Irvine
• University of California San Diego
• University Iowa
• University Kentucky
• University Minnesota
• University Texas Medical Branch
• University Virginia
• University Wisconsin
• Vanderbilt University
• Wake Forest University
• Washington University St. Louis
• Winthrop University
Conflict of Interest
Grant support was from the Cancer Prevention Research Institute of Texas Grant no. RP101207-P03, UTMB Clinical and Translational Science Award #UL1TR000071, NIH T-32 Grant no. T32DK007639, and AHRQ Grant no. 1R24HS022134. The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. BLH is an Associate Director of the ACS NSQIP for the American College of Surgeons and receives a consultant fee from ACS for this role.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Mark P. Callery (Boston, MA): “Dr. Cooper, by using a large NSQIP database, you have shown that neoadjuvant therapy prior to pancreatic cancer resection is safe. It does not increase a patient’s risk of death and overall morbidity. Let us hope your data can help dispel the myth that neoadjuvant therapy is dangerous. The low rate (12.7 %) of such therapy identified in your dataset is not surprising. That said, your work is important as it can now inform the reluctant that neoadjuvant therapy is a safe sensible approach in select patients, especially those with borderline resectable disease. Why do you think the mortality rates in this study are lower than most published postoperative mortality rates? Why do you think the rates of organ space infection are lower in the patients treated with neoadjuvant therapy? Finally, do you believe the participating institutions in this demonstration project somehow confound the findings and their interpretation? Thank you and congratulations to you and your coauthors.”
Closing Discussant
Dr. Cooper (Houston, TX): “Dr. Callery, thank you for your kind comments and insightful questions. I think the mortality rates in this study are likely lower than those in most published studies because a significant majority of the institutions that participated in the Pancreatectomy Demonstration Project meet the Leapfrog criteria for high-volume institutions for pancreatectomy.
I initially struggled a bit myself to understand why the rates of organ space infections are lower in the patients treated with neoadjuvant therapy; however, I was able to come up with two possible explanations. First, the rates of pancreatic fistula for the entire neoadjuvant therapy group were lower than those for the initial surgery group. Even though this difference only reached significance for the subset of patients treated with radiation, this may have been a key contributing factor as pancreatic fistulae are the most common cause of organ space infection in patients undergoing pancreatectomy. A second potential contributing factor may be that the patients in the neoadjuvant therapy group may have benefitted from some “prehabilitation” effect during the neoadjuvant therapy period that is not adequately measured by albumin, ASA class, and the other preoperative variables captured within NSQIP and that provided some protection against organ space infections.
I think the characteristics of the institutions participating in the Pancreatectomy Demonstration Project should certainly be kept in mind when interpreting the results of this study. As previously mentioned, many of these were high-volume institutions; however, it should also be noted that the institutions which are most well-known for and have the longest standing experience with the use of neoadjuvant therapy for treatment of pancreatic cancer were not participants in the Demonstration Project. In addition, 31 of the 43 participating hospitals contributed at least one patient to the neoadjuvant therapy group, so these patients were treated at a variety of different hospitals, which suggests that these results may be more generalizable than they might initially seem.”
Rights and permissions
About this article
Cite this article
Cooper, A.B., Parmar, A.D., Riall, T.S. et al. Does the Use of Neoadjuvant Therapy for Pancreatic Adenocarcinoma Increase Postoperative Morbidity and Mortality Rates?. J Gastrointest Surg 19, 80–87 (2015). https://doi.org/10.1007/s11605-014-2620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2620-3