Abstract
Background
Resection of the superior mesenteric vein (SMV)-portal vein (PV)-splenic vein (SV) confluence during pancreatectomy for pancreatic cancer requires management of the SV.
Discussion
Simple SV ligation can result in sinistral portal hypertension if the inferior mesenteric vein (IMV) enters the confluence and is thereby resected, or if the IMV is insufficient to drain the SV. We describe herein three patients whose clinical course confirms the importance of the IMV decompressing the SV to avoid sinistral hypertension.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Venous resection during pancreatectomy for pancreatic cancer is now performed with much greater frequency for otherwise resectable primary tumors that involve the superior mesenteric vein (SMV), portal vein (PV), or SMV-PV confluence. 1 When tumor abutment or, more commonly, encasement involves the splenic vein (SV)-SMV-PV confluence, complete tumor resection often requires the distal SV to be divided, thereby facilitating increased exposure to the proximal superior mesenteric artery (SMA) (for tumor resection) and creating increased length of the SMV-PV to allow for segmental venous resection and primary anastomosis. SV ligation may result in sinistral hypertension, and venous congestion in the stomach may be visibly apparent (even at the time of operation) especially when a distal gastrectomy is performed as part of a standard pancreaticoduodenectomy. The anecdotal observation that SV ligation can result in clinically significant sinistral hypertension and gastrointestinal hemorrhage (often months after the initial surgery) has caused some surgeons to consider either reimplanting the SV into the venous conduit when a venous conduit is used to reconstruct the SMV-PV or to perform a distal splenorenal shunt to decompress the SV.2 These techniques for SV decompression can be technically challenging but in some patients may be preferred when SV ligation is associated with inadequate collateral flow to decompress the stomach and spleen.3
Sinistral, or left-sided, portal hypertension in upper gastrointestinal and pancreatic surgery is caused by reversal of venous flow from the spleen through collaterals, resulting in gastric and esophageal varices.4 Why this is clinically significant in some, but not all, patients who undergo distal SV ligation is often due to the variable anatomy of the inferior mesenteric vein (IMV). When the IMV enters the SV and the distal SV is ligated at the time of pancreatic resection, the SV decompresses via retrograde flow in the IMV; this obviously does not occur when the IMV enters directly into the SMV. The importance of the IMV in decompressing the SV is not well described but, in our opinion, is a critically important part of the surgical planning for Whipple procedures which require resection of the SV-SMV-PV confluence. We describe herein three patients whose clinical course confirms the importance of the IMV in decompressing the SV and preventing clinically significant sinistral hypertension.
Patients and Methods
Case 1
A 74-year-old man presented with worsening hyperglycemia followed by obstructive jaundice and was diagnosed as having borderline resectable pancreatic head cancer due to tumor encasement of the SMV-PV confluence. He was treated with neoadjuvant gemcitabine/cisplatin followed by gemcitabine-based chemoradiation and, after final restaging, was taken to surgery. Pancreaticoduodenectomy with resection of the SV-SMV-PV confluence was completed, and the SV was ligated. The distal SV was ligated because the IMV entered the SV, thereby providing retrograde decompression of the SV. Because a long segment of SMV-PV was resected, venous reconstruction was performed using an internal jugular vein (IJV) conduit. His postoperative course was unremarkable, and he was discharged on postoperative day 8. Postoperative restaging revealed a wide open SV-IMV (Fig. 1a). Six months later, the SV-IMV was narrowed (Fig. 1b). Two years later, he presented with anemia and evidence of gastrointestinal blood loss. Gastroscopy documented gastric varices secondary to sinistral hypertension. On CT imaging (Fig. 1c, d), the previously visualized patent IMV-SV junction had become occluded by what appeared to be retroperitoneal fibrosis; a diagnosis of exclusion as recurrent cancer could not be demonstrated by multiple imaging studies. He underwent embolization of the proximal splenic artery (fibered platinum coils) which reduced sinistral hypertension by decreasing arterial inflow and thereby stopped further blood loss. The patient has remained asymptomatic to date (an additional 1 year of follow-up) with no evidence of cancer recurrence. This patient’s clinical course clearly demonstrated adequate IMV decompression of the SV with subsequent progressive IMV occlusion followed by development of symptomatic sinistral portal hypertension. By reducing inflow via embolization of the splenic artery, sinistral hypertension resolved.
a Case 1: coronal CT images 14 months postoperative demonstrating patency of IMV-SV junction (Aug 2011). b Case 1: narrowing of IMV-SV junction 20 months postoperative (Feb 2012) by coronal MIP imaging and 3D volume rendering. c–d Occlusion of IMV-SV junction 24 months postoperative (June 2012) as shown by coronal MIP and 3D volume rendering. SV splenic vein, IMV inferior mesenteric vein
Case 2
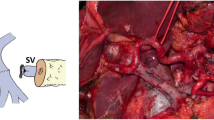
A 76-year-old woman was diagnosed with borderline resectable pancreas head cancer to include encasement of the SMV-PV confluence. She was treated with neoadjuvant gemcitabine, Taxotere, and capecitabine followed by capecitabine-based chemoradiation (5,040 cGy). Pancreaticoduodenectomy was then performed including resection of the SMV-PV confluence and ligation of the SV. Primary end-to-end repair of the SMV-PV confluence was possible without the need for interposition grafting due to the mobility afforded to the PV after dividing the SV (Fig. 2). The IMV entered the SV and appeared to adequately decompress the spleen and stomach. Her postoperative course was complicated by Clostridium difficile infection, but was otherwise uneventful, and she remained well for over 1 year when she then presented with bleeding per rectum. Investigation with gastroscopy and colonoscopy did not identify a bleeding source. However, on CT imaging, she was noted to have cecal and gastric varices. The patient was admitted for angiography (Fig. 3a) which demonstrated both gastric and cecal varices and documented the back pressure transmitted from the IMV through venous collaterals between the left colic and middle colic veins. Subsequent venous hypertension in the middle colic/right colic trunk was transmitted back along the right colic vein manifesting as varices in the cecal region (Fig. 3b). This was despite having a patent SV-IMV (Fig. 3c); our working diagnosis was that the IMV was simply insufficient to adequately decompress the spleen and stomach. She underwent coil embolization of the proximal splenic artery in an effort to decrease arterial inflow. Recovery from this procedure was uneventful, and the patient was discharged 2 days later. She has had no further gastrointestinal bleeding over the 1 year of subsequent follow-up and remains asymptomatic and disease-free. Sinistral portal hypertension resulting in gastric varices and blood loss approximately 1 year after SV ligation was clearly documented in this patient; it was effectively treated with splenic artery embolization.
Case 2 intraoperative photograph demonstrating direct end-to-end venous reconstruction following pancreaticoduodenectomy and resection of the SMV-PV confluence with ligation of the SV. SMV superior mesenteric vein, PV portal vein, SV splenic vein, CHA common hepatic artery, SMA superior mesenteric artery
a Portal venogram demonstrating gastric and colonic varices and SV decompression via the IMV in Case 2. Note the collateralization from the IMV to the SMV via venous anastomoses between the left colic vein (branch of IMV) and the middle colic vein (branch of SMV) at the splenic flexure. b Coronal and 3D volume-rendered images of gastric and cecal varices that developed in Case 2. c Curved planar reformat shows patent SV-IMV in this patient who developed cecal and gastric varices. SMV superior mesenteric vein, IMV inferior mesenteric vein, SV splenic vein
Case 3
This 68-year-old woman presented with abdominal pain which led to a CT diagnosis of a borderline resectable pancreatic head cancer due to abutment of the SMA and near-complete occlusion of the SMV just caudal to the SV confluence. The diagnosis was confirmed on EUS-guided FNA biopsy at the time of metal stent placement. She was treated with FOLFIRINOX (eight cycles) followed by gemcitabine-based chemoradiation (5,040 cGy) and then proceeded to pancreaticoduodenectomy with resection of the SMV-PV confluence. Her IMV entered just at the confluence of the SV and SMV, and the SV-IMV confluence was able to be preserved (Fig. 4). Prior to surgery, she had developed collateral venous flow through omental channels secondary to a nearly occluded SMV, but importantly, she did not have gastric varices (Fig. 5a). On repeat imaging 2 months postoperatively (Fig. 5b, c), she continued to adequately decompress her SV through the IMV and preexisting omental channels and had not developed any dilation of perigastric or gastric veins within the gastric fundus. We present this case as perhaps our best example of a robust IMV-SV junction allowing for SV ligation rather than, for example, performing a distal splenorenal shunt.
Intraoperative photograph demonstrating direct end-to-end venous reconstruction following pancreaticoduodenectomy and resection of the SMV-PV confluence with ligation of the SV in Case 3. Note the prominent IMV-SV junction that was preserved decompressing the left portal system. SMV superior mesenteric vein, PV portal vein, SV splenic vein, CHA common hepatic artery, SMA superior mesenteric artery, GDA gastroduodenal artery, IVC inferior vena cava
a Case 3: preoperative CT images demonstrating a lack of gastric varices and preexisting venous collaterals through omentum (asterisk). b–c Postoperative coronal MIP and 3D volume-rendered images 2 months following surgery showing no interval development of gastric varices, dilation of IMV compared to preoperative images and persistence of flow through omental collaterals. IMV inferior mesenteric vein, SV splenic vein
Discussion
This small case series clearly demonstrates the importance of the IMV in decompression of the SV and the clinical significance of inadequate decompression of the SV resulting in sinistral hypertension and gastrointestinal hemorrhage. The most powerful evidence in support of this clinical observation comes from Case 1, in which an initially patent IMV-SV confluence was not associated with sinistral hypertension, but following occlusion of the IMV, the patient presented with gastrointestinal hemorrhage. Successful reduction in arterial inflow by proximal splenic artery embolization completed the experimental paradigm wherein cause and effect were linked. This patient’s course nicely demonstrated adequate decompression of the SV by the IMV which was followed by sinistral hypertension when the IMV-SV junction was interrupted.
Importantly, as illustrated in Case 1, even if the SV-IMV junction is patent, the IMV may be inadequate to decompress the spleen and stomach. This is likely quite uncommon but may be partially explained by some degree of hepatic fibrosis with resultant increased portal pressures related to a lengthy course of chemotherapy. This is especially true if the left gastric vein remains connected to the PV, thereby allowing for the right and left portal systems to remain in continuity. Decompression of the SV via other venous channels with the development of colonic varices has also been described 3 and is nicely illustrated in this case. This clinical observation supports the fundamental concept that back pressure transmitted from inadequate SV decompression will result in the development of collateral venous circulation often accompanied by abnormal dilatation of venous channels; such varices can then become clinically evident if hemorrhage occurs. Importantly, varices other than those in the stomach may develop from SV ligation. In contrast, Case 1 demonstrates that an adequate IMV-SV conduit may effectively eliminate the development of the superior collateral venous pathway through the stomach,4 thereby minimizing the risk to such patients for postoperative upper gastrointestinal hemorrhage. Interestingly, if we consider the IMV to be the major avenue for decompression of the ligated SV, the other minor collateral connections between the right and left portal systems such as the left gastric vein (often, but not always ligated) and retroperitoneal collaterals may be a benefit in the setting of a normal liver, or in fact, a liability in the setting of hepatic fibrosis.
Ligation of the SV at the time of pancreaticoduodenectomy requires that the surgeon considers the subsequent venous return from the spleen. In the scenario whereby the IMV joins the SMV-PV confluence or the SMV proper and the SV is ligated, there is no mechanism for SV decompression by retrograde flow in the IMV. Reimplantation of the SV into the reconstructed SMV-PV or diversion of the SV into the left renal vein (via a distal splenorenal shunt) is necessary to prevent sinistral portal hypertension. We prefer splenorenal shunting as described by Warren et al., 5 , 6 as reimplantation of the SV into the newly created venous reconstruction may distort the SMV-PV confluence. If the IMV joins the SV, retrograde flow in the IMV will usually provide sufficient outflow to adequately decompress the spleen. However, as reported herein, the caliber of the IMV may occasionally be inadequate to decompress the SV. Should subsequent gastrointestinal bleeding occur, proximal splenic artery embolization can be curative by reducing inflow into the left-sided portal system. High-quality cross sectional imaging can be used to assess the IMV-SV confluence as this anatomic location is vulnerable to compression/occlusion if local recurrence of pancreatic cancer should occur.
The physiological significance of distal SV ligation or thrombosis when combined with pancreaticoduodenectomy and its potential to lead to the development of sinistral hypertension has been questioned. However, we believe that the cases provided herein support this clinical reality, and we encourage surgeons to consider how the SV will be decompressed when the SMV-PV-SV requires resection to include ligation of the SV.
References
Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr., Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1736-1744.
Christians KK, Riggle K, Keim R, et al. Distal splenorenal and temporary mesocaval shunting at the time of pancreatectomy for cancer: Initial experience from the Medical College of Wisconsin. Surgery. 2013;154(1):123-31.
Strasberg SM, Bhalla S, Sanchez LA, Linehan DC. Pattern of venous collateral development after splenic vein occlusion in an extended Whipple procedure: comparison with collateral vein pattern in cases of sinistral portal hypertension. J Gastrointest Surg. 2011;15(11):2070-2079.
Koklu S, Coban S, Yuksel O, Arhan M. Left-sided portal hypertension. Dig Dis Sci. 2007;52(5):1141-1149.
Warren WD, Zeppa R, Fomon JJ. Selective trans-splenic decompression of gastroesophageal varices by distal splenorenal shunt. Ann Surg. 1967;166(3):437-455.
Warren WD, Fomon JJ, Zeppa R. Further evaluation of selective decompression of varices by distal splenorenal shunt. Ann Surg. 1969;169(5):652-660.
Acknowledgments
The authors thank Leah Pitts RT (R) (CT) and Maureen Levenhagen RT (R) for image acquisition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilgrim, C.H.C., Tsai, S., Tolat, P. et al. Optimal Management of the Splenic Vein at the Time of Venous Resection for Pancreatic Cancer: Importance of the Inferior Mesenteric Vein. J Gastrointest Surg 18, 917–921 (2014). https://doi.org/10.1007/s11605-013-2428-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-013-2428-6