Abstract
Introduction
Trends in the use of modern chemotherapeutic regimens, primary tumor resection, and the timing of chemotherapy and resection in older patients with stage IV colorectal cancer have not been evaluated.
Methods
We used Cancer Registry- and Medicare-linked data (2000–2009) to describe time trends in resection of the primary tumor and receipt of chemotherapy in patients ≥66 presenting with stage IV colorectal cancer (N = 16,168).
Results
The mean age was 77.8 ± 7.3 years; 53.8 % were women and 82.9 % were white. Primary cancer sites were colon in 83.4 % and rectum in 16.6 %. Resection of the primary tumor decreased from 64.6 to 57.1 % (P < 0.0001) from 2001 to 2009. Systemic chemotherapy was given to 45.1 % of the patients. While the use of chemotherapy was stable over time (P = 0.48), the use of modern regimens containing oxaliplatin or irinotecan increased from 40.9 to 75.4 % (P < 0.0001). Bevacizumab use increased from 0.10 to 54.2 % (P < 0.0001). Survival improved by 4 % per year even after controlling for treatment and tumor location (HR = 0.96, 95 % CI 0.95–0.97).
Conclusions
Survival in older patients with stage IV disease is improving over time. Surgical resection is still performed in the majority of patients. Resection rates decreased while modern chemotherapy was rapidly adopted perhaps suggesting a shift in practice patterns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Twenty percent of patients with colorectal cancer will present with metastatic (stage IV) disease at the time of diagnosis.1 For stage IV disease, treatment with curative intent is only possible in the small subset of patients presenting with limited metastatic disease burden. While long-term survival has been reported after aggressive treatment in highly selected patients with limited synchronous or metachronous metastatic disease, the overall 5-year survival in patients presenting with stage IV disease is only 6 %.2
Prior to the year 2000, 5-fluorouracil (5-FU)/leucovorin (LV) was the standard chemotherapeutic regimen for patients with stage IV disease. In 2000, phase III studies and randomized clinical trials demonstrated a survival benefit in patients receiving oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) combined with 5-FU/LV when compared to 5-FU/LV alone.3 – 5 Consequently, FOLFOX and FOLFIRI became first-line chemotherapy for advanced colorectal cancer. Several other agents have subsequently been approved for treatment in combination with FOLFOX or FOLFIRI. These include capecitabine, bevacizumab, and cetuximab.6 – 10
Historically, patients with stage IV colorectal cancer underwent resection of the primary tumor to minimize tumor-related complications such as obstruction, bleeding, or perforation. A previous study using Surveillance Epidemiology and End Results (SEER)-Medicare data from 1991 to 1999 demonstrated a 72 % cancer-directed surgery rate in older patients.11 The mortality of cancer-directed surgery in older patients has been reported to range from 10 to18%.11 , 12 Given the advances in chemotherapy and palliative techniques such as endoluminal stenting since this study and the high mortality of cancer-directed surgery, the role of elective resection in stage IV disease has become controversial.10 – 13
The goal of our study was to evaluate trends in the management and outcomes of older patients presenting with stage IV colorectal cancer since the introduction of modern chemotherapeutic agents. First, we evaluated the adoption of newer cytotoxic regimens including oxaliplatin, irinotecan, and bevacizumab. Second, we described the trends in use of surgical resection of the primary tumor since the previous report and after the introduction of more efficacious systemic therapy. Finally, to assess the influence of these practice changes on survival, we evaluated disease-specific survival over this same time period.
Methods
The Institutional Review Board at the University of Texas Medical Branch determined this study to be exempt from review. The Texas Department of State Health Services approved the study as did the privacy review board of the Centers for Medicare and Medicaid Services. Data use agreements have been signed with both data providers.
Data Source
Data from the Texas Cancer Registry (TCR) and SEER-Medicare-linked database were used for the analysis. The TCR dataset provides detailed information about elderly adults with cancer in Texas. SEER collects data on cancer cases from population-based cancer registries covering approximately 28 % of the US population. Both registries collect data on patient demographics, primary tumor site, stage, first course of treatment, tumor morphology, cause of death, and survival.14 , 15 Through the National Cancer Institute and Center for Medicare and Medicaid Services, approximately 98 % of all people aged 65 and older in TCR and 93 % in SEER are matched with Medicare enrollment and claims files.16 , 17 The Medicare claims data include information on hospital stays, physician services, and hospital outpatient visits.18 The Medicare files used for this study included the Denominator file (demographics and eligibility), the Medicare Provider Analysis and Review file (MEDPAR, inpatient claims), the Carrier claim file (claims from non-institutional service providers), and the Outpatient Standard Analytical File (OutSAF, claims from institutional outpatient providers).18
Study Sample and Outcome Measures
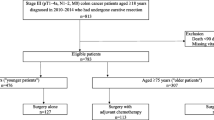
The cohort selection is shown in Fig. 1. The final sample included 16,168 patients (Fig. 1). We included cancer patients diagnosed with stage IV colorectal cancer between 2001 and 2007 and their Medicare claims from 2000 through 2009. This allowed us to determine patient comorbidity in the year prior to diagnosis and to follow all patients for 2 years or until death.
Resection of the primary tumor was identified from the Medicare claims (MEDPAR, carrier, outpatient SAF) using International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) procedure and Current Procedural Terminology, Fourth Edition (CPT-4) codes for colorectal resection (Table 1). These codes included colon and rectal resections, both open and laparoscopic, with or without colostomy. Patients who underwent stoma formation without resection or stent placement were not classified as having resection of the primary tumor. Emergent resection was defined as follows: a colorectal resection classified as “emergent” in the MEDPAR file, or any colorectal resection performed prior to or subsequent to systemic treatment with chemotherapy with a diagnosis code for obstruction, bleeding, or perforation (or related diagnosis; Table 1).
Chemotherapy was identified using Healthcare Common Procedure Coding System Codes, ICD-9 procedure and diagnosis codes, J codes, and revenue center codes for administration of chemotherapy as defined by SEER-Medicare.19 A beneficiary was considered to have received chemotherapy if he/she had a claim for chemotherapy after the diagnosis of colorectal cancer (Table 1). Specific agents were identified using J codes (Table 1). We defined “standard” chemotherapy as 5-fluorouracil ± leucovorin and “modern” chemotherapy as any regimen containing oxaliplatin or irinotecan. We were unable to assess the use of capecitabine, an oral analog of 5-fluorouracil, as orally administered agents cannot be identified in the Medicare parts A and B claims data. If a claim for leucovorin without 5-fluorouracil, oxaliplatin, or irinotecan was found, the patient was assumed to have received standard chemotherapy, as it is possible they may have been treated with capecitabine. Regimens not meeting these definitions were classified as “other”. Standard or modern chemotherapy regimens as defined above were given in 84.3 % of patients identified as having received chemotherapy.
Covariates
Patient characteristics included age, sex, race, Charlson comorbidity index (0, 1, 2, and 3 or more), and year of diagnosis. Median income and percent of residents with <12 years education were determined at the zip code level. Based on these variables, quartiles of education and income were established with quartile one being the lowest education/income and quartile four, the highest. Tumor characteristics included type (colon versus rectum), site (right, left, transverse, rectum, and unspecified), nodal status (negative, positive, no nodes, or unknown), and tumor differentiation (well/moderately versus poorly versus other). Rectal cancer was defined by site code for rectal cancer (26) or site code for rectosigmoid cancer (25) plus a rectal cancer operation (low anterior resection or abdominoperineal resection) and/or radiation.
Analysis
We calculated summary statistics for the overall cohort and determined the percentage of patients undergoing each treatment modality. The number of patients undergoing resection of the primary tumor was determined. For patients who received chemotherapy, we determined the percentage receiving standard chemotherapy and modern chemotherapy. Bevacizumab received Food and Drug Administration (FDA) approval for use in advanced colorectal cancer in 2004. For this analysis, its use was evaluated independently of other chemotherapeutic regimens.
We used a Cochran–Armitage test for trends to evaluate the trends in resection of the primary tumor, use of chemotherapy, and chemotherapy type. A logistic regression model was used to evaluate the independent association between year of diagnosis and resection of the primary tumor. In this model, year was defined as a continuous variable with the odds ratio representing the percent increase or decrease in resection per year of diagnosis. Unadjusted disease-specific survival was evaluated using a Kaplan–Meier analysis. A Cox proportional hazards model was used to evaluate improvements in 5-year disease-specific survival over time.
All P values were from two-sided tests. All analyses were performed with SAS version 9.2 (SAS Inc., Cary, NC, USA). Statistical significance was accepted at the P < 0.05 level.
Results
Patient and Tumor Characteristics
We identified 16,168 beneficiaries with stage IV colon cancer on presentation who met the inclusion criteria (Fig. 1). The mean age of the study population was 77.8 ± 7.3 years. Females comprised 53.8 % of the cohort. The majority of patients were white (82.9 %) and had a Charlson comorbidity score of zero (57.7 %). The colon was the primary site of cancer in 83.4 % of patients and the rectum in 16.6 % of patients. Further breakdown of the distribution of cancers throughout the colon is listed in Table 2.
Treatment
The characteristics of the treated and untreated patients are shown in Table 2. Resection alone was performed in 27.4 % of patients, chemotherapy alone was administered to 11.1 % of patients, and 27.5 % of patients did not receive treatment. Thirty-four percent of patients (N = 5,500) received chemotherapy and underwent resection of the primary tumor. In patients undergoing both treatment modalities, resection was performed prior to chemotherapy in 91.2 % of patients.
Resection of the primary tumor with or without chemotherapy was performed in 9,935 patients (61.5 %). Resection was emergent in 26.8 % of the 9,935 patients. The 30-day operative mortality was 10.2 % for patients undergoing elective resection and 21.5 % for patients undergoing emergent resection.
Systemic chemotherapy was administered to 7,292 patients (45.1 %). Of the 7,292 patients, 4,081 (56.0 %) were treated with modern regimens containing oxaliplatin or irinotecan, 2,069 (28.4 %) were treated with the standard regimen and 1,142 (15.7 %) were treated with other regimens. The most common agents identified in those receiving other chemotherapeutic regimens included: carboplatin, cisplatin, gemcitabine, cetuximab, and docetaxel.
Bevacizumab was given to 27.4 % of patients treated with systemic chemotherapy. Bevacizumab was administered in combination with a modern chemotherapy regimen in 83.1 % of patients, with a standard chemotherapy regimen in 11.8 %, and another regimen in 5.1 % of patients.
Liver resection was performed in 17.6 % of patients (N = 2,846). Ablation was performed in 3.2 % (N = 515) and chemoembolization in 1.2 % of patients (N = 193).
Time Trends in Treatment
Resection rates decreased from 64.6 % in 2001 to 57.1 % in 2007 (P < 0.0001). The rate of elective resection decreased from 49.5 % in 2001 to 40.9 % in 2007 (P < 0.0001; Fig. 2a).
a Time trends (2001–2007) in resection of the primary tumor for the overall cohort (solid line, N = 9,935), elective resection of the primary tumor (dotted line, N = 7,274). b Trends in use of standard, modern, and other chemotherapeutic regimens in patients with stage IV colorectal cancer. Solid line modern chemotherapy, dashed line standard chemotherapy, dotted line other chemotherapy. c Trends in use of bevacizumab in older patients presenting with stage IV colorectal cancer. d Time trends in treatments. Solid line resection of primary tumor only, dashed line chemotherapy only, dotted line chemotherapy and resection of primary tumor, dot and dash line no treatment
The overall administration of chemotherapy remained stable over the study period (P = 0.48); however, among patients who received any chemotherapy, the use of oxaliplatin or irinotecan containing regimens increased from 40.9 % in 2001 to 75.4 % in 2007 (P < 0.0001; Fig. 2b). Use of bevacizumab increased over time, with the greatest rate of increased use noted in 2003 shortly before it received FDA approval for use in advanced colorectal cancers (Fig. 2c).
The percent of patients who underwent both resection and chemotherapy remained stable, with approximately 30–35 % of the cohort receiving both treatments across time (Fig. 2d). The use of chemotherapy as the only treatment modality increased over time from 9.8 to 13.9 % from 2001 to 2007 (P < 0.0001) while at the same time, the proportion of patients undergoing resection alone decreased (29.8–26.0 %, P < 0.0001; Fig. 2d).
Factors Associated with Resection of the Primary Tumor
After controlling for demographics and receipt of chemotherapy, the year of diagnosis remained a significant predictor of resection of the primary tumor, with a 3 % decrease in resection with each increasing year of diagnosis (OR, 0.97; 95 % CI, 0.95–0.99). Younger patients and those with poorly differentiated colonic primaries had an increased likelihood of undergoing resection of the primary tumor (Table 3).
Survival
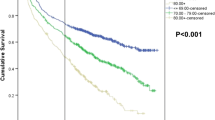
The 2- and 5-year disease-specific survival rates for the entire cohort were 27.4 and 12.9 %, respectively. In an unadjusted analysis, survival improved over time with a 25.2 % two-year disease-specific survival in the early time period (2001–2004) compared to a 30.7 % two-year disease-specific survival in the later time period (2005–2007; P < 0.0001). In the Cox proportional hazards model for the overall cohort (Table 4), for each 1-year increase in the diagnosis year, the hazard of death decreased by an estimated 4 % (HR, 0.96; 95 % CI, 0.95–0.97). Resection of the primary tumor (emergent or elective), receipt of chemotherapy, and receipt of bevacizumab were independently associated with improved survival. Advancing age at diagnosis, colon cancers, and poorly differentiated tumors were associated with worse prognosis.
Discussion
Our study is the first to evaluate treatment patterns and outcomes in older colorectal cancer patients presenting with stage IV disease in the era of modern chemotherapy. Time trends demonstrate an increase in the use of oxaliplatin- and irinotecan-containing regimens after studies in 2000 demonstrated their efficacy and superiority in improving survival when compared to the standard 5-FU and leucovorin regimen.3 – 5 Similarly, the use of bevacizumab has increased since it received FDA approval for use in stage IV colorectal cancer in 2004.
Through 2007, surgical resection was performed in the majority of patients presenting with advanced disease and was the first treatment modality in most patients receiving combination surgical resection and chemotherapy. However, we observed a statistically significant decrease in the rate of surgical resection of the primary tumor from 64.6 % in 2001 to 57.1 % in 2007. This decrease is even more dramatic when compared to the 72 % rate of resection of the primary tumor reported in a study using SEER-Medicare linked data from 1991 to 1999.11 A 2010 study of 103,744 patients from the Netherlands Cancer Registry also demonstrated a decline in primary tumor resection rates from 66 to 56 % (P < 0.001) and a dramatic rise in chemotherapy use from 2 % in 1989–1993 to 40 % in 2004–2006 (P < 0.001) in older patients with stage IV disease.20
In addition to the treatment trends, we observed an improvement in survival over time, consistent with other studies using tumor registry data.21 , 22 As in our study, the Netherlands Cancer Registry study found an independent association between year of diagnosis and survival.20 The improved survival over time in our study was not entirely mediated by treatment, as year of diagnosis remained significantly associated with survival even after adjusting for chemotherapy, resection of the primary tumor, and metastasectomy. Taken together, the decreased resection rates, increased use of modern chemotherapeutic agents, and the improved survival over time suggest better allocation of patients to appropriate treatment groups. These data suggest that we are aggressively treating the patients who will benefit most and avoiding unnecessary operations or aggressive therapy in those who are not likely to benefit. Additional factors that may explain improvements in survival above those attributed to treatment include advances in surgical technique, improvements in prevention, recognition, and management of complications, improved imaging leading to more accurate staging and diagnosis of treatable metastases and subsequently, more appropriate treatment allocation.
Our analysis shows that the number of patients receiving chemotherapy alone increased. This increase in chemotherapy alone may represent the beginning of a paradigm shift in the treatment of stage IV disease, allowing us to reserve elective resection of the primary tumor for patients with limited disease burden or those who exhibit a good response to chemotherapy. Continued evaluation of these trends as more data are available will confirm changes based on the current National Comprehensive Cancer Network (NCCN) recommendations, where immediate colon resection is reserved for patients at imminent risk for obstruction or significant bleeding.23
Our study has several limitations. We evaluated the management of stage IV disease in older patients; therefore, the results may not be generalizable to younger patients presenting with advanced colorectal cancer. However, older patients are often not included in randomized controlled trials and have a high-risk of treatment-related morbidity and mortality. As such, it is important to study the comparative effectiveness of different treatment strategies in this vulnerable population. We were unable to evaluate the use of newer chemotherapeutic agents, such as panitumumab and aflibercept, which were introduced after the study period. In addition, we could not capture the use of capecitabine, using SEER-Medicare data from 2001 to 2007, as this is administered orally. If a patient was only treated with leucovorin, we placed them in the standard chemotherapy group because it is possible they were treated with capecitabine; this occurred in only 0.4 % of patients receiving chemotherapy. These data do not allow us to evaluate the intent of treatment. For example, we cannot determine which patients received chemotherapy with the intent to undergo resection in the future versus those who received chemotherapy purely with palliative intent. Similarly, we are unable to determine which resections were performed to palliate symptoms and which were performed in asymptomatic patients, but we were able to identify emergent versus elective resections. Lastly, there is selection bias; aggressive treatment is more likely to be pursued in healthier patients and patients with lower burden of disease. The observed improved survival over time partly reflects proper patient selection for surgical resection and aggressive therapy.
Our study demonstrates that practitioners are rapidly adopting the use of newer chemotherapeutic agents and employing elective surgical resection less often. These changes are associated with improved survival over time. Colorectal cancer is primarily a disease of the elderly, yet older patients account for only 40 % of patients included in clinical trials.24 While there is no question as to the role of surgical resection in symptomatic patients, the high operative mortality associated with elective resection in older patients presenting with stage IV disease makes elective resection controversial in the setting of modern chemotherapy. Further studies are needed to determine if we are, in fact, observing a paradigm shift. As more data becomes available, we can evaluate adherence to the current NCCN treatment guidelines and evaluate the comparative effectiveness of a chemotherapy first approach in this vulnerable population of patients.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29.
Colorectal Cancer Detailed Guide. Available at: http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-survival-rates. Accessed 13 Mar 2013
de Gramont A, Figer A, Seymour M et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–2947.
Saltz LB, Cox JV, Blanke C et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000; 343: 905–914.
Douillard JY, Cunningham D, Roth AD et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041–1047.
Van Cutsem E, Köhne CH, Hitre E et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360: 1408–1417.
Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342.
Rinaldi F, George E, Adler AI. NICE guidance on cetuximab, bevacizumab, and panitumumab for treatment of metastatic colorectal cancer after first-line chemotherapy. Lancet Oncol 2012; 13: 233–234.
Hoff PM, Ansari R, Batist G et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: Results of a randomized phase III study. J Clin Oncol 2001; 19: 2282–2292.
Van Cutsem E, Tabernero J, Lakomy R et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012; 30: 3499–3506.
Temple LK, Hsieh L, Wong WD et al. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol 2004; 22: 3475–3484.
Basili G, Lorenzetti L, Biondi G et al. Colorectal cancer in the elderly. Is there a role for safe and curative surgery? ANZ J Surg 2008; 78: 466–470.
Konyalian VR, Rosing DK, Haukoos JS et al. The role of primary tumour resection in patients with stage IV colorectal cancer. Colorectal Dis 2007; 9: 430–437.
Texas Cancer Registry. Available at: http://www.dshs.state.tx.us/tcr/.
Surveillance Epidemiology and End Results (SEER). Available at: http://seer.cancer.gov/about/overview.html. Accessed 22 March 2013
Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Texas Cancer Registry Division. Available at: http://www.dshs.state.tx.us/tcr/default.shtm. Accessed 6 May 2013
National Cancer Institute SEER-Medicare 2013. Available at: http://healthservices.cancer.gov/seermedicare/overview/linked.html. Accessed 6 May 2013
Research Data Assistance Center (ResDAC).Medicare Claims. Available at: http://www.resdac.org/cms-data/file-family/Medicare-Claims
Procedure codes for SEER-Medicare Analysis. Available at: http://healthservices.cancer.gov/seermedicare/considerations/procedure_codes.html. Accessed 18 Mar 2013
van Steenbergen LN, Elferink MA, Krijnen P et al. Improved survival of colon cancer due to improved treatment and detection: a nationwide population-based study in The Netherlands 1989–2006. Ann Oncol 2010; 21: 2206–2212.
Sun E, Lakdawalla D, Reyes C et al. The determinants of recent gains in cancer survival: an analysis of the Surveillance, Epidemiology, and End Results (SEER) database. In ASCO. 2008.
Kopetz S, Chang GJ, Overman MJ et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27: 3677–3683.
NCCN Clinical Practice Guidelines. Version 3.2013 edition. 2013.
Cen P, Liu C, Du XL. Comparison of toxicity profiles of fluorouracil versus oxaliplatin regimens in a large population-based cohort of elderly patients with colorectal cancer. Ann Oncol 2012; 23: 1503–1511.
Acknowledgments
The collection of cancer incident data used in this study was supported by the Texas Department of State Health Services and Cancer Prevention Research Institute of Texas as part of the statewide cancer reporting program and the Centers for Disease Control and Prevention’s National Program of Cancer Registries Cooperative Agreement #5U58/DP000824-05. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the DSHS, CPRIT, or CDC.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and +Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries under agreement No. U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding
Cancer Prevention Research in Texas grant #RP101207-P03, Clinical and Translational Science Award #UL1TR000071, and NIH T-32 grant #5T32DK007639.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vargas, G.M., Sheffield, K.M., Parmar, A.D. et al. Trends in Treatment and Survival in Older Patients Presenting with Stage IV Colorectal Cancer. J Gastrointest Surg 18, 369–377 (2014). https://doi.org/10.1007/s11605-013-2406-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-013-2406-z