Abstract
Introduction
Increased lymph node (LN) retrieval for gastric cancer has been associated with improved overall survival (OS). This study examines the impact of number of examined LN (eLN) and lymph node ratio.
Methods
Patients referred for surgical care of gastric cancer were stratified by number of eLN, positive LNs (LN+), and lymph node ratio (LN+/eLN). Clinicopathologic factors were compared; OS and disease-free survival (DFS) were the primary endpoints.
Results
From 1997 to 2012, 222 patients, median age 67 (range, 17–92) years, were analyzed. Of 220 (99 %) explored, 164 (74 %) underwent resection. Median OS was 22 (range, 0.3–140) months. Perineural and lymphovascular invasion and poor differentiation adversely affected OS, p < 0.05. A median 14 eLN (range, 0–45), with median 1 LN+ (range, 0–31), was observed. There were no OS or DFS differences when comparing the eLN groups. Both OS and DFS were impacted by LN+. Lymph node ratio demonstrated worse median OS with increasing ratio: 49 months (0) to 37 months (0.01–0.2), 27 months (0.21–0.5), and 12 months (>0.5), p < 0.0001. DFS was similar: 35 months (0), decreasing to 22 months (0.01–0.2), 13 months (0.21–0.5), and 7 months (>0.5), p < 0.0001.
Conclusion
Number of eLN did not impact survival, while LN+ adversely affected survival. Lymph node ratio may predict prognosis better than number of eLN or LN+ in gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of gastric cancer in the USA has decreased remarkably in the last decade. However, over 21,000 new cases are diagnosed each year, and over 10,000 deaths are attributed to gastric cancer annually.1 The current National Comprehensive Care Network (NCCN) guidelines recommend surgical resection and D2 lymphadenectomy (regional lymph nodes along with those along the named vessels of the celiac axis), with the goal of retrieving and examining at least 15 lymph nodes (examined lymph node (eLN)) to provide adequate surgical staging.2 The current American Joint Committee on Cancer (AJCC) seventh edition staging criteria for gastric cancer, however, does not take into account the number of eLN. Rather, the number of positive lymph nodes (LN+), in addition to tumor size and presence of distant metastases, is used to determine stage and prognosis.3
The extent of lymphadenectomy had previously been the subject of debate, with proponents in Asia, especially Korea and Japan, which have the highest global gastric cancer incidence, advocating “extended” (>D1) lymphadenectomy according to the Japanese lymph node station classification.4 , 5 Surgeons in the USA and Europe had previously found mixed results, with several large randomized studies finding no survival benefit and heightened morbidity with > D1 lymphadenectomy.6 , 7 A 15-year analysis from the prospective Dutch D1/D2 trial demonstrated similar overall survival rates between the two groups. The D2 group, however, had a lower locoregional recurrence rate and gastric cancer-related death rate but did have a higher operative mortality and complication rate.8 Therefore, while D2 lymphadenectomy is recommended, it is not required per the current NCCN guidelines.3 Numerous studies, however, both from Asia and the USA, have shown improved survival associated with an increased number of eLN,9 – 11 with many studies showing improved survival with >15 lymph nodes retrieved.
In the last several decades, lymph node ratio (ratio of LN+ to eLN) has been proposed as an independent prognostic factor in determining overall survival (OS) in gastric cancer. Some groups have shown that lymph node ratio may predict survival more accurately than the current AJCC staging system, particularly in patients when <15 lymph nodes have been examined.12 – 16 Few studies from the USA, however, have been performed,12 , 15 and the incorporation of lymph node ratio in determining prognosis of patients remains undetermined. This study evaluates lymph node ratio as well as lymph node retrieval as they relate to prognosis in gastric cancer.
Methods
After institutional review board approval, a prospectively maintained database of patients referred for surgical care of biopsy-proven gastric adenocarcinoma from 1997 to 2012 was reviewed. All patients with gastroesophageal junction tumors were excluded. Demographic, clinical, and pathologic factors were compared. Subgroup analysis was also performed according to various lymph node parameters. Patients were stratified by number of examined lymph nodes: <5, 6–10, 11–15, and >15. The eLN value 15 was chosen to reflect the NCCN guideline, and the lower eLN cut points were chosen to determine if fewer eLN resulted in differences in endpoints. The number of positive lymph nodes were grouped per the AJCC staging criteria into 0–2, 3–6, 7–15, and >15. Lymph node ratio (LN+/eLN) was also stratified by 0, 0.01–0.2, 0.21–0.5, and >0.5 cut points; these levels have previously been demonstrated in studies from Asia to have prognostic value.17 , 18 Disease-free survival (DFS) and OS were the primary endpoints.
Statistical Analyses
The statistical analysis was conducted using the SAS 9.3 software, SAS Institute, Cary, North Carolina. Demographic, clinical, and pathologic factors were compared using the Pearson correlation and chi-square analysis. Survival analyses were performed using the Kaplan–Meier method, and comparisons across different strata were made with the log-rank test. Multivariate adjusted factor analysis was performed using the Cox proportional hazard modeling. For this analysis, age was stratified into two groups: <45 vs. ≥45 years. Lymph node retrieval was treated as a continuous variable; the other factors were considered as categorical variables. Statistical significance was determined at the two-tailed p value of ≤0.05. Factors considered in the adjusted model included gender, tumor size, tumor differentiation, perineural invasion, lymphovascular invasion, and lymph node ratio.
Results
Patient Characteristics
Of 222 patients who presented for surgical evaluation, 122 (55 %) were male and 100 (45 %) were female. The median age was 67 years (range, 17–92). The majority (N = 165, 74 %) were Caucasian, 20 (9 %) Hispanic, 12 (5 %) black, and 25 (11 %) other. Of 213 (96 %) patients with known smoking status, 109 (51 %) were nonsmokers, 28 (13 %) were active smokers, and 76 (36 %) had a positive smoking history but were not smoking at the time of evaluation.
Two-hundred twenty (99 %) underwent surgical exploration with 164 (74 %) undergoing resection. Of those undergoing resection, 87 (53 %) underwent distal or subtotal distal gastrectomy, 72 (44 %) total gastrectomy, 4 (2 %) proximal gastrectomy, and 1 (<1 %) palliative partial gastrectomy. Forty-six (28 %) had received neoadjuvant chemotherapy, and 73 (45 %) received adjuvant chemotherapy. Final pathologic stage was recorded in 155 (95 %). Stage 0/1 disease was found in 53 (34 %), stage II in 55 (35 %), stage III in 37 (24 %), and stage IV in 10 (6 %). Of 159 (97 %) patients with data available regarding margins, the majority (N = 126, 79 %) had a R0 resection and 33 (21 %) had a R1 resection.
Overall and Disease-Free Survival
Median follow-up for the cohort was 36 months, and median OS was 22 months. Although men tended to have a worse OS than females (median 18 vs. 28 months, respectively), this was not statistically significant, p = 0.073. Ethnicity and active or positive history of smoking also did not impact OS (Table 1). Older age demonstrated worse OS; patients ≤45 years had improved median OS compared with >45 years: 34 vs. 20 months, respectively, p = 0.045. Median OS was lower in patients with the presence of perineural invasion (PNI), lymphovascular invasion (LVI), and poor differentiation (Table 1). Ninety-six patients had LVI demonstrated on pathology; median OS was 21 vs. 56 months for patients without evidence of LVI, p < 0.0001. Similarly, 69 patients had presence of PNI and showed decreased median OS, 19 vs. 47 months, p < 0.0001. Well-differentiated tumors demonstrated a 124-month median OS. This decreased substantially to 37 months for moderately differentiated tumors and 19 months for poorly differentiated tumors, p = 0.015 (Table 1). Positive surgical margins were also associated with worse OS, with a median OS of 37 months for negative surgical margins vs. 17 months for positive surgical margins, p = 0.0002. The use of neoadjuvant chemotherapy did not impact overall survival, p = 0.721.
Of patients who underwent resection, 51 (31 %) developed disease recurrence. The most common site was the peritoneum (N = 30, 59 %), followed by local recurrence (N = 7, 14 %), the liver (N = 7, 14 %), or other distant sites (N = 7, 14 %). Median DFS was 12 months for the cohort. There was again a trend towards worse DFS in men (median 10 vs. 16 months in females), although this was not significant, p = 0.091. Ethnicity and smoking status did not affect DFS (Table 1). As seen with OS, median DFS also was adversely affected by LVI (median DFS, 14 vs. 35 months in the absence of LVI, p = 0.0005) and PNI (13 vs. 26 months, p = 0.0002). Median DFS was 120 months for well-differentiated tumors, decreasing to 22 months for moderate differentiation and 10 months for poor differentiation, p = 0.0005 (Table 1). Similar to OS, DFS was not affected by the use of neoadjuvant therapy, p = 0.169.
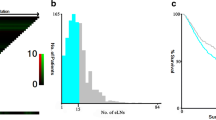
One hundred sixty (98 %) resected patients had lymph node data recorded. The median number of eLN was 14 (range, 0–45). Eighty-nine (56 %) had <15 eLN at the time of resection. There were no OS (p = 0.66) or DFS (p = 0.58) when the <15 eLN vs. ≥15 eLN groups were compared. Further subgroups were created with 1–5 eLN (N = 27), 6–10 eLN (N = 34), 11–15 eLN (N = 28), and >15 eLN (N = 71). There were no OS or DFS differences between the groups, p = 0.31 and 0.18, respectively (Fig. 1). The median number of LN+ was 1 (range, 0–31). The study period was divided into 5-year intervals; there was a significant increase in the median eLN from the earliest to the latest period, 13 to 14.5, respectively, p = 0.0267. Using AJCC staging criteria, subgroups were created to include 0 LN+ (N = 70), 1 and 2 LN+ (N = 33), 3–6 LN+ (N = 19), 7–15 LN+ (N = 17), and >15 LN+ (N = 20). Patients without lymph node metastasis showed an improved OS and DFS compared with any of the LN+ categories (Fig. 2). Administration of neoadjuvant chemotherapy did not impact either number of eLN (p = 0.762) or number of LN+ (p = 0.936). Lymph node ratio was calculated for 155 (95 %); median lymph node ratio was 0.09 (range, 0–0.97). Ratio cut points of 0 (N = 67), 0.01–0.2 (N = 25), 0.21–0.5 (N = 31), and >0.5 (N = 32) were used. Increasing lymph node ratio was associated with both worse OS and DFS, p < 0.0001 (Fig. 3).
Number of eLN had no impact on OS or DFS. Median OS and DFS were better differentiated using lymph node ratio than the number of LN+. Median OS decreased from 52 months with 0 LN+ to 11.5 months with >15 LN+; a median OS of 21 months was observed with 1 and 2 LN+, 34 months with 3–6 LN+, and 25 months with 7–15 LN+. A similar trend was seen with DFS and LN+ (Table 2). Lymph node ratio showed a steady correlation with both OS and DFS. Median OS was improved with a LN ratio of 0 (49 months) and declined to 37 months with a ratio of 0.01–0.2, 27 months with a ratio of 0.21–0.5, and 12 months with a ratio >0.5 (Table 2). Disease-free survival showed a similar correlation: 35 months with ratio 0, 22 months with ratio 0.01–0.2, 13 months with ratio 0.21–0.5, and 7 months with ratio >0.5 (Table 2).
When stratifying the cohort into <15 vs. ≥15 eLN, median OS and DFS also showed a steady decline with increasing LN ratio, particularly in the <15 eLN group. Median OS in this group decreased from 49 months in the 0 LN ratio group to 37 months in the 0.01–0.2, 17 months in the 0.21–0.5, and 13 months in the >0.5 LN ratio group (Table 2). Disease-free survival in the <15 eLN group similarly showed a trend with a median DFS of 31 months in the LN ratio 0 group, 11 months in the 0.01–0.2, 13 months in the 0.21–0.5, and 6 months in the >0.5 group, p < 0.001. The median OS and DFS followed similar trends with LN ratio groups in the ≥15 eLN group, albeit the median OS and DFS were longer than in the <15 eLN group (Table 2).
Multivariate Analysis
Factors considered into the univariate model to determine impact on overall or disease-free survival were gender, age, tumor size and differentiation, perineural invasion, lymphovascular invasion, number of eLN, number of LN+, and LN ratio. For OS, a LN ratio of 0.2–0.5 was significantly associated with worse OS, with a hazard ratio (HR) of 2.87 (confidence interval 1.02–8.06), p = 0.045 (Table 3). Although there was a trend towards worse OS with the other LN ratios, these were not statistically significant. One to two LN+ and >15 LN+ were also adversely associated with OS; however, the number of eLN did not impact OS (Table 3). Male gender, age younger than 45 years, and presence of lymphovascular invasion were also associated with worse OS (Table 3). Disease-free survival was significantly impacted only by 3–6 LN+, with a HR 4.24 (confidence interval (C.I.) 1.1–16.7), p = 0.039 (Table 3). Notably, the number of eLN and LN ratio was not associated with DFS on univariate analysis. On multivariate analysis, male gender, poor histologic differentiation, and LN ratio of >0.5 were associated with worse OS (Table 4). Male gender and LN ratio of >0.5 also adversely impacted DFS (Table 4).
Discussion
The overall survival of patients undergoing gastrectomy for gastric adenocarcinoma has been previously associated with the number of both positive and negative lymph nodes in a validated nomogram for patients undergoing R0 resections.9 , 19 However, a recent nomogram, proposed and validated with a large series of patients in South Korea by Han et al. has incorporated the number of examined lymph nodes as a prognostic factor.20 Surgeons in Asia have long advocated the standard performance of D2 lymphadenectomy for gastric adenocarcinoma; in comparison with a high-volume U.S. cancer center, the surgeons in South Korea retrieved a greater number of lymph nodes and demonstrated improved 5-year survival although did have a significantly greater incidence of early stage gastric cancer.21
Contrary to many reports demonstrating improved survival with extensive lymphadenectomy,9 – 11 this study found no OS or DFS difference when stratifying by <15 vs. ≥15 lymph nodes, similar to a previous U.S. study from Massachusetts General Hospital.15 Although surgeons and oncologists in the USA generally advocate the administration of neoadjuvant chemotherapy prior to resection, only 28 % of this cohort received neoadjuvant therapy. There was no independent effect of either neoadjuvant or adjuvant therapy on OS or DFS.
Lymph node ratio has been reported to predict overall survival and has been evaluated in patients with an insufficient (<15) lymph node retrieval,15 , 22 , 23 showing a greater survival prediction than the number of eLN. This current study spans over a decade within a single U.S. cancer center, during a time when the surgical paradigm had shifted from performance of D1 lymphadenectomy to routine D2 lymphadenectomy for gastric cancer. Within this cohort, 56 % had an insufficient number of eLN after surgical resection. Although no survival differences were seen with <15 eLN, there is a possibility of stage migration with insufficient staging information being obtained.
Despite large series in Asia describing lymph node ratio and its correlation with survival, a few Western studies have reported its utility.15 , 24 A study from Massachusetts General Hospital utilized the ratio cut points of 0.3 and 0.7 in a cohort of 257 patients and found greater sensitivity for predicting OS with ratio than number of LN+.15 A larger study from the Polish Gastric Cancer Study Group determined that a lymph node ratio of 0.4 was an independent prognostic factor for survival.24 This current study utilized the lymph node ratio cut point values of 0.2 and 0.5, similar to other Asian studies.17 , 18 , 25 On multivariate analysis, a ratio of >0.5 was significantly associated with worse OS and DFS. Although poor pathologic characteristics such as the presence of perineural and lymphovascular invasion as well as poor differentiation were correlated with a worse median overall and disease-free survival, similar to previous reports,26 – 28 only poor differentiation predicted worse overall survival on multivariate analysis. Both number of LN+ and lymph node ratio demonstrated significant survival differences with an increasing value. Lymph node ratio demonstrated a similar association with survival, when evaluated in the group with <15 eLN, suggesting that the LN ratio may be used as a predictor of outcome, even when insufficient staging information is obtained.
Several limitations are inherent in this study, being both retrospective in nature as well as small in size; statistical analyses, in part, were confounded by small sample size. In contrast to South Korea where gastric cancer care is centralized, the USA sees a more distributed approach, with nearly 60 % of patients treated at community hospitals or community cancer centers.29 Additionally, this study spans a time frame whereupon the standard lymphadenectomy for gastric cancer shifted from routine D1 to D2 lymphadenectomy, a paradigm shift that has resulted in a greater number of eLN. This study, however, represents one of the larger series of a single-institution, U.S. academic cancer center's experience with gastric cancer patients evaluating lymph node ratio.
Conclusion
In our series, number of examined lymph nodes did not impact OS or DFS. Lymph node ratio, in particular a ratio of >0.5, appeared to offer prognostic information and was associated with worse OS and DFS with an increasing ratio. This effect was also seen when specifically evaluating a subgroup of patients with <15 eLN. Further investigation is warranted to determine whether lymph node ratio could be incorporated into the gastric cancer staging system.
References
American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Gastric Cancer. Version 2.2013. NCCN.org.
Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, p 120.
Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. The Lancet Oncology, 2006; 7(4): 309–315.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer, 2011; 14: 113–123.
Hartgrink HH, van de Velde CJH, Putter J, et al. Extended Lymph Node Dissection for Gastric Cancer: Who May Benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. Journal of Clinical Oncology, 2004; 22: 2069–2077.
Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized survival trial. British Journal of Cancer, 1999; 79: 1522–1530.
Songun I, Putter H, Kranenbarg EMK, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncology, 2010; 11: 439–49.
Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. Journal of Clinical Oncology, 2003; 21 (19): 3647–50.
Son T, Hyung WJ, Lee JH, et al. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer, 2012; 118 (19): 4687–93.
Xu D, Huang Y, Geng Q, et al. Effect of lymph node number on survival of patients with lymph node-negative gastric cancer according to the 7th edition UICC TNM system. PLoS ONE, 2012; 7(6): e38681.
Wang J, Dang P, Raut CP, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Annals of Surgery, 2012; 255(3): 478–85.
Lemmens VEPP, Dassen AE, van der Wurff AAM, et al. Lymph node examination among patients with gastric cancer: Variation between departments of pathology and prognostic impact of lymph node ratio. European Journal of Surgical Oncology, 2011; 37(6): 488–496.
Wang W, Xu DZ, Li YF, et al. Tumor-ratio-metastasis staging system as an alternative to the 7th edition UICC TNM system in gastric cancer after D2 resection-results of a single-institution study of 1343 Chinese patients. Annals of Oncology, 2011; 22(9): 2049–56.
Maduekwe UN, Lauwers GY, Fernandez-Del-Castillo C, et al. New metastatic lymph node ratio system reduces stage migration in patients undergoing D1 lymphadenectomy for gastric adenocarcinoma. Annals of Surgical Oncology, 2010; 17(5): 1267–77.
Fukuda N, Sugiyama Y, Midorikawa A, Mushiake H. Prognostic significance of the metastatic lymph node ratio in gastric cancer patients. World Journal of Surgery, 2009; 33(11): 2378–82.
Zhou Y, Zhang J, Cao S, and Li Y. The evaluation of metastatic lymph node ratio staging system in gastric cancer. Gastric Cancer, 2012; 16(3): 309–17.
Sun Z, Zhu GL, Lu C, et al. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Annals of Oncology, 2009; 20(5): 897–905.
Peeters KC, Kattan MW, Hartgrink HH, et al. Validation of a nomogram for predicting disease-specific survival after an R0 resection for gastric carcinoma. Cancer, 2005; 103(4): 702–7.
Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after D2 gastrectomy for gastric cancer. Journal of Clinical Oncology, 2012; 30(31): 3834–40.
Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Annals of Surgery, 2010; 251(4): 640–6.
Latengbaolide A, Lin D, Li Y, et al. Lymph Node Ratio Is an Independent Prognostic Factor in Gastric Cancer After Curative Resection (R0) Regardless of the Examined Number of Lymph Nodes. American Journal of Clinical Oncology, 2012; 36(4): 325–30.
Deng J, Liang H, Wang D, et al. Enhancement the prediction of postoperative survival in gastric cancer by combining the negative lymph node count with ratio between positive and examined lymph nodes. Annals of Surgical Oncology, 2010; 17(4): 1043–51.
Kulig J, Sierzega M, Kolodziejczyk P, et al. Ratio of metastatic to resected lymph nodes for prediction of survival in patients with inadequately staged gastric cancer. British Journal of Surgery, 2009; 96(8): 910–8.
Kong SH, Lee HJ, Ahn HS, et al. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: the reappraisal of positive lymph node ratio as a proper N-staging. Annals of Surgery, 2012; 255(1): 50–8.
Du C, Zhou Y, Cai H, et al. Poor prognostic factors in patients with stage I gastric cancer according to the seventh edition TNM classification: a comparative analysis of three subgroups. Journal of Surgical Oncology, 2012; 105(3): 323–8.
Lee IS, Yook JH, Kim TH, et al. Prognostic factors and recurrence pattern in node-negative advanced gastric cancer. European Journal of Surgical Oncology, 2013; 39(2): 136–40.
Bilici A, Seker M, Ustaalioglu BB, et al. Prognostic significance of perineural invasion in patients with gastric cancer who underwent curative resection. Annals of Surgical Oncology, 2010; 17(8): 2037–44.
Reid-Lombardo KM, Gay G, Patel-Parekh L, et al. Treatment of Gastric Adenocarcinoma May Differ Among Hospital Types in the United States, a Report from the National Cancer Data Base. Journal of Gastrointestinal Surgery, 2007; 11(4): 410–420.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, J., Rahman, S., Saeed, N. et al. Prognostic Impact of Lymph Node Retrieval and Ratio in Gastric Cancer: a U.S. Single Center Experience. J Gastrointest Surg 17, 2059–2066 (2013). https://doi.org/10.1007/s11605-013-2380-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-013-2380-5






