Abstract
Objective
Laparoscopic feeding jejunostomy is a safe and effective means of providing enteral nutrition in the preoperative phase to esophageal cancer patients.
Design
This research is a retrospective case series.
Setting
This study was conducted in a university tertiary care center.
Patients
Between August 2007 and April 2012, 153 laparoscopic feeding jejunostomies were performed in patients 10 weeks prior to their definitive minimally invasive esophagectomy.
Main Outcome Measures
The outcome is measured based on the technique, safety, and feasibility of a laparoscopic feeding jejunostomy in the preoperative phase of esophageal cancer patients.
Results
One hundred fifty-three patients underwent a laparoscopic feeding jejunostomy approximately 1 and 10 week(s) prior to the start of their neoadjuvant therapy and definitive minimally invasive esophagectomy, respectively. Median age was 63 years. Of the patients, 75 % were males and 25 % were females. One hundred twenty-seven patients had gastroesophageal junction adenocarcinoma and 26 had squamous cell carcinoma. All patients completed their neoadjuvant chemoradiation therapy. The median operative time was 65 min. We had no intraoperative complications, perforation, postoperative bowel necrosis, bowel torsion, herniation, intraperitoneal leak, or mortality as a result of the laparoscopic feeding jejunostomy. Four patients were noted to have superficial skin infection around the tube, and 11 patients required a tube exchange for dislodgment, clogging, and leaking around the tube. All patients progressed to their definitive surgical esophageal resection.
Conclusion
A laparoscopic feeding jejunostomy is technically feasible, safe, and can provide appropriate enteral nutrition in the preoperative phase of esophageal cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophagectomy, whether for benign or malignant disease, is a complex operation which carries significant morbidity and mortality both for open and for minimally invasive techniques.1,2 Furthermore, esophageal cancer patients are prone to malnutrition due to weight loss resulting from dysphagia and side effects from neoadjuvant chemoradiation therapy.3,4 Ryan et al. reported that 34 % of patients undergoing esophagectomy had presented with severe weight loss as defined by >10 % in 6 months or >5 % in 1 month.5 The Department of Veteran Affairs National Surgical Quality Improvement Program data reported that 32 % of patients undergoing esophagectomy had presented with severe weight loss.6 In fact, both severe weight loss and hypoalbuminemia, markers of severe malnutrition, have been shown to be associated with higher postoperative complication rates.6,7 Furthermore, neoadjuvant chemoradiation therapy commonly utilized for non-early stage esophageal malignancy in order to reduce tumor size, increase resectability, and prevent further metastases is frequently associated with gastrointestinal side effects such as nausea, emesis, diarrhea, mucositis, and decreased appetite—all of which can compromise a patient’s fluid, electrolyte, and nutritional status.4,8 Despite the complexity of the operation and the potential for malnutrition and, therefore, higher complication rates, maintaining optimal nutrition in patients undergoing esophagectomy is important throughout the treatment period.
In a study by Gianotti et al., 305 patients undergoing surgery for gastrointestinal cancer were randomized to three arms: (1) preoperative nutrition alone (1,000 kcal a day of an oral liquid diet containing omega-3 fatty acids and arginine), (2) pre- and perioperative nutrition, and (3) no nutritional supplementation. A significant reduction in infection rates (30 to 13 %) and median length of hospital stay (14 to 11 days) was seen with preoperative nutrition compared with no nutritional supplementation.9 This study demonstrated the benefit of preoperative nutrition before gastrointestinal cancer resection. Additionally, prior reports have suggested the theoretical beneficial effects of enteral feeding, compared with parenteral feeding, on gastrointestinal immune function.10,11 Mazaki and Ebisawa performed a meta-analysis of 29 randomized controlled trials comparing various routes of nutritional support and found that enteral nutrition significantly reduced the rate of any complication, any infectious complication, anastomotic leak, intra-abdominal abscess, and duration of hospital stay.12
Jejunostomy is a means of enteral nutrition that can be accomplished via a multitude of described techniques, such as laparotomy, percutaneous, endoscopic or laparoscopy.13 Feeding jejunostomy tubes (JT) have been commonly utilized either in the preoperative phase or at the time of an operation in patients undergoing esophagectomy.14–16 Due to potential complications of JT placement, the need for routine use of feeding JTs in foregut surgery has been studied.17–19 If morbidity and mortality from JT placement could be minimized, this would potentially justify its routine use in patients undergoing esophagectomy—given the substantial benefit of maintaining optimal nutrition. A landmark study of needle catheter jejunostomy by Myers et al. reported 2,022 needle catheter jejunostomies performed during laparotomy over a 16-year period. There were 34 complications (1.5 %), the most common of which was a minor complication—premature loss of catheter (N = 15, 0.74 %)—and the most devastating of which was bowel necrosis (N = 3, 0.15 %).20 Controversy exists whether or not standard tube jejunostomy is associated with more complications compared with needle catheter jejunostomy.21 Now with the availability of laparoscopic techniques of jejunostomy, routine use of laparoscopic feeding JT may be justified in the preoperative or neoadjuvant phase for esophageal cancer patients, when they are most at risk of becoming malnourished before undergoing definitive surgical therapy. We describe our technique and outcomes of laparoscopic JT placement.
Methods
Methods
Under an IRB protocol, we evaluated all of our esophageal cancer patients who have had a minimally invasive esophagectomy between August 2007 and April 2012. We identified 153 patients whose evaluation included an endoscopic ultrasound followed by a positron emission tomography and computerized tomography scans. We included all patients who were staged as T2–T4 or regional-node positive, received preoperative neoadjuvant chemoradiation, and had a laparoscopic feeding jejunostomy prior to their definitive esophageal resection.
Operative Description
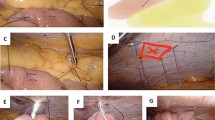
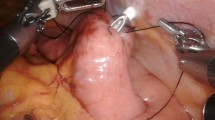
Under general anesthesia, the abdominal cavity is entered via a 5-mm left subcostal incision under direct visualization. Three additional trocars are placed with the use of a 5-mm 30° angle scope. Our technique is similar to the methods previously described.22 A jejunotomy was performed 40–50 cm distal to the ligament of Treitz (Fig. 1). A 16 Fr T-tube is cut so that the back wall is hemisected in order to prevent occlusion (Fig. 2). The T-tube is inserted through the 12-mm trocar and advanced into the jejunotomy (Fig. 3a, b). The T-tube is soft and pliable, especially when a portion of the back wall is removed. The enterotomy is widened with a Maryland dissector. The T-tube is placed into the small bowel utilizing the Maryland. The T-tube is secured with an absorbable purse-string suture (Fig. 4a, b). The distal end of the T-tube is brought out through a separate skin incision in the left upper quadrant. The seromuscular layer of the jejunum is fixed to the abdominal wall at four points surrounding the T-tube site using non-absorbable suture with the aid of the Carter–Thomason device; a single additional suture is placed proximal and distal to the T-tube site in order to prevent kinking or twisting of the small bowel mesentery (Fig. 5a, b). The T-tube is flushed with saline in order to assess for patency and for leak. The T-tube is brought out through the left abdominal wall in the standard location for a feeding jejunostomy since this does not interfere with a subsequent minimally invasive esophagectomy in our experience (Fig. 6). The tube is secured externally on the skin in a circular fashion using interrupted sutures.
Statistics were performed utilizing t test or chi-square when appropriate. Significance was defined as P < 0.05.
Results
Between August 2007 and April 2012, we identified 221 patients who had a minimally invasive esophagectomy. One hundred fifty-three patients who were staged as T2 to T4, or regional-node positive, scheduled to receive neoadjuvant chemoradiation therapy had a laparoscopic feeding jejunostomy tube placement prior to their definitive esophageal resection. Of them, 127 patients had adenocarcinoma of the distal esophagus, and 26 had squamous cell carcinoma. The demographics, underlying esophageal disease, cancer type, clinical stage, and pathological features are shown in Table 1.
All 153 patients had a successful laparoscopic feeding jejunostomy placed utilizing a 16 Fr T-tube as described in this article. The mean operative time was 65 min. No intraoperative complications, perforation, postoperative bowel necrosis, bowel torsion, herniation, intraperitoneal leak, or mortality. The postoperative complications are listed in Table 2. Four patients were noted to have superficial skin infection around the tube, and 11 patients required a tube exchange for dislodgment, clogging, and leaking around the tube, for an overall minor complication rate of 10 %. Although neoadjuvant treatment interruptions did occur, rates of completion of planned chemoradiotherapy were greater than 90 % in those patients undergoing a laparoscopic feeding jejunostomy placement. Tube complications or placement did not compromise future access for laparoscopic esophagectomy.
During this time period, an additional 25 patients underwent laparoscopic feeding jejunostomy placement for potentially resectable esophageal cancer. However, these patients did not undergo surgical resection due to either complications of chemoradiotherapy, progression of disease, or patient refusal of surgery. None of these patients were deemed unresectable due to complications of laparoscopic feeding jejunostomy placement. The mortality rate of the 153 patients undergoing feeding placement and subsequent esophageal resection was less than 1 %. The morbidity rates for the majority of these esophagectomy patients has been previously reported.25
In general, patients were admitted postoperatively for 1–2 days to obtain a nutritional consult and help set up appropriate nutritional intake via the feeding jejunostomy tube. Regardless of their dysphasia severity, they were all counseled and provided 40 to 60 g of daily protein intake with 1,500 to 1,800 kcal per 12 to 24 h. During the course of their neoadjuvant chemoradiation therapy, the patient’s weight, body mass index (BMI), and albumin improved from their initial presentation to the time prior to their esophageal resection while receiving supplemental nutrition via their laparoscopic feeding jejunostomy (approximately a 10-week course) as noted in Table 3.
Discussion
Feeding jejunostomy complication rates have a reported incidence of 1 to 20 % and often include bowel torsion, herniation, dislodgement, intraperitoneal leak, bowel necrosis, infection, and an associated mortality rate of 2 to 9.6 %.17–19 In fact, Date et al. performed a retrospective analysis of patients undergoing either palliative or adjunctive feeding jejunostomy at the time of major esophageal or gastric surgery and found that 9 of 42 patients (21.4 %) had procedure-related complications; 7 (16.7 %) of which were minor, and 2 (4.7 %) of which were major requiring emergency laparotomy.19 Fenton et al. published a series of 143 feeding JTs placed in 151 patients undergoing esophagectomy for esophageal carcinoma or high-grade dysplasia. Of the 143 JTs, 33 (23.1 %) were being used for nutritional support at the time of discharge, and further analysis showed that only BMI less than 18.5 was predictive of the need for JT on discharge. Postoperative JT-specific complications occurred in 26 patients (18.2 %) and included 3 cases (2.1 %) of ileus or small bowel obstruction, 5 cases (3.6 %) of JT dysfunction (i.e., irreversible clogging or technical error in suture securing the JT), and 18 cases (12.8 %) of skin or subcutaneous infection.14
Llaguna et al. reported a series of 73 patients who underwent placement of JT at the time of esophagectomy. There were 21 complications (28.8 %), 10 major—as defined by requiring a reoperation—and 11 minor. Two patients (2.7 %) required reoperation for small bowel obstruction, and 8 complications (11 %) were managed by an interventional radiologist. Of the minor complications, five (6.8 %) were managed by bedside procedure, and six (8.2 %) required no intervention. On discharge, 39 patients (54 %) required JT feeding.15 The authors described several technical considerations in potentially reducing JT-associated morbidity: (1) utilizing a more lateral entry site for the J-tube, perhaps making small bowel volvulus less likely due to the tube lying more laterally, rather than anteriorly; (2) fixing the bowel in at least three locations: the jejunostomy site, 2 cm proximal and 2 cm distal; (3) utilizing a smaller 12 Fr tube with a 3 to 5-mL retention balloon (as opposed to their previous 14 Fr tube with a 7 to 10-mL retention balloon), thus reducing the likelihood of bowel obstruction secondary to filling of the balloon, which is done to prevent dislodgement. Consequently, we have been using a 16 Fr T-tube, eliminating the need for filling of a retention balloon. Additionally, we cut along the horizontal limbs of the T-tube, to remove the back wall, in order to minimize the potential for clogging as well as allowing wire access if ever needed for outpatient tube exchange. Lastly, the longitudinal configuration of the T-tube within the small bowel further minimizes the potential for the bowel, or its mesentery, to volvulize around the fixed exit site of the feeding tube. An additional non-absorbable fixation suture is placed proximal and distal to the initial seromuscular sutures to the abdominal wall in order to prevent this from happening.
Han-Geurts et al. studied 1,387 patients who underwent esophagectomy, of which 1,166 patients underwent concurrent needle catheter feeding jejunostomy, and reported 13 (1.1 %) JT-associated complications requiring re-laparotomy for intraperitoneal leakage (5), dislodgement (4), herniation (3), and torsion (1). There were five mortalities (0.4 %) resulting from JT-associated complications.23,24 In our patient series, all feeding J-tubes were performed laparoscopically, completely reducing the incidence of herniation from a laparotomy. However, one of the limitations in attempting to derive from the literature expected complication rates of JTs is the variability in the outcome measures reported and the likelihood of underestimating and not capturing certain complications. The major complications of death, reoperation, and bowel ischemia are more clinically significant and relevant and, at the same time, less likely to be missed in retrospective reviews. The rate of major complications such as reoperation ranges from 1.1 to 2.7 %, and JT-associated mortality rate ranges from 0.4 to 2.4 %.14,15,17–19,23 Thus, we chose to report our outcomes in terms of these uniformly agreed-upon major complications in what is one of the largest series of laparoscopic feeding JTs in esophageal cancer patients. Our low morbidity and mortality suggest the safety and feasibility of routine use of feeding JT in esophageal cancer patients either in the preoperative phase for those undergoing neoadjuvant therapy or at the time of esophagectomy. In fact, most of our patients had neoadjuvant chemoradiation followed by minimally invasive esophagectomy, and we previously have reported a series of 105 consecutive patients who underwent minimally invasive esophagectomy with a mortality rate of 1 %.25 We have also demonstrated that there were no significant differences in postoperative morbidity between patients who received chemoradiation and those who did not.26 We believe that the establishment of preoperative JT and maintenance of optimal nutrition via supplemental enteral feeding were important factors in reducing morbidity and mortality of definitive surgical therapy. This is likely due to an improvement in the patient’s weight, BMI, and preoperative albumin when they are maintained on cycled or daily feeding as previously described during their chemoradiation treatment phase.
Markides et al. performed a systematic review that included five randomized controlled trials and one case–control study to evaluate the most effective means of supplemental nutrition in patients undergoing esophagectomy. These six studies compared different routes of nutritional support, and although none of these studies used identical methods, the systematic review suggested that if enteral feeding is to be used during post-esophagectomy, feeding jejunostomy should be performed over nasojejunal or nasoduodenal tubes due to lower dislodgement rates.27 Additionally, Mistry et al. in India described a similar technique to ours, indicating that this method of placing a feeding jejunostomy is cost-effective since it does not require special equipment or a specially designed or manufactured feeding tubes.28 In fact, the actual cost of a 16 Fr T-tube at our institution is US$9 versus a US$127 charge for a manufactured feeding tube requiring operative placement.
Conclusion
In conclusion, there has been much debate regarding the risks and/or benefits of routine use of feeding JTs in esophageal cancer patients. Part of the controversy stems from the variability in reported outcomes, and the safety of JTs should be assessed in terms of the major complications of reoperation, bowel ischemia, or death—especially given the feasibility of salvage techniques for minor complications such as dislodged or obstructed JTs. Although the literature has demonstrated the benefits of maintaining optimal nutrition in patients undergoing major gastrointestinal surgery, routine use of feeding JTs has not been universally adopted due to the risk of devastating complications and/or death, albeit low. We reported our technique of laparoscopic JT and its associated outcomes in one of the largest series of laparoscopic JTs in esophageal cancer patients. Devastating morbidity and mortality have not occurred, and we would argue that given the known benefits of maintaining optimal nutrition in patients undergoing esophagectomy, routine use of laparoscopic feeding JT should be strongly considered in this patient population.
References
Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 1999;230:392–403.
Kim T, Hochwald SN, Sarosi GA, Caban AM, Rossidis G, Ben-David K. Review of minimally invasive esophagectomy and current controversies. Gastroenterol Res Pract. 2012; 2012:683213.
Davies AR, Forshaw MJ, Khan AA, Noorani AS, Patel VM, Strauss DC, Mason RC. Transhiatal esophagectomy in a high volume institution. World J Surg Oncol 2008;6:88.
Kight CE. Nutrition considerations in esophagectomy patients. Nutr Clin Pract 2008;23:521–8.
Ryan AM, Rowley SP, Healy LA, Flood PM, Ravi N, Reynolds JV. Post-oesophagectomy early enteral nutrition via a needle catheter jejunostomy: 8-year experience at a specialist unit. Clin Nutr 2006;25:386–93.
Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, Daley J, Henderson WG, Krasnicka B, Khuri SF. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217–22.
Kudsk KA, Tolley EA, DeWitt RC, Janu PG, Blackwell AP, Yeary S, King BK. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enteral Nutr 2003;27:1–9.
Kim T, Grobmyer SR, Smith RD, Ben-David K, Ang DN, Vogel SB, Hochwald SN. Esophageal cancer – the five year survivors. J Surg Oncol 2010;103:179–83.
Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002;122:1763–70.
Alverdy J, Chi HS, Sheldon G. The effect of parenteral nutrition on gastrointestinal immunity: the importance of enteral stimulation. Ann Surg 1985;202:681–4.
Langkamp-Henken B, Glezer JA, Kudsk KA. Immunologic structure and function of the gastrointestinal tract. Nutr Clin Pract 1992;7:100–8.
Mazaki T, Ebisawa K. Enteral versus parenteral nutrition after gastrointestinal surgery: a systematic review and meta-analysis of randomized controlled trials in the English literature. J Gastrointest Surg 2008;12:739–55.
Tapia J, Murguia R, Garcia G, de los Monteros PE, Oñate E. Jejunostomy: techniques, indications, and complications. World J Surg 1999;23:596–602.
Fenton JR, Bergeron EJ, Coello M, Welsh RJ, Chmielewski GW. Feeding jejunostomy tubes placed during esophagectomy: are they necessary? Ann Thorac Surg 2011;92:504–12.
Llaguna OH, Kim HJ, Deal AM, Calvo BF, Stitzenberg KB, Meyers MO. Utilization and morbidity associated with placement of a feeding jejunostomy at the time of gastroesophageal resection. J Gastrointest Surg 2011;15:1663–9.
Gerndt SJ, Orringer MB. Tube jejunostomy as an adjunct to esophagectomy. Surgery 1994;115:164–9.
Brock MV, Venbrux AC, Heitmiller RF. Percutaneous replacement jejunostomy after esophagogastrectomy. J Gastrointest Surg 2000;4:407–10.
Finley FJ, Lamy A, Clifton J, Evans KG, Fradet G, Nelems B. Gastrointestinal function following esophagectomy for malignancy. Am J Surg 1995;169:471–5.
Date RS, Clements WD, Gilliland R. Feeding jejunostomy: is there enough evidence to justify its routine use? Dig Surg 2004;21:142–5.
Myers JG, Page CP, Stewart RM, Schwesinger WH, Sirinek KR, Aust JB. Complications of needle catheter jejunostomy in 2,022 consecutive applications. Am J Surg 1995;170:547–51.
Haun JL, Thompson JS. Comparison of needle catheter versus standard tube jejunostomy. Am Surg 1985;51:466–9.
Allen JW, Ali A, Wo J, Bumpous JM, Cacchione RN. Totally laparoscopic feeding jejunostomy. Surg Endosc 2002;16:1802–5.
Han-Geurts IJ, Verhoef C, Tilanus HW. Relaparotomy following complications of feeding jejunostomy in esophageal surgery. Dig Surg 2004;21:192–6.
Han-Geurts IJ, Hop WC, Verhoef C, Tran KT, Tilanus HW. Randomized clinical trial comparing feeding jejunostomy with nasoduodenal tube placement in patients undergoing oesophagectomy. Br J Surg 2007;94:31–5.
Ben-David K, Sarosi GA, Cendan JC, Howard D, Rossidis G, Hochwald SN. Decreasing morbidity and mortality in 100 consecutive minimally invasive esophagectomies. Surg Endosc 2012;26:162–7.
Ben-David K, Rossidis G, Zlotecki RA, Grobmyer SR, Cendan JC, Sarosi GA, Hochwald SN. Minimally invasive esophagectomy is safe and effective following neoadjuvant chemoradiation therapy. Ann Surg Oncol 2011;18:3324–9.
Markides GA, Al-Khaffaf B, Vicker J. Nutritional access routes following oesophagectomy – a systematic review. Eur J Clin Nutr 2011;65:565–73.
Mistry RC, Mehta SS, Karimundackal G, Pramesh CS. Novel cost-effective method of laparoscopic feeding-jejunostomy. J Minim Access Surg 2009;5:43–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben-David, K., Kim, T., Caban, A.M. et al. Pre-therapy Laparoscopic Feeding Jejunostomy is Safe and Effective in Patients Undergoing Minimally Invasive Esophagectomy for Cancer. J Gastrointest Surg 17, 1352–1358 (2013). https://doi.org/10.1007/s11605-013-2231-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-013-2231-4