Abstract
Background
Most data on large studies of superior mesenteric artery syndrome (SMAS) were published over 30 years ago. New studies are needed so that current medical progress can influence SMAS diagnosis and improve therapeutic outcomes.
Methods
This study was conducted to report the clinical features and outcomes of SMAS. From January 2000 to December 2009, 80 cases (53 females, median age 28 years) of SMAS were collected retrospectively from seven university hospitals in South Korea.
Results
The median body mass index at diagnosis was 17.4 kg/m2, with a range of 10–22.1. Forty (50 %) of the 80 SMAS patients had co-morbid conditions including mental and behavioral disorders, infectious disorders, and disorders of the nervous system (21.3, 12.5, and 11.3 %, respectively). Computerized tomography was most commonly (93.8 %) used to diagnose SMAS. The overall medical management success and recurrence rates were 71.3 and 15.8 %, respectively. Surgical management had a high 92.9 % (13/14) success rate. The most common surgical procedure for SMAS was laparoscopic duodenojejunostomy.
Conclusions
This is the largest case series to document the clinical features and changes in diagnostic modalities, medical and surgical managements, and their outcomes in SMAS patients. Laparoscopic duodenojejunostomy is the preferred surgical procedure when medical management of the disease fails.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superior mesenteric artery syndrome (SMAS) is an uncommon disease resulting from the compression of the third portion of the duodenum by the superior mesenteric artery. In 1861, Rokitansky1 was the first to observe that superior mesenteric vessels may compress and obstruct the duodenum over the lumbar spine. Subsequently, in 1927, Wilkie2 published the first comprehensive series of 75 patients. Therefore, SMAS is also known as Wilkie's syndrome, arteriomesenteric duodenal compression, chronic duodenal ileus, or cast syndrome.3 The typical symptoms of SMAS are anorexia, nausea, vomiting, early satiety, abdominal pain, and postprandial fullness. Diagnosis requires radiologic studies in patients with symptoms suggestive of SMAS.
Upper gastrointestinal (GI) barium studies combined with simultaneous angiography have been established as definitive diagnostic tools for SMAS. Upper GI barium studies show a dilated proximal duodenum with an abrupt termination of the barium column in the third portion. Angiography has been suggested as the “gold standard” procedure for the assessment of the aortomesenteric angle and distance.4 The SMA angle to the aorta is normally 45° (range, 38–56°), whereas in SMAS the SMA angle is decreased to 6–25°. Accordingly, the distance between the SMA and the aorta normally ranges from 10 to 20 mm, whereas in SMAS this distance is decreased to 2–8 mm.5 SMAS therapy includes weight gain to increase the aortomesenteric angle, but surgery is indicated in symptomatic patients when conservative management approaches fail.6
A PubMed search (www.ncbi.nlm.nih.gov/PubMed; 1950 to December 2011) using a combination of the MeSH term including “superior mesenteric artery syndrome” and additional words (Wilkie's syndrome, aortomesenteric compression, arteriomesenteric duodenal compression, or duodenal vascular compression) yields more than 500 articles.
However, most studies investigating SMAS include only a small sampling of patients, and the largest studies date back to 1960–1980.3 Given the advances in diagnosis and nutritional and surgical management, an updated study of SMAS in a large patient population is needed. Thus, we analyzed the clinical characteristics, diagnostic tools, and outcomes of SMAS in South Korea during the previous decade.
Methods
Patient Population
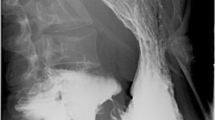
This study was approved by the Institutional Review Board of all participating university hospital centers (IRB #2010-110). SMAS was defined using the following criteria: (1) gastrointestinal symptoms of anorexia, nausea, vomiting, early satiety, abdominal pain, or postprandial fullness, (2) radiologic findings suggestive of extrinsic compression of the third portion of the duodenum between the SMA and the aorta, a dilated duodenum, an aortomesenteric distance of ≤8 mm, or an aortomesenteric angle of ≤25°. The aortomesenteric distance was defined as the minimum distance at the level where the duodenum passes between the SMA and aorta. The aortomesenteric angle was measured as the angle between the aorta and SMA on sagittal images. These parameters are shown in Fig. 1 in an SMAS patient. Eighty-three cases of SMAS were collected retrospectively from university hospital centers over a 10-year period beginning on January 1, 1999. Three cases were excluded due to the absence of any gastrointestinal symptoms. Thus, we analyzed a total of 80 cases of SMAS.
Data Collection
The following information was collected from the complete review of medical records:
-
1.
Demographics: sex, age at time of initial diagnosis, alcohol drinking (non-imbibers/social drinkers/heavy drinkers), and smoking status (non-smoker/past smoker/current smoker).
-
2.
Date of symptom onset, initial diagnosis, and last follow-up (date of death if deceased): early diagnosis was defined as less than 30 days from symptom onset to initial diagnosis, while late diagnosis was considered as undiagnosed or more than 30 days from symptom onset to initial diagnosis. Body mass index (BMI) at SMAS diagnosis: BMI was classified as underweight (< 18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (≥ 23 kg/m2), or obese (≥ 25 kg/m2) according to Asian-Pacific criteria.7
-
3.
Symptoms, including anorexia, nausea, vomiting, early satiety, abdominal pain, and postprandial fullness.
-
4.
Radiologic methods used in the diagnosis: upper GI barium study, abdominal ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI).
-
5.
Upper endoscopy: the presence of esophagitis or peptic ulcer.
-
6.
Co-morbidity: We used two definitions of co-morbidity in the patient population: (1) to indicate simultaneously existing medical conditions independent of another condition in a SMAS patient and (2) to indicate a medical condition in a patient that causes or is otherwise related to another condition in the same SMAS patient. These co-morbid conditions had been diagnosed by specialists in all related departments, including psychiatry, neurosurgery, and neurology. We classified diseases and other health problems in medical records according to the International Statistical Classification of Diseases (ICD)-10, version 2010.8
-
7.
Medical therapy: supportive care such as total parenteral nutrition, medication, or tube decompression.
-
8.
Surgical therapy: open duodenojejunostomy, section of the ligament of Treitz (Strong's operation), open gastrojejunostomy, laparoscopic duodenojejunostomy, and laparoscopic gastrojejunostomy.
-
9.
Outcome: Success was defined as both improvement of gastrointestinal symptoms and weight gain.
-
10.
Recurrence: Recurrence was defined as a return of gastrointestinal symptoms accompanying a radiologic finding suggestive of SMAS.
-
11.
Cause of death: primary cause of death.
Statistical Analysis
The patient's clinical data were summarized as a percentage or median (with range), according to the characteristics of each variable. Subgroup analyses of the above-mentioned variables were performed according to gender, age, and lag time (time interval from symptom onset to initial diagnosis). Significant differences in the subgroup analyses were determined by using Pearson's chi-square test, Fisher's exact test, and Mann–Whitney U test as appropriate. Data were analyzed using a commercially available statistical software package (SPSS version 12.0, SPSS Inc. Chicago, IL, USA). A p value of less than 0.05 was considered to be statistically significant.
Results
Clinical Characteristics
There were 80 SMAS patients between January 2000 and December 2009; 53 (66.3 %) of these were female. The median age at diagnosis was 28 years, with a range of 11–92 years. Fifteen of the patients were teenagers (Table 1).
Seventy-two patients (90 %) were non-drinkers, while seven (8.8 %) were social drinkers and one was a heavy drinker (1.3 %). Seventy patients (87.5 %) were non-smokers, and three (3.8 %) and seven (8.8 %) were past and heavy smokers, respectively.
The median time interval from symptom onset to initial diagnosis was 30 days, with a range of 0–900 days. Thirty-four patients (42.5 %) were diagnosed early. The median follow-up period was 5 months, with a range of 1–84 months. The median BMI at diagnosis was 17.4 kg/m2 with a range of 10–22.1. Sixty-one patients (76.3 %) were underweight and 19 (23.7 %) had a normal BMI.
Vomiting, nausea, and abdominal pain were the most common symptoms (70, 66.3, and 65 %, respectively). Other major contributing symptoms were anorexia (33.8 %), postprandial fullness (33.8 %), and early satiety (12.5 %). On a one to six scale, the number of patients with each symptom was: one (17.5 %), two (22.5 %), three (40 %), four (10 %), five (5 %), and six (5 %).
CT was the most common diagnostic modality (93.8 %) for SMAS, followed by upper barium studies (53.8 %), ultrasonography (27.5 %), and MRI (5 %). When CT was used as gold standard, the false negative rate of the upper GI series was 18.6 % in eight of 43 patients. Upper endoscopy was performed in 51 patients (63.8 %) at diagnosis. A pulsatile compressive lesion in the third portion of the duodenum was noted in all patients who underwent upper endoscopy. Esophagitis or peptic ulcers were found in 21 (41.2 %) of these 51 patients.
The median aortomesenteric angle and distance were 10.5° (range 7.2–19.7°) and 0.5 cm (range 0.3–0.8 cm).
Half of the SMAS patients were free of co-morbid conditions. Mental and behavioral disorders, infectious diseases, and diseases of the nervous system were the most common co-morbid conditions among patients with SMAS (21.3, 12.5, and 11.3 %, respectively; Fig. 2). Details of the co-morbid conditions are shown in Table 2. SMAS patients with one to three co-morbid conditions were one (31.3 %), two (12.5 %), and three (7.5 %), respectively.
Treatment and Outcomes
Figure 3 shows the management and outcomes for the 80 SMAS patients. All seven patients (8.8 %) with outpatient care had positive responses and no recurrence, with a median follow-up period (range) of 2 months (1–6 months). Seventy-three patients (91.2 %) were admitted for inpatient care at diagnosis. The median number of admissions was one (range one to 12) during the median follow-up period of 5 months (range 1–84 months).
Vomiting was the only significant difference in clinical features presented by patients with inpatient care relative to outpatients. There was a significant difference in the incidence of vomiting between patients with outpatient and inpatient care [two (28.6 %) vs. 54 (74 %), respectively].
Inpatient care began with conservative management. Total parenteral nutrition was indicated in 51 patients (69.9 %). Sixty-four patients (87.7 %) were managed medically at initial admission, with success being observed in 50 (78.1 %). There were recurrences in nine of the inpatients (18 %). Six of the nine patients (66.7 %) with recurrences had success with medical re-treatment.
Surgery was performed on 15 SMAS patients (18.8 %). Of these, one had a colectomy for ischemic colitis that developed during hospitalization a month after medical treatment of SMAS. The remaining 14 of 80 patients (17.5 %) underwent surgery for the relief of SMAS. The most common surgical procedure for SMAS was laparoscopic duodenojejunostomy (eight), followed by open duodenojejunostomy (four), and open gastrojejunostomy (two). The success rate of surgery was 92.9 % (13/14). However, a patient with an open gastrojejunostomy died of a surgical complication (peritonitis due to an anastomotic leak).
Five patients (6.3 %) died: two from muscular dystrophy, one from ischemic colitis, one from tracheostomy bleeding, and one due to a surgical complication.
In summary, a higher overall success rate of 92.9 % in 13 of 14 patients was observed in patients who underwent surgical management, compared with 71.3 % in 57 of 80 patients who underwent medical management. The recurrence rate (0 %) of surgical management during the median follow-up period of 12 months was also lower compared with that (15.8 %) of medical management.
Subgroup Analyses
Table 3 shows the subgroup analyses of clinical characteristics, treatment modalities, and outcomes according to gender, age, and lag time. There were no significant differences between male and female patients with SMA in age, lag time, BMI, type of care, treatment modality, and success. No significant differences with respect to these results were observed between pediatric and adult patients with SMAS. In the comparison of the early and late diagnosis subgroups, female patients were more common in the early diagnosis subgroup, compared with the late diagnosis subgroup (52.9 vs. 76.1 %, p = 0.030). A higher surgery rate was also found in the early diagnosis subgroup compared with the late diagnosis subgroup (5.9 vs. 28.3 %, p = 0.011).
Discussion
Clinical Characteristics
SMAS is a rare disease, and the vast majority of available literature describes case reports or a small series of patients. In the present study, we describe 80 patients affected by SMAS that were diagnosed in South Korean tertiary hospitals over the last decade. Our data suggest that females are affected more frequently by SMAS than males and in our study; two-thirds of the patients were between 10 and 39 years of age. These findings agree with earlier studies.2,4,9,10 The duodenum is surrounded by a mesenteric fat pad and lymphatic tissue as it crosses the aortomesenteric interval. This serves as a cushion, allowing for sufficient space and preventing extrinsic compression of the small bowel caused by the SMA. Low BMI or weight loss is a well-known risk factor for SMAS.3
Interestingly, our study showed that 19 of 80 SMAS patients (23.7 %) had normal BMIs. The reason for the development of SMAS in patients with normal BMIs is currently not known. An earlier study10 reported a mean BMI of 21.3 kg/m2 and no weight loss was recorded in 50 % of the 22 pediatric SMAS cases. These results suggest that low BMI or enduring weight loss is not a requirement for the development of SMAS. A growth spurt might be related with a change in the SMA configuration without weight loss. BMI as a measure of obesity in children and adolescents has limited accuracy, and interpretation of data that rely solely on BMI across different age groups should be done cautiously. However, in our study, there was no significant difference in BMI between pediatric and adult SMAS patients.
Laffont et al.11 reported the occurrence of SMAS in paraplegic patients 3 months after injury. They hypothesized that an imbalance in the autonomic nervous system results in unchecked parasympathetic activity in the acute setting and may lead to the development of SMAS. Furthermore, prior neurological injury may induce the hyperextension of the spine with increased lumbar lordosis, prolonged supine positioning, or increased flaccidity of the abdominal wall musculature, which may predispose patients to developing SMAS.10 SMAS is commonly described following corrective spinal surgery for scoliosis.3 This procedure lengthens the spine cranially, displacing the SMA origin, which decreases the mesenteric artery's lateral mobility and reduces the acuteness of the aortomesenteric angle. Anatomical conditions such as a short ligament of Treitz,3 low origin of the SMA,12 or esophagectomy13 may bias patients towards SMAS.
A case report of identical twins 14 with SMAS and another case in utero15 suggest that genetics may predispose some patients towards SMAS. Therefore, neurological injury, anatomical variation, or genetic susceptibility may contribute to this condition. Larger case studies are needed for the identification of SMAS in normal-BMI individuals.
Our study showed that SMAS was diagnosed late in 46 patients (57.5 %). The clinical manifestations were similar to functional gastrointestinal disorders, especially in chronic SMAS. The result suggests that SMAS may be misdiagnosed as functional gastrointestinal disorders. Delayed diagnosis of SMAS can result in malnutrition, electrolyte imbalance, dehydration, and even death. Therefore, patients with gastrointestinal symptoms who are underweight should be thoroughly evaluated for SMAS, despite the fact that SMAS is rare. Diagnosis depends on a high index of suspicion since symptoms can be non-specific.
In our study, most patients presented with a constellation of symptoms, typically including vomiting, nausea and abdominal pain. Vomiting was likely to cause SMAS patients to seek inpatient care because it might induce dehydration and electrolyte imbalance, which require hospitalization.
Diagnosis
The current study demonstrated that contemporary medical progress influences diagnosis. The traditional method of diagnosis of SMAS was an upper barium study.16 In contrast, an SMAS diagnosis was most commonly confirmed by CT in our study. CT measurements are very similar to conventional and CT angiographic measurements and have a high diagnostic rate.17 CT is advantageous over upper gastrointestinal barium studies because there is relatively little patient discomfort. Its relative non-invasiveness is advantageous over conventional angiography. The change in diagnostic modality appears to be linked closely to the expanded availability, technical advances, and non-invasiveness of CT.
When CT was used as gold standard, the false negative rate of the upper GI series was 18.6 % (eight of 43 patients). Duodenal peristalsis was suppressed using an antiperistaltic agent (e.g., Buscopan) in hypotonic duodenography. Hypotonic duodenography was not used in all patients with a false negative result. However, the use of antiperistaltic agents has been reported to improve the diagnostic accuracy of SMAS.4
Co-morbid Conditions
Our data indicated that the most common diseases that were co-morbid with SMAS were mental and behavioral disorders, including major depression, mental retardation, and anorexia nervosa. Frequent associations of SMAS with psychiatric disorders, such as anorexia nervosa, have been reported.18–21 Whether mental and behavioral disorders predispose patients towards developing SMAS is unclear. These conditions can cause malnutrition and weight loss, which can lead to loss of aortomesenteric fat.
Interestingly, certain infectious diseases, such as tuberculosis or acute gastroenteritis, were common co-morbidities among SMAS patients. Tuberculosis is endemic in South Korea and also is a chronic wasting disease; thus, the fact that it was co-morbid with SMAS was not unexpected. However, it is not clear whether acute gastroenteritis is related to SMAS because there are few reports of acute gastroenteritis being associated with this condition.22,23 Acute weight loss was identified in our SMAS patients with acute gastroenteritis. However, the outcomes of these patients were more favorable than either the other SMAS patients in the present study or those from previous studies. These patients responded 100 % of the time to medical treatment and had a recurrence rate of 0 %. These findings suggest that SMAS might be found incidentally during their investigations rather than as a result of acute gastroenteritis. Thus, the association of weight loss secondary to acute gastroenteritis with SMAS cannot be ruled out. Diseases of the nervous system, including epilepsy, muscular dystrophy, dystonia, paraplegia, Parkinson's disease, MELAS syndrome, and cerebral palsy, were often co-morbid with SMAS. Paraplegia and cerebral palsy have been reported to be predisposing conditions for the development of SMAS.10,11 Diseases of the nervous system might affect food intake, leading to malnutrition, and thus be predisposing factors. The cross-sectional design used in this study does not allow for the establishment of causal relationships between co-morbid diseases and SMAS.
Medical and Surgical Managements
If symptoms of SMAS were mild, small-volume meals with oral supplements were used to increase caloric intake and correct weight loss. In those patients with significant symptoms, a medical approach was pursued with either enteral or parenteral hyperalimentation, postural change, and nasogastric decompression. Some drugs, including antacids, H2 receptor blockers, proton pump inhibitors, prokinetics, and anti-depressants, were used depending on the symptoms and co-morbidity conditions present.
The overall success rate of medical management in our study was 71.3 % (57 of 80 patients) during the median follow-up period of 5 months (range 1–84 months), while the overall recurrence rate was 15.8 % (nine of 57 patients). In comparison to two similar pediatric cohorts published in 1974 and in 2006, the need for surgical treatment decreased from 70 to 14 %.10,24 Recent advances in nutritional treatments have led to a substantial shift towards medical management rather than surgery. Interestingly, compared to pediatric cohorts, the success rate of medical treatment in our cohort of mostly adult patients was lower. In adult chronic patients, conservative treatment is often a prolonged in-hospital therapy with a low success rate .6 Most of SMAS patients following scoliosis surgery recovered using conservative therapy.25 Different populations or chronicity between studies might account for the differences observed.
Surgical interventions include gastrojejunostomy, duodenojejunostomy, and section of the ligament of Treitz (Strong's operation).26 Gastrojejunostomy provides adequate gastric decompression but may fail to completely release duodenal obstruction, leading to the persistence of symptoms that can necessitate duodenojejunostomy in some cases.26 Strong's procedure has the advantage of maintaining bowel integrity but has a failure rate of 25 %, presumably due to the short branches of the inferior pancreaticoduodenal artery not permitting the duodenum to fall inferiorly.26 Duodenojejunostomy is a simple procedure with a low risk of post-operative adhesions and a high success rate (~80–100 %).
Most surgeons prefer duodenojejunostomy and consider it superior to other options. Recent advances in laparoscopic surgery have led to reports of laparoscopic duodenojejunostomy.27 It reduces post-operative pain, shortens the duration of hospital stay, and limits the risk of incisional hernia.28,29 In our cases, laparoscopic duodenojejunostomy was performed in eight patients (57.1 %) for the relief of SMAS, with a success rate of 100 %. Open duodenojejunostomy was performed in four patients with a success rate of 100 %, and open gastrojejunostomy was performed in two patients with a success rate of 50 %. Given these results, laparoscopic duodenojejunostomy is the first-choice surgical option.
The Limitations of our Study
Our study has some important limitations. First, it was based on a retrospective medical chart review of patients in a tertiary center. In addition, the role of referral bias cannot be negated completely. Also, the patients with SMAS seen at academic centers may have more severe symptoms than those of SMAS in the general population. This study did not assess whether there is a positive correlation between symptoms and the aortomesenteric angle/distance. Thus, a further prospective study should be performed to evaluate the relationship between symptoms and the SMA/aorta anatomy. Lastly, the short follow-up period of our study is another limitation. However, we believe that the strength of our study is the large SMAS patient population since it is a relatively rare disease. Additionally, our study provides much-needed recent information about the clinical presentation and treatment of SMAS. Future long-term follow-up is needed to substantiate our findings.
Conclusions
In summary, these data describe the largest case study describing the clinical features, treatments, and outcomes of SMAS. SMAS occurred more frequently in female patients between 10 and 39 years of age. SMAS was observed in patients with a normal BMI, although low BMI appeared to be a predisposing factor for its development. In our study, surgical management is superior to medical management with respect to success and recurrence. However, these findings do not mean that surgery can be indicated in symptomatic patients first, given the potential complications and mortality of surgery. Surgery should be indicated in symptomatic patients when medical management fails. There is no clear time limit for medical management. However, in our study, the median duration of treatment of 9 days (range 2–62 days) was observed in patients that were successful after medical management. Further work should be addressed to provide the time limit for medical management.
References
yon Rokitanski C. Lehrbuch der Pathologischen Anatomie. Braumttller & Seidel, Vienna, 1861, p 187.
Wilkie DPD. Chronic duodenal ileus. Am J Med Sci. 1927;173:643–9.
Welsch T, Buchler MW, Kienle P. Recalling superior mesenteric artery syndrome. Dig Surg. 2007;24:149–56.
Gustafsson L, Falk A, Lukes PJ, Gamklou R. Diagnosis and treatment of superior mesenteric artery syndrome. Br J Surg. 1984;71:499–501.
Konen E, Amitai M, Apter S, Garniek A, Gayer G, Nass S, Itzchak Y. CT angiography of superior mesenteric artery syndrome. AJR Am J Roentgenol. 1998;171:1279–81.
Merrett ND, Wilson RB, Cosman P, Biankin AV. Superior mesenteric artery syndrome: diagnosis and treatment strategies. J Gastrointest Surg. 2009;13:287–92.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63.
http://apps.who.int/classifications/icd10/browse/2010/en. Accessed March 28, 2012.
Ylinen P, Kinnunen J, Hockerstedt K. Superior mesenteric artery syndrome. A follow-up study of 16 operated patients. J Clin Gastroenterol. 1989;11:386–91.
Biank V, Werlin S. Superior mesenteric artery syndrome in children: a 20-year experience. J Pediatr Gastroenterol Nutr. 2006;42:522–5.
Laffont I, Bensmail D, Rech C, Prigent G, Loubert G, Dizien O. Late superior mesenteric artery syndrome in paraplegia: case report and review. Spinal Cord. 2002;40:88–91.
Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol. 2002;37:640–3.
Cho KR, Jo WM. Superior mesenteric artery syndrome after esophagectomy with cervical esophagogastrostomy. Ann Thorac Surg. 2006;82:e37-8.
Iwaoka Y, Yamada M, Takehira Y, Hanajima K, Nakamura T, Murohisa G, Hirai R, Kitagawa M. Superior mesenteric artery syndrome in identical twin brothers. Intern Med. 2001;40:713–5.
Caspi B, Deutsch H, Grunshpan M, Flidel O, Hagay Z, Appelman Z. Prenatal manifestation of superior mesenteric artery syndrome. Prenat Diagn. 2003;23:932–4.
Goin LS, Wilk SP. Intermittent arteriomesenteric occlusion of the duodenum. Radiology. 1956;67:729–37.
Unal B, Aktas A, Kemal G, Bilgili Y, Guliter S, Daphan C, Aydinuraz K. Superior mesenteric artery syndrome: CT and ultrasonography findings. Diagn Interv Radiol. 2005;11:90–5.
Froese AP, Szmuilowicz J, Bailey JD. The superior-mesenteric-artery syndrome: cause or complication of anorexia nervosa? Can Psychiatr Assoc J. 1978;23:325–7.
Lee CW, Park MI, Park SJ, Moon W, Kim HH, Kim BJ, Shim IK, Park SS. [A case of superior mesenteric artery syndrome caused by anorexia nervosa]. Korean J Gastroenterol. 2011;58:280–3.
Jordaan GP, Muller A, Greeff M, Stein DJ. Eating disorder and superior mesenteric artery syndrome. J Am Acad Child Adolesc Psychiatry. 2000;39:1211.
Elbadaway MH. Chronic superior mesenteric artery syndrome in anorexia nervosa. Br J Psychiatry. 1992;160:552–4.
Okugawa Y, Inoue M, Uchida K, Kawamoto A, Koike Y, Yasuda H, Otake K, Miki C, Kusunoki M. Superior mesenteric artery syndrome in an infant: case report and literature review. J Pediatr Surg. 2007;42:E5-8.
Lee S-B, Kang H-C, Yoon Y-J. A case report of superior mesenteric artery syndrome after acute gastroenteritis. Korean J Fam Med. 2010;31:862–6.
Burrington JD. Superior mesenteric artery syndrome in children. Am J Dis Child. 1976;130:1367–70.
Altiok H, Lubicky JP, DeWald CJ, Herman JE. The superior mesenteric artery syndrome in patients with spinal deformity. Spine (Phila Pa 1976). 2005;30:2164–70.
Lee CS, Mangla JC. Superior mesenteric artery compression syndrome. Am J Gastroenterol. 1978;70:141–50.
Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181:377–8.
Morris TC, Devitt PG, Thompson SK. Laparoscopic duodenojejunostomy for superior mesenteric artery syndrome—how I do it. J Gastrointest Surg. 2009;13:1870–3.
Kim IY, Cho NC, Kim DS, Rhoe BS. Laparoscopic duodenojejunostomy for management of superior mesenteric artery syndrome: two cases report and a review of the literature. Yonsei Med J. 2003;44:526–9.
Acknowledgments
This study was supported by a grant of the Korean Society of Neurogastroenterology and Motility.
Conflict of Interest
No conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, T.H., Lee, J.S., Jo, Y. et al. Superior Mesenteric Artery Syndrome: Where Do We Stand Today?. J Gastrointest Surg 16, 2203–2211 (2012). https://doi.org/10.1007/s11605-012-2049-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-012-2049-5