Abstract
Background
The human cervical cancer oncogene HCCR-2 is overexpressed in various malignant tumors and cell lines, and might function as a negative regulator of the p53 tumor suppressor. Here, we used RNA interference strategies to evaluate the role of HCCR-2 in liver cancer, and to explore its potential therapeutic effect.
Methods
Changes of HepG2 cells stably transfected by an HCCR-2 RNA interference vector were detected by real-time PCR, MTT staining, plate colony formation, flow cytometry, and cell migration experiments. Apoptosis-related protein Bcl-2 and Bax levels were measured by Western blot.
Results
Our results showed that of the three siRNA-expressing vectors, siRNA-H3 had a suppressive effect on the expression of HCCR-2 mRNA, interfering with proliferation and migration of HCCR-2. Moreover, the apoptotic rate also increased, and cells transfected by siRNA-H3 were blocked in the G0/G1 stage. Plate colony formation experiments demonstrated that the single cell clone formation capacity of HepG2-H3 cells was clearly lower than that of HepG2 and HepG2-N cells. Western blot results indicated that the expression of Bcl-2 was inhibited, and the expression of Bax was increased.
Conclusions
In summary, RNAi targeting HCCR-2 could be an effective means for suppressing malignant features of hepatocellular carcinoma cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common neoplasm worldwide, with over half a million new cases per year,1 and the third most common cancer killer.2,3 Asia has a disproportionately large share of the world's HCC, mainly because of the endemicity of hepatitis B and C viruses, infections which can lead to liver cirrhosis and an increased risk of HCC.4 Although surgery (partial hepatectomy or total hepatectomy with orthotopic liver transplantation) can be curative for localized small liver tumors, therapeutic options for patients with advanced or metastatic HCC are limited, and survival in surgically incurable HCC patients has not increased significantly over the past 30 years.5 Many chemotherapeutic agents have been tested in HCC, with reported response rates ranging between 10% and 15%, but no survival advantage has been demonstrated. Most of the patients with advanced cancer have poor prognoses due to high rates of recurrence and metastasis. Invasion and metastasis are fundamental properties of HCC cells. Therefore, to improve the overall long-term survival of patients with HCC, more active treatment of recurrence and metastases is necessary.
Adhesion to an appropriate extracellular matrix is important for normal cells to survive, and detachment from such supportive matrices usually triggers a specific type of apoptosis termed anoikis.6 Loss of sensitivity to anoikis has been shown to directly contribute to the ability of tumor cells to metastasize.7,8 In animal models, tumors that are resistant to anoikis show a higher incidence of metastases and increased cell survival in the blood circulation.9 Suppression of anoikis, therefore, is likely to be a prerequisite for tumor cells to successfully metastasize to distant sites.10 A recent study indicated that anoikis-resistant hepatoma cells acquired increased invasiveness, evading therapeutic agents and escaping from immune surveillance after anchorage removal. Acquisition of anoikis resistance may act as a selective pressure to develop more metastatic potential in the development of hepatomas.11 Therefore, restoring anoikis sensitivity could help limiting the uncontrolled spread of metastatic tumors.
The human cervical cancer oncogene (HCCR) is overexpressed in many human tumors, including liver cancers.12,13 The HCCR gene is classified into two species, HCCR-1 and HCCR-2, according to their molecular characteristics. HCCR-2 lacks exon 1 of HCCR-1, and is identical in sequence from nucleotides 129 to 2,118.14 Previous work suggests that cells expressing HCCR-1 or HCCR-2 were tumorigenic in nude mice. Their functional roles in tumorigenesis may be as negative regulators of the p53 tumor suppressor gene.15,16 The HCCR is elevated according to disease progression from liver cirrhosis to carcinoma, which is more frequently positive in patients with early, small hepatocellular carcinoma.17 HCCR2-transgenic mice have been shown to develop breast cancers and metastasis.14 The crucial role of HCCR-2 in the anoikis resistance of HCC cells is still unclear. We hypothesize that HCCR-2 could also be involved in anoikis-resistant HCC cell metastasis. To investigate that possibility, we studied the effects of HCCR-siRNA on anoikis-resistant HCC cells.
Methods
Construction and Transfection of a siRNA Expression Vector
We selected interference sequences targeting HCCR-2 using an online design tool according to the principle of RNAi design to generate three different HCCR-2 siRNAs: siRNA-H1, an interference sequence targeting the HCCR-2 mRNA coding sequence from 475 to 493 bp; siRNA-H2, an interference sequence targeting the HCCR-2 mRNA coding sequence from 611 to 629 bp; and siRNA-H3, an interference sequence targeting the HCCR-2 mRNA coding sequence from 854 to 872 bp. The oligonucleotides 5′-GATCCGACAGATCTGTGCACCAAGATCAAGACGTCTTGGTGCACAGATCTGTTTTTTTA-3′ and 3′-GCTGTCTAGACACGTGGTTCTAGTTCTGCAGAA CCACGTGTCTAGACAAAAAAATTCGA -5′ were used to generate siRNA-H1 oligonucleotides. 5′-GATCCGTAAGATGTGAGAAGCATGGTCAAGACGCCAT GCTTCTCACATCTTATTTTTTA-3′ and 3′-GCATTCTACACTCTTCGTACCAG TTCTGCGGTACGA AGAGTGTAGAATAAAAAAT TCGA-5′ used to generate siRNA-H2 oligonucleotides. 5′-GATCCGTTGTGCAGCAAGAGAGACATCAAGA CGTGTCTCTCTTGCTGCACAATTTTTTA-3′ and 3′-GCAACACGTCGTTC TC TCTGTAGTTCTGCACAGAGAGAACGACGTGTTAAAAAATTCGA-5′ used to generate siRNA-H3 oligonucleotides. A scrambled sequence was used as the control siRNA ACTACCGTTGTATAGGTGT. Each oligonucleotide pair (100 pmol) was annealed by incubation at 95°C for 5 min and cooled slowly, and was ligated separately into the pGenesil-1 vector (Genesil Biotechnology Co., Ltd. Wuhan, China) which had been digested with BamHI and HindIII. As a result, four vectors, siRNA-H1, siRNA-H2, siRNA-H3, and a negative control vector, were successfully generated and verified by sequencing. These vectors were transfected into the cell line HepG2 (ATCC, Rockville, MD, USA) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Real-time PCR
Total RNA was extracted from cell lines with Tripure (Invitrogen) according to the manufacturer's instructions. Following DNase I treatment using a DNA-free kit (Ambion), cDNA was generated from total RNA using the Perfect Real Time RT-PCR Kit (Takara, Tokyo, Japan). Real-time quantitative PCR experiments were done using the SYBR Green PCR Core kit (Applied Biosystems) according to the vendor's instructions, using an ABI 7900HT (Applied Biosystems) real-time PCR instrument. The following primer pairs were used to amplify HCCR-2 and actin: HCCR-2 forward, 5′-GGGAGATGGAG CATTTGAG-3′ and reverse, 5′-GCTTCCGGAAAG CATGATAG-3′; actin forward, 5′-GTTGCGTTACACCCTTTCTTGACA3′ and reverse, 5′-GCACGAAG GCTCATCATTCAAAA3′. Transcript expression levels were normalized using actin levels as an endogenous control. Cycle conditions were 95°C for 5 min followed by 40 cycles of 95°C for 30 s, 58°C for 30 s, and extension for 45 s at 72°C.
Proliferation Assay
MTT experiments were performed according to the manufacturer's protocol. Parental HepG2 cells, negative control cells, and HCCR-2 siRNA cells were seeded at densities of 1 × 104 cells per well in 96-well plates. Three wells of each group of cells were picked out and added to 20 μL MTT (5 mg/mL) every 24 h. DMSO 200 μl was then added to each well to dissolve the crystals after incubation for 4 h. Incubation went on at 37°C for 10 min. Absorbance was measured at 490 nm using a plate reader.
Soft Agar Colony Formation Assay
Tumor cells (2.0 × 102) were grown in triplicate on 10-cm2 dishes in a suspension of 0.6% low melting point agarose (Life Technologies) and DMEM supplemented with 10% FCS. After 2 weeks, the plates were photographed under a phase-contrast microscope and assayed for colony number and size. Clones containing at least 50 cells were counted.
Migration and Invasion Assays
Confluent cells grown in 10-cm2 dishes were wounded using a sterile pipette tip and grown in DMEM with 5% FCS. Eight hours after the wound was made, images of the cells capable of migrating across the scratch were taken with a Nikon Eclipse TE2000-U using Metaview™ software (Universal Imaging Corporation). Cell invasion assays were performed in Boyden chambers with 8-μm pore filter inserts for 24-well plates (BD Bioscience). After 12 h of incubation at 37°C, cells on the underside of the filter were fixed and stained with Giemsa and examined by light microscopy (×200 magnification). Three microscopic fields were counted for each well.
Suspension Culture and Anoikis Assay
Cells were prevented from adhering to the plastic dishes by culturing the cells in dishes coated with PolyHEMA (Sigma, St Louis, MO, USA) as described previously.18 Briefly, culture of transient- or stable-transfected cells was trypsinized and plated onto 6-well polyHEMA plates (made by applying 1.5 ml of 10 mg/ml solution of polyhydroxyethylmethacrylate in ethanol onto the plate, and drying in a tissue culture hood). After 24 h of growth in suspension, cells were harvested for apoptosis measurements using an annexin V–FITC detection kit (Sigma) as described.19
Western Blot Analysis
Proteins were prepared by homogenization of cells in lysis buffer (10 mmol/L Tris–HCl, pH 8.0; 140 mmol/L NaCl; 5 mmol/L EDTA; 0.25 g/L NaN3; 10 g/L Triton X-100; 10 g/L deoxycholate; 1 g/L SDS; 0.5 mmol/L PMSF; 1 g/L leupeptin; and 1 g/L protinin). Protein concentration was determined by the Coomassie brilliant blue method. Proteins were separated by SDS–PAGE, transferred to PVDF membranes (Millipore, Temecula, CA, USA), blocked with milk/BSA, then probed with the specific mouse antibodies against caspase-8 (Cell Signaling, USA), Bcl-2, Bax, p53, caspase-9, cleaved caspase-3, and β-actin (Santa Cruz, USA) at 1:500 dilution. After washing, the blots were incubated with secondary horseradish peroxidase-conjugated goat anti-mouse, goat anti-rat, and goat anti-rabbit antibodies; visualized using an enhanced chemiluminescence reagent (Pierce Biotechnology, Rockford, IL, USA); and subsequently exposed to autoradiographic film.
Statistical Analysis
Data are presented as the mean ± SD for the indicated number of experiments and evaluated by a t test. P values below 0.05 were regarded as statistically significant. All data were analyzed with SPSS 10.0 software.
Results
siRNA Inhibits the mRNA Expression of HCCR-2 in HepG2 Cells
The three siRNAs targeting the open reading frame of HCCR-2 mRNA were tested to inhibit the expression of HCCR-2 mRNA in HepG2 cells. Real-time quantitative PCR and the relative standard curve method were used to analyze the amounts of HCCR-2 mRNA isolated from parental cells, the negative control cells, and siRNA-H1, siRNA-H2, and siRNA-H3 cells. The generated linear regression equations describing the relative standard curves were y = −0.31x + 9.26 (R 2 = 0.989) for HCCR-2 mRNA and y = −0.29x + 7.53 (R 2 = 0.999) for β-actin mRNA. After normalization with these equations, the calculated values of HCCR-2 mRNA relative copies are shown in Fig. 1. These results suggested that siRNA-H1 and siRNA-H3 can attenuate HCCR-2 activity at the mRNA level in HepG2 cells. siRNA-H3 was found to be the most effective. Therefore, we selected siRNA-H3 for subsequent experiments.
RNAi of HCCR-2 Inhibits the Proliferation and Colony Formation Capacity of HepG2 Cells
Cell colony formation capacity of HepG2-si cells was clearly lower (29.6 ± 3.21) than that of parental HepG2 (129.3 ± 13.57) and negative control cells (120.3 ± 9.86) (Fig. 2a). To investigate the impact of siRNA on the proliferation of HepG2 cells, we performed MTT assays. As illustrated in Fig. 2b, negative control cells had similar growth rates compared with parental HepG2 cells, and both were clearly higher than HepG2 siRNA (HepG2-si) cells. These results demonstrated that HCCR-2 RNAi suppressed HepG2 cell proliferation in cell culture.
HCCR-2 RNAi Inhibits Cell Migration and Invasion of HepG2 Cells
Cell migration and matrigel invasion assays showed that HepG2-si cells migrated much more slowly than negative control cells and parental cells (Fig. 3a), suggesting that HCCR-2–siRNA can inhibit cell migration of HepG2 cells culture. The invasive potential of the HepG2-si cells, assessed by measuring the ability of cells to transverse a reconstituted basement membrane of matrigel, was significantly decreased (55.33 ± 3.05), compared with that of the parental (86 ± 4.58) and control cells (81.33 ± 7.37, P < 0.05) (Fig. 3b).
HCCR-2 RNAi effects on cell migration and invasion of HepG2 cells. a Representative photos of haptotactic migration assay with HCCR-2-siRNA, control, and parental HepG2 cells. b Matrigel chemoinvasion assay. The number of HCCR-2–siRNA cells that transversed the transwell membranes in matrigel chemoinvasion assays (P < 0.05). (×200)
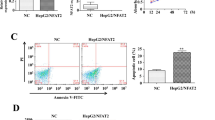
HCCR-2 RNAi Promote the Anoikis in HepG2 Cells
To confirm the effect of HCCR-2 RNAi on anoikis sensitivity and apoptosis frequency of HepG2-si cells, control and parental cells cultured in poly-HEMA-treated wells for 24, 48, and 72 h were quantified (Fig. 4a). Apoptosis frequency of HepG2-si cells was significantly lower (21.72% ± 2.23%) than in those for the control (1.56 ± 1.22) and parental cells (2.10% ± 1.58%, P < 0.05).
HCCR-2 RNAi effects on anoikis and regulation of the expression of associated proteins in resistant HCC cells. a HCCR-2 RNAi effects on the anoikis of HCC cells. The percentages of dead cells were calculated and compared. Error bars indicate means ± SD of three independent experiments with duplicate plates. b HCCR-2 RNAi regulation of the expression of procaspase-8, procaspase-9, and Bcl-2, and Bax in resistant HCC cells. A densitometric analysis of levels of associated proteins (relative to β-actin levels expressed as mean ± SD of three experiments) was used to show the difference among groups (*P < 0.05, versus HepG2 or HepG2/N)
HCCR-2 RNAi Upregulates the Expression of Procaspase-8, Procaspase-9, and Bcl-2, and Downregulates the Expression of Bax in Resistant HCC Cells
To investigate the mechanism by which HCCR-2 RNAi promote anoikis, we checked the mitochondrial pathway. As shown in Fig. 4b, HCCR-2 siRNA clearly increased the expression of procaspase-8, procaspase-9, and Bcl-2 (P < 0.05) and decreased the expression of Bax in HepG2 cells (P < 0.05). However, expression of procaspase-3 and p53 in HCCR-2 siRNA cells was not notably different compared with parental and negative control cells (P > 0.05) (Fig. 4b).
Discussion
The effect of downregulation of HCCR-2 on the invasion of HCC is still unclear. Our results indicate that silencing of the HCCR2 gene induces anoikis-like apoptosis and suppresses the proliferation and invasion of HCC cells in culture. Western blot results indicated that the expression of Bcl-2 was inhibited, and the expression of Bax was increased. Our results, along with others, suggest that RNAi targeting HCCR-2 could be an effective means for suppressing proliferation and invasion of HepG2 cells.
To successfully metastasize, cancer cells must acquire the ability to resist anoikis and survive after detachment from their matrix contacts.20 Anoikis, apoptotic cell death due to loss of cell adhesion, is critical for regulation of tissue homeostasis in tissue remodeling, development, fibrosis, and tumor metastasis.21 Resistance to anoikis is likely involved in the process of metastasis, specifically during the tumor cell migration through lymph or vascular channels. Here, we showed that decreased HCCR-2 was associated with an increase of anoikis in HCC cells. We postulate that HCCR-2 overexpression may help malignant cells survive in an anchorage-independent manner, leading to a poor prognosis of HCC patients, while silencing of HCCR2 gene induces anoikis-like apoptosis, leading to a good prognosis of HCC patients.
The molecular mechanisms involved in anoikis induced by HCCR-2 siRNA are not well understood. Most cells undergo apoptosis through the intrinsic, or mitochondrial pathway.22,23 This is dependent on mitochondrial outer membrane permeabilization, which is mediated by the pro-apoptotic Bcl-2 family proteins, Bax and Bak. During apoptosis, Bax translocates from the cytosol to the outer mitochondrial membrane, wherein it contributes to the formation of pores to release cytochrome-C and a second mitochondrial activator of caspases, which activate the caspases to drive cell death.24–26 It was found that HCCR encodes a mitochondrial outer membrane protein and suppresses the UVC-induced apoptosis.27 To identify the molecular mechanism that underlies the enhanced anoikis in HCCR-2 siRNA-treated HCC cells, we tested the effects of HCCR-2 siRNA on Bax and Bcl-2 expression. Results from the Western blot analysis showed that the levels of Bax was increased, while Bcl-2 was decreased in HCCR-2 siRNA HCC cells, compared with parental and negative control cells, indicating that Bax and Bcl-2 is involved in the regulation of anoikis induced by HCCR-2 siRNA in HCC cells. Caspase, as the executioner of apoptosis, plays an important role in the process of apoptosis. There are two pathways in the caspase cascade:28,29 the cell-surface-death-receptor pathway and the mitochondrion-initiated pathway. In the death-receptor pathway, activation of caspase-8 following its recruitment to the death-inducing signaling complex is the critical event. In the mitochondrion-initiated pathway, caspase-9 is activated. They cleave and activate downstream caspases and executioner caspases such as caspase-3. Therefore, caspase-3, caspase-8, and caspase-9 are the three key caspases in the process of apoptosis. Accordingly, our results showed that the cleavage of proenzymes into the active fragments of caspase-8 and caspase-9 and caspase-3 was only found in HCCR-2 siRNA cells, but not in parental and negative control cells, thus enhancing procaspase-8, procaspase-9, and procaspase-3 expression. These results indicate the importance of an intrinsic mitochondria pathway in HCCR-2 RNAi mediated anoikis.
As a major apoptosis regulator, p53 also plays a critical role in anoikis and metastasis. p53-dependent anoikis has been demonstrated in many cell types.30,31 Inactivation of p53 promotes metastasis in a number of mouse tumor models. HCCR-2 is overexpressed in HCC, and its functional role in tumorigenesis may reside as a negative regulator of the p53 tumor suppressor gene.12 However, in this study, we did not find that HCCR-2 siRNA altered p53 protein level in HCC cells. These contradictory roles of p53 in modulating apoptosis could be explained by the fact that cells derived from various tissues might have different genetic backgrounds. In addition, various cell lines derived from the same type of tumors could have different signaling networks, which may result in a different response.
Taken together, it is strongly suggested that HCCR-2 is a crucial regulatory molecule of anoikis resistance in HCC cells. HCCR-2 siRNA induces anoikis-like apoptosis and suppresses the aggressive phenotype of HCC cells in cell culture through the intrinsic or mitochondrial pathway. A better understanding of the mechanism that regulates anoikis sensitivity may help identify targets for HCC therapy.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94(2):153–156.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362(9399):1907–1917.
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27(9):1485–1491.
Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, Khin MW, Koo WH; Asian Oncology Summit. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 2009;10(11):1111–1118.
Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol 2005;11(47):7391–7400.
Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol 1997;139(3):575–578.
Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol 1997;9(5):701–706.
Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 2004;430(7003):1034–1039.
Wewer UM, Shaw LM, Albrechtsen R, Mercurio AM. The integrin alpha 6 beta 1 promotes the survival of metastatic human breast carcinoma cells in mice. Am J Pathol 1997;151(5):1191–1198.
Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol 2009;29(13):3722–3737.
Cao L, Han L, Zhang Z, Li J, Qu Z, Du J, Liang X, Liu Y, Liu H, Shi Y, Liu S, Gao L, Sun W. Involvement of anoikis-resistance in the metastasis of hepatoma cells. Exp Cell Res 2009;315(7):1148–1156.
Ko J, Lee YH, Hwang SY, Lee YS, Shin SM, Hwang JH, Kim J, Kim YW, Jang SW, Ryoo ZY, Kim IK, Namkoong SE, Kim JW. Identification and differential expression of novel human cervical cancer oncogene HCCR-2 in human cancers and its involvement in p53 stabilization. Oncogene 2003;22(30):4679–4689.
Chung YJ, Kim JW. Novel oncogene HCCR: its diagnostic and therapeutic implications for cancer. Histol Histopathol 2005;20(3):999–1003.
Ko J, Shin SM, Oh YM, Lee YS, Ryoo ZY, Lee YH, Na DS, Kim JW. Transgenic mouse model for breast cancer: induction of breast cancer in novel oncogene HCCR-2 transgenic mice. Oncogene 2004;23(10):1950–1953.
Jung SS, Park HS, Lee IJ, Namkoong H, Shin SM, Cho GW, Ha SA, Park YG, Lee YS, Ko J, Kim JW. The HCCR oncoprotein as a biomarker for human breast cancer. Clin Cancer Res 2005;11(21):7700–7708.
Yoon SK, Lim NK, Ha SA, Park YG, Choi JY, Chung KW, Sun HS, Choi MJ, Chung J, Wands JR, Kim JW. The human cervical cancer oncogene protein is a biomarker for human hepatocellular carcinoma. Cancer Res 2004;64(15):5434–5441.
Ha SA, Lee YS, Shin SM, Kim HK, Kim S, Namkoong H, Kim HJ, Jung SM, Lee YS, Chung YJ, Jung SS, Kim JW. Oncoprotein HCCR-1 expression in breast cancer is well correlated with known breast cancer prognostic factors including the HER2 overexpression, p53 mutation, and ER/PR status. BMC Cancer 2009;9:51.
Bourguignon LY, Zhu H, Zhou B, Diedrich F, Singleton PA, Hung MC. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem 2001;276(52): 48679–48692.
Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, Peterse JL, Cardiff RD, Berns A, Jonkers J. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 2006;10(5):437–449.
Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer 2006;6(6):449–458.
Valentijn AJ, Zouq N, Gilmore AP. Anoikis. Biochem Soc Trans 2004;32(Pt3):421–425.
Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2001;2(1):63–67.
Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol 2000;149(2):431–446.
Yamaguchi H, Wang HG. Bcl-XL protects BimEL-induced Bax onformational change and cytochrome C release independent of interacting with Bax or BimEL. J Biol Chem 2002;277(44):41604–41612.
Owens TW, Valentijn AJ, Upton JP, Keeble J, Zhang L, Lindsay J, Zouq NK, Gilmore AP. Apoptosis commitment and activation of mitochondrial Bax during anoikis is regulated by p38MAPK. Cell Death Differ 2009;16(11):1551–1562.
Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 2004;116(2):205–219.
Cho GW, Shin SM, Kim HK, Ha SA, Kim S, Yoon JH, Hur SY, Kim TE, Kim JW. HCCR-1, a novel oncogene, encodes a mitochondrial outer membrane protein and suppresses the UVC-induced apoptosis. BMC Cell Biol 2007;8:50.
Grossmann J. Molecular mechanisms of “detachment-induced apoptosisd Anoikis”. Apoptosis 2002;7(3):247–260.
Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev 2005;24(3):425–439.
Ravid D, Maor S, Werner H, Liscovitch M. Caveolin-1 inhibits cell detachment-induced p53 activation and anoikis by upregulation of insulin-like growth factor-I receptors and signaling. Oncogene 2005;24(8):1338–1347.
Cheng H, Liu P, Wang ZC, Zou L, Santiago S, Garbitt V, Gjoerup OV, Iglehart JD, Miron A, Richardson AL, Hahn WC, Zhao JJ. SIK1 couples LKB1 to p53-dependent anoikis and suppresses metastasis. Sci Signal 2009;2(80):ra35.
Acknowledgments
We thank Dr. Su Yongyue (Institute of Burn Research, Southwest Hospital, Third Military Medical University, Chongqing 400038, People's Republic of China) for generously providing the vector pGenesil-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jun Guo and Liuqin Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, J., Yang, L., Zhang, Y. et al. Silencing of the HCCR2 Gene Induces Apoptosis and Suppresses the Aggressive Phenotype of Hepatocellular Carcinoma Cells in Culture. J Gastrointest Surg 15, 1807–1813 (2011). https://doi.org/10.1007/s11605-011-1633-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-011-1633-4