Abstract
Introduction
The implementation of laparoscopic pancreaticoduodenectomy (LPD) has been appropriately met with apprehension, and concerns exist regarding outcomes early in a program’s experience. We reviewed our early experience and outcomes of LPD.
Methods
A retrospective review of patients undergoing LPD was compared to a matched cohort of open pancreaticoduodenectomy (OPD) patients. The endpoints are as follows: age, gender, ASA score, BMI, operative time, estimated blood loss, perioperative transfusion requirement, intensive care unit stay, margin status, lymph node count, 90 day morbidity and mortality, length of stay, and adjuvant therapy treatment.
Results
Fourteen patients underwent an attempted LPD. The median operative time was 456 min (interquartile range (IQR), 109.5), median estimated blood loss was 300 ml (IQR, 225), and 29% of the patients required a perioperative blood transfusion. A conversion was necessary in two patients (14%). A malignancy was present in 12 patients. The mean tumor size was 2.2 cm (standard deviation (SD), 1.1), the mean lymph node count was 18.5 (SD 6.2), and an R0 resection was achieved in all 12 cases. Clavien grade I/II complications occurred in 42% of the patients, and Clavien grade III/IV complications occurred in three (20%). There was one late postoperative death. The median length of stay was 8 days. Compared to OPD, LPD took longer to perform, but no differences were noted with respect to blood loss, morbidity, mortality, R0 resection rate, and LN harvest.
Conclusions
LPD can be implemented in a high-volume pancreatic surgery center with acceptable oncologic and patient outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic radical pancreaticoduodenectomy (LPD) had been met by an appropriate degree of skepticism following an initial report in 1994 by Gagner.1 However, concerns regarding the feasibility and oncologic integrity of LPD have now been tempered by three recent reports of success2–4, and others have generated enthusiasm through the utilization of robotic assistance to perform the procedure. Furthermore, a laparoscopic distal pancreatectomy has been successfully introduced into multiple high-volume pancreatic surgery centers with superior results.5,6 In this setting, numerous centers are now considering the introduction of LPD with or without robotics to their pancreatic surgery programs.
Shorter hospital stays, reduced analgesia requirements, rapid return to baseline performance status, and reduced morbidity have been observed in the laparoscopic treatment of various gastrointestinal malignancies.6–9 Radical pancreaticoduodenectomy has been historically plagued by high rates of morbidity. This compromises the quality of life and precludes the administration of adjuvant chemotherapy to an unacceptably high number of cancer patients. Thus, the potential benefit of LPD compared to traditional, open techniques warrants exploration.
Due to the inherent learning curve required to master novel procedures, there are significant concerns that patient safety and operative outcomes will be compromised as surgeons with varying pancreatic and/or laparoscopic surgical experience begin to perform LPD, and the existing literature does not address this issue. To this end, we reviewed our initial experience with LPD as performed by a single, high-volume pancreatic surgeon with extensive laparoscopic surgical experience in a tertiary care setting. We present the preparation taken prior to the performance of LPD, the criteria utilized for patient selection and report on postoperative oncological surrogate markers and clinical outcomes. We conclude that laparoscopic pancreaticoduodenectomy can be safely implemented in a high-volume pancreatic surgery center without subjecting patients to an unacceptably higher risk of complications or a compromise of oncologic surgical principles.
Methods
A retrospective review of a prospectively maintained database was performed to identify all LPD performed at the University of Pittsburgh Medical Center (UPMC) between September 2008 and March 2010. An open pancreaticoduodenectomy (OPD) cohort matched for age, gender, comorbidities, body mass index (BMI), pathological diagnosis, and tumor stage was subsequently obtained from cases performed between January 2006 and August 2008. An approval by the University of Pittsburgh Institutional Review Board was obtained to perform this study, but was not required prior to the initiation of LPD given prior publication of the procedure. Rather a full disclosure regarding the surgical team’s status with respect to LPD was provided to all of the patients. All cases were performed by a single surgeon (SJH) in conjunction with a surgical oncology fellow serving as first assistant. The primary surgeon is a high-volume pancreatic surgeon with extensive experience in minimally invasive surgical oncology having performed, exclusive of laparoscopic cholecystectomy, over 500 cases of minimally invasive procedures on the gastrointestinal tract, including gastric, hepatic, pancreatic, and colorectal resections.
Patient Selection
Patient selection was determined, in part, using the UPMC image-based mathematical model predictive of margin negative resection (R0).10 This predictive model utilizes computed tomography (CT) and endoscopic ultrasound (EUS) imaging features to establish the probability of an R0 resection. All patients with lesions predicted by the model to have an increased risk of a positive margin and patients undergoing neoadjuvant therapy as part of a clinical trial were excluded.
Staged Development of Operative Technique and Experience
In order to achieve technical experience with LPD, the surgical team performed four LPD procedures on fresh frozen cadavers. These efforts focused upon trochar placement and methods of exposure and reconstruction. After comfort was reached in these regards, LPD resection with intentional conversion to standard open technique was performed on two patients. This step was taken to ensure an acceptable operative time for the resection (lap OR time <300 min), and to assess the adequacy of the resection by open technique. These patients were excluded from the current analysis.
Operative Technique
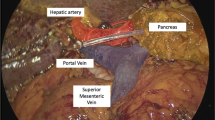
Port placement and utilization is depicted in Fig. 1. The sequence of the dissection is altered in that the inferior border of the pancreas and superior mesenteric vein dissection is performed prior to the Kocher maneuver. All arterial branches are controlled with clips or ligatures in addition to bipolar electrocautery or stapling. The uncinate process is dissected along the adventitia of the superior mesenteric artery. An antrectomy, rather than pylorus preservation, is routinely performed. The specimen is placed in a bag for retrieval. Additional prophylactic antibiotics are administered based upon the operative time prior to the formation of a muscle-sparing, right lower quadrant utility incision. A wound protector is also utilized.
The abdomen is entered using the Veres needle technique at port E. The remaining ports are placed under direct vision, and the camera is moved to port C. (1) Entry into the lesser sac, establishment of the plane between the middle colic and gastroepiploic vessels, mobilization of hepatic flexure/right colon, infrapancreatic portal vein dissection, and cholecystectomy: ports D and E with surgeon on the left. (2) Kocher maneuver, portal dissection, bile duct division (scissors), GDA ligation (linear stapler): ports A and B with surgeon on the right, fixed liver retractor through port E. (3) Mobilization of the ligament of Treitz: ports A and B. (4) Division of antrum (linear stapler): port D by first assistant on the left. (5) Pancreatic neck division (bipolar electrocautery with scissors at pancreatic duct), uncinate resection (bipolar electrocautery and clips): ports A, B, and D. (6) Specimen extraction: right lower quadrant 5-cm muscle-sparing incision (F) with wound protector. (7) Pancreaticojejunostomy (reconstructive limb brought behind the root of the mesentery to create a neoduodenum): ports D and E with a surgeon on the left, liver retractor removed. (8) Hepaticojejunostomy: ports A and B with surgeon back on the right, liver retractor replaced in port E. (9) Stapled gastrojejunostomy: ports A and B with surgeon on the right
For reconstruction, an end-to-side duct to the mucosa pancreaticojejunostomy is fashioned in two running layers of absorbable monofilament suture (polydiaxone) modified from the technique described by Ohwada.11 An end-to-side hepaticojejunostomy using a running 4–0 polydiaxone suture is subsequently fashioned. The gastrojejunostomy is performed antecolic using a stapled technique. Two drains are routinely left in the vicinity of the pancreaticojejunostomy and the hepaticojejunostomy.
Endpoints
Data were obtained from both the electronic medical record and outpatient clinic charts and included operative time (minutes), blood loss (milliliters), intraoperative blood transfusion, final pathological diagnosis, lymph node harvest (n), margin status (R0 versus R1), postoperative complications, hospital length of stay (days), administration of adjuvant therapy, and disease-specific and overall survival. Identical data from a group of 14 OPD patients were compared to the LPD cohort.
Statistical Analysis
Using the SPSS (Chicago, IL), data were imported and verified. Descriptive statistics were performed to characterize the sample. With the exception of tumor size and number of harvested lymph nodes, the data were nonnormally distributed therefore nonparametric statistics were performed. Mann–Whitney U tests were used to test between group differences with continuous variables and chi-square analyses for categorical variables.
Results
Between September 2008 and March 2010, 14 patients underwent a planned LPD. A matched cohort of 14 OPD patients was treated between January 2006 and August 2008. The patient characteristics and operative data for both groups are presented in Table 1. With respect to overall health, 35.7% of the LPD patients were American College of Anesthesiology Score (ASA) class II, and 64.3% of the patients were ASA class III. The median LPD operative time was 456 min (range, 334–583 min; interquartile range (IQR), 109.5), and the median estimated blood loss (EBL) was 300 ml (range, 150–1,300 ml; IQR, 225). Immediate postoperative intensive care unit (ICU) care was deemed appropriate for five LPD patients (36%), and four patients received a perioperative (within 72 h) blood transfusion (29%). The LPD median ICU stay was 0 days and the median length of hospital stay was 8 days (range, 5–28 days; IQR, 8.5). When compared to the OPD group, only operative times were significantly different: LPD, 456 min (range, 334–583 min) and OPD 372.5 min (range, 290–628 min) (P = 0.01).
The primary indication for LPD proved to be malignancy (12 out of 14, 88%). Table 2 summarizes the final pathological diagnoses. Initial patient selection intentionally attempted to exclude ductal adenocarcinoma of the pancreatic head, and of the eight cases of pancreatic cancer, four had preoperative clinical diagnoses of the distal bile duct or ampullary cancer. The other four cases represented subsequent cases and lacked any features by imaging of portal vein or celiac or superior mesenteric artery encroachment by the neoplasm. Table 3 summarizes stage, margin, and lymph node harvest data for both groups. Regarding the LPD cohort, an R0 resection margin was achieved in all 12 cases of malignancy. The average tumor size was smaller in the LPD group (LPD, 2.2 cm; range, 0.8–4.7 cm versus OPD, 3.6; range, 3–5 cm; p = 0.02), and the mean number of retrieved lymph nodes was comparable: LPD, 18.5 (range, 12–31); OPD, 19.1 (range, 10–36) (p = 0.85). The one T4N1 lesion in the LPD group was a duodenal adenocarcinoma with invasion of the pancreatic parenchyma and common bile.
The postoperative outcomes and complications are summarized in Table 4. No significant differences were noted with regard to postoperative morbidity between the two groups. A conversion to an open procedure was necessary in two cases (14%). Regarding these cases, the first patient with a BMI of 37 was converted due to failure to progress during exposure of the third portion of the duodenum. The second patient required conversion secondary to intraoperative bleeding from the portal vein in the setting of chronic pancreatitis (EBL, 1,300 ml); this patient subsequently required resectioning of a 2-cm segment of the portal vein with primary reconstruction secondary to the poor quality of the tissues.
Within the LPD cohort, there was a single mortality that occurred 44 days postoperatively (grade V Clavien12) due to a multisystem organ failure secondary to sepsis (aspiration on POD 5 resulting in bilateral pneumonia). Three other LPD patients had a major complication (Clavien grade III or IV, necessitating radiological, endoscopic, or operative intervention and/or causing organ failure). These included gastric staple line bleeding necessitating reoperation (performed laparoscopically) on postoperative day 1 (n = 1), pulmonary embolus and aspiration pneumonia requiring reintubation (n = 1), and upper gastrointestinal hemorrhage from marginal ulcer 30 days postoperation requiring therapeutic gastroscopy (n = 1).
Six LPD patients suffered a minor complication (Clavien grade I or II, not necessitating radiologic, endoscopic, or operative intervention and not causing organ failure). A detailed listing of these complications includes infection of the utility incision (n = 2), delayed gastric emptying prolonging hospital stay (n = 2), delayed gastric emptying and portal vein thrombosis (n = 1), and wound infection and prolonged ileus necessitating TPN and antibiotics for 1 week (n = 1). Finally, a pancreatic leak occurred in five LPD patients (36%). All leaks were grade A (ISGPF)13 and were adequately controlled by the intraoperatively placed drains. These leaks were diagnosed by checking a drain amylase on the third postoperative day regardless of effluent character or volume. All of these leaks had been sealed and the drains removed within 5 weeks of the operative date.
Of the 12 LPD patients with malignancy, an adjuvant treatment was indicated in nine of the cases based upon histological diagnosis and stage. Five of the nine (55.5%) patients commenced adjuvant treatment with a mean time from surgery to onset of chemotherapy of 60 days (range, 41–80). The reasons for not commencing adjuvant chemotherapy included surgical mortality (n = 1), poor postoperative functional status (n = 1), patient refusal (n = 1), and loss to follow-up (n = 1). At median follow-up of 9.5 months (range, 4–21 months), only one patient has had a recurrence.
In Table 5, the operative details and outcomes between the first and last seven LPD cases are compared to ascertain evidence of a learning curve. Using the Mann–Whitney U and chi-square analyses, trends of reduced operative time, blood loss, and shorter hospitalization were observed, but these differences did not reach statistical significance. No significant differences in complication rates or pancreatic fistula rates were noted.
Discussion
Given the potential of a reduced morbidity, we embarked on implementing LPD at a high-volume pancreatic surgery center. Our approach was cautious due to concerns that patient safety and operative outcomes could be compromised during the early experience. We aimed to meet the standards as published by Winter et al. who reported overall perioperative mortality and morbidity rates of 2% and 38%, respectively (1% and 45% in the last decade) including a reoperative rate of 3% and a median length of stay of 9 days (8 days in the last decade) in a series of 1,175 OPD performed for pancreatic cancer.14
Our results from a small series of patients did not quite meet this exceptional standard; however, our single mortality unrelated to surgical technique, a single reoperation, and the morbidity rate and median length of stay do compare favorably. This data suggests that a laparoscopic pancreaticoduodenectomy can be implemented in a high-volume pancreatic surgery center without subjecting patients to an unacceptably higher risk of complications early in the surgeon’s experience. Our intraoperative and immediate oncological parameters such as blood loss, resection margin, and lymph node harvest are comparable to this and other large open series.14,15 Thus, our results further suggest that LPD can be implemented without compromising oncologic principles of the procedure. We conclude that the learning curve of a surgeon embarking on the performance of LPD impacts the duration of the procedure, but does not negatively impact complication rates, margin status, lymph node harvest, blood loss, need for transfusion, or need for intensive care. Furthermore, the early operative experience does not necessarily equate with a high conversion rate as we were able to perform an LPD in 12 out of 14 patients successfully (86%).
Since Gagner’s first report of LPD in 1994,1,16 there have only been three series of patients undergoing LPD reported (Table 6).2–4 It is unclear from these prior manuscripts how these centers embarked upon the performance of LPD, whether cases performed during their initial experience were excluded, or whether a disproportionate number of complications occurred early in their experience. Our data compares favorably with most of the outcomes reported in these prior studies, particularly with regard to immediate oncological surrogate markers, morbidity, and length of stay. Our overall pancreatic fistula rate (36%) is higher than that reported by others, but may represent differences in diagnosis criteria and patient selection bias that resulted in a high percentage of the patients in our series having normal, soft pancreata. Four of our five LPD leaks occurred in soft glands with small caliber ducts. None of these leaks was associated with a clinical event, nor did the pancreatic leak rate appear to be influenced by a learning curve.
One impetus for LPD is the hope it will result in a reduced postoperative morbidity, and thus the successful institution of adjuvant therapy will be an important endpoint to determine its superiority to OPD. The administration of chemotherapy has proven beneficial in pancreatic cancer patients,17–19 but its delivery is limited to approximately 40–60% of surgical patients due to postoperative complications, prolonged convalescence, patterns of referral, and the location/nature of the treatment facility.20,21 In this series, 55% of patients received adjuvant chemotherapy, and the average time to its institution was 60 days. A larger series and longer follow-up will be necessary to determine any benefit of LPD with respect to the successful initiation of adjuvant treatment, reduction in complication rates, or improvement in quality of life.
The impact of LPD on health care delivery costs is an important unanswered issue, but we did not perform a cost analysis on this early experience for a number of reasons. First, we observed a decrease in operative times with increasing experience, had not reached a nadir, and this is a major component of cost. Furthermore, this variable is dependent upon the individual surgeon, and the conclusions from a single surgeon’s experience may not prove to be applicable to a population of surgeons. Finally, the length of stay for this cohort was artificially prolonged to ensure safe hospital discharge early in our experience. Future study in this regard is clearly warranted.
The philosophy regarding patient selection and the performance of LPD for malignancy early in a surgeons experience is not straightforward. The potential for a compromised dissection must be weighed against the ease of reconstruction. Thus, ampullary pathology leading to dilation of both the biliary and pancreatic ducts and pancreatic fibrosis without compromise of retroperitoneal or vascular margins may represent the ideal situation for initial attempts at LPD. Our R0 resection rate of 100% supports the use of our previously published preoperative predictive model of negative margin resection that employs findings of CT and EUS imaging.10 Obese patients and patients with chronic calcific pancreatitis pose additional technical challenges that may be best avoided early in a surgeon’s experience.
Finally, these results may not be generalized, and questions remain regarding what training and experience is best to prepare a surgeon to safely perform LPD. We anticipate that a number of experienced pancreatic surgeons will embark upon LPD in the near future. We found that the dissections and reconstructions on cadavers to be very helpful. The subsequent performance of our initial LPD with the intent of laparoscopic resection and open reconstruction helped us gain efficiency and confidence in the setting of intended conversion to an open procedure. In our opinion, familiarity with the anatomy, and technical assessment of the adequacy of the anastamoses were more important than minimally invasive surgical skill.
Conclusion
In summary, we have presented an early experience in performing total laparoscopic pancreaticoduodenectomy. Our results suggest safety and feasibility in the implementation of this procedure when performed at a high-volume tertiary care center by a surgical team experienced in open pancreatic and minimally invasive surgery. Early postoperative outcomes and oncologic surrogate results will not be compromised if appropriate surgical expertise is coupled to careful patient selection.
References
Gagner, M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8(5):408–10.
Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145(1):19–23.
Palanivelu C, Rajan PS, Rangarajan M, Vaithiswaran V, Senthilnathan P, Parthasarathi R, Praveen Raj P. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg 2009;16(6):731–40.
Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc 2006;20(7):1045–50.
Kooby DA, Gillespie T, Bentrem DJ, Nakeeb A, Schmidt MC, Merchant NB, Parikh AA, Martin RC 2nd, Scoggins CR, Ahmad S, Kim HJ, Park J, Johnston F, Strouch MJ, Menze A, Rymer J, McClaine R, Strasberg SM, Talamonti MS, Staley CA, McMasters KM, Lowy AM, Byrd-Sellers J, Wood WC, Hawkins WG. Left-sided pancreatectomy:a multicenter comparison of laparoscopic and open approaches. Ann Surg 2008;248:438–446.
Borja-Cacho D, Al-Refaie WB, Vickers SM, Tuttle TM, Jensen EH. Laparoscopic distal pancreatectomy. J Am Coll Surg 2009;209(6):758–65.
Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350(20):2050–9
Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, Oba H, Aoki T, Minami M, Hirakawa K. A meta-analysis of randomized controlled trials that compared laparoscopy-assisted and open distal gastrectomy for early gastric cancer. J Gastrointest Surg 2010;14(6):958–64.
Vanounou T, Steel JL, Nguyen KT, Tsung A, Marsh JW, Geller DA, Gamblin TC. Comparing the clinical and economic impact of laparoscopic versus open liver resection. Ann Surg Oncol 2010;17(4):998–1009.
Bao P, Potter D, Eisenberg DP, Lenzner D, Zeh III HJ, Lee KKW, Hughes SJ, Sanders MK, Young J, and Moser AJ. Validation of a prediction rule to maximize curative (R0) resection of early-stage pancreatic adenocarcinoma. HPB. 11: 606–611, 2009.
Ohwada S, Iwazaki S, Nakamura S, Ogawa T, Tanahashi Y, Ikeya T, Lino Y, Morishita Y. Pancreaticojejunostomy-securing technique: duct-to-mucosa anastomosis by continuous running suture and parachuting using monofilament absorbable thread. J Am Coll Surg 1997;185(2):190–4
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M.The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250(2):187–96.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138(1):8–13.
Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ.1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006; 10(9):1199–210; discussion 1210–1.
Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Grochow LB, Abrams RASix hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226(3)248-57; discussion 257–60.
Gagner M, Pomp A. Laparoscopic pancreatic resection: Is it worthwhile? J Gastrointest Surg 1997;1(1):20–5; discussion 25–6.
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297(3):267–77.
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350(12);1200–10
Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, Doi R, Monden M, Hatori T, Tanaka M, Shimada M, Kanemitsu K. A randomized phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer 2009;101(6):908–15.
Vanderveen KA, Chen SL, Yin D, Cress RD, Bold RJ. Benefit of postoperative adjuvant therapy for pancreatic cancer: A population-based analysis. Cancer 2009;115(11):2420–9.
Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic surgery in the United States: utilization, outcomes, and the effect of hospital volume. Cancer 2006;110(6);1227–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zureikat, A.H., Breaux, J.A., Steel, J.L. et al. Can Laparoscopic Pancreaticoduodenectomy Be Safely Implemented?. J Gastrointest Surg 15, 1151–1157 (2011). https://doi.org/10.1007/s11605-011-1530-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-011-1530-x