Abstract
Introduction
Positive volume–outcome relationships in esophagectomy have prompted support for regionalization of care; however, outcomes have not recently been analyzed. This study examines national trends in provision of esophagectomy and reassesses the volume–outcome relationship in light of changing practice patterns and training paradigms.
Methods
The Nationwide Inpatient Sample was queried from 1998 to 2006. Quantification of patients’ comorbidities was made using the Charlson Index. Using logistic regression modeling, institutions’ annual case volumes were correlated with risk-adjusted outcomes over time, as well as presence or absence of fellowship and residency training programs.
Results
A nationwide total of 57,676 esophagectomies were recorded. In-hospital unadjusted mortality fell from 12% to 7%. Adjusting for comorbidities, greater esophagectomy volume was associated with improvements in the incidence of most measured complications, though mortality increased once greater than 100 cases were performed. Hospitals supporting fellowship training or a surgical residency program did not have higher rates of mortality or total complications.
Conclusions
The current national mortality rate of 7% following esophagectomy is higher than is reported in most contemporary case series. A greater annual esophagectomy volume improves outcomes, but only up to a point. Current training paradigms are safe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Through the turn of the millennium, the USA has experienced a steady rise in the incidence of esophageal adenocarcinoma, with annual increases of more than 2% per year between 1998 and 2003.1 Age-adjusted incidence rates of esophageal cancer now approximate 4.5 cases per 100,000 population,2 placing it seventh among causes of cancer death.3
For over 30 years, surgeons have pondered the association between case volume and patient outcomes for high-risk surgical procedures.4–8 Esophagectomy, because of its high risk and relatively low volume, has been embraced as a procedure warranting regionalization of care within specialty centers.9,10
As a consequence, systems to drive cases to high-volume centers have emerged. For example, the Leapfrog group (Washington, DC), a collaboration of healthcare purchasing organizations that works to initiate improvements in the safety, quality, and affordability of healthcare,11 has established definitions for case volume requirements12 and tracks outcomes. Investigators have attempted to support or refute case volume thresholds for esophagectomy.13–15 The definition of what constitutes a high-volume center varies markedly in the literature and is usually arbitrarily defined.16,17
On the surface, recent data seem to support improved outcomes in the era of regionalization in esophageal surgery. High-volume centers show superior esophagectomy outcomes,18 with the best centers reporting mortality rates from 1% to 4%.16,19–25 However, as systems supporting regionalization gain traction, it remains vital to track national outcomes, since high-volume reporting centers may not represent the rate of actual mortality across the USA. To date, broad efforts to confirm case volume as a surrogate for quality have usually stratified hospital case volume as a categorical variable when comparing statewide or nationwide outcomes via administrative datasets.26–30
Paradigm shifts may bring unintended consequences. High-volume centers are also usually the seats of surgical training. Rising numbers of esophageal operations will require these institutions increase both clinical and educational missions. However, since esophageal surgery is often within the domains of specialist surgeons focused on minimally invasive, thoracic, and oncologic practice, the structure of advanced training is heterogeneous and difficult to evaluate. The impact of fellowship programs on patient outcomes after esophagectomy has not been evaluated outside of single-institution experience.31 The effect of general surgery training programs has rarely been assessed.
Finally, ongoing advances in both surgical and nonsurgical therapeutic modalities and protocols mandate periodic reassessment of our systems intended to regulate delivery of care. Therefore, we report the current state of esophageal surgery in this country with regards to national trends in provision and the impact of case volume and training programs on the safety of esophagectomy.
Methods
The most recently available Nationwide Inpatient Sample (NIS)32 databases covering the years 1998–2006 were queried. These are the largest all-payer inpatient care databases in the USA, containing data from approximately eight million hospital stays each year. The latest release, the 2006 database, contains all discharge data from 1,045 hospitals located in 38 states, approximating a 20% stratified sample of all nonfederal, short-term, general, and other specialty hospitals in the USA.32 A dataset was created by merging core and hospital files and filtered to identify esophagectomies using the ICD-9-CM procedure codes 42.4 (esophagectomy), 42.40 (esophagectomy, not otherwise specified), 42.41 (partial esophagectomy), 42.42 (total esophagectomy, excluding esophagogastrectomy), and 43.99 (esophagogastrectomy, also including complete gastroduodenectomy, esophagoduodenostomy with complete gastrectomy esophagojejunostomy with complete gastrectomy, radical gastrectomy, and other total gastrectomy). While these are standard codes for esophagectomy, they also include some gastrectomies without esophagectomy. To correct for this, gastric operations were assumed if associated with a diagnosis code for malignant neoplasm of stomach (151–151.9) or for gastric ulcer (531–531.9) and were excluded. Pediatric patients less than or equal to 17 years of age were excluded. To calculate nationwide case volume totals, the NIS-supplied discharge-level weight was applied. At all other times, the unweighted NIS cohort was utilized for calculating standard errors and performing regression analyses.
Information regarding the presence of a Fellowship Council (FC)-accredited fellowship program in each year of the study period was taken from the Fellowship Council’s webpage.33 The Fellowship Council is an association of minimally invasive, endoscopic, and combined gastrointestinal surgery fellowship directors formed to address the unique needs of fellowship applicants and programs. In 2006, there were 89 listed programs. Information regarding the presence of a thoracic surgery fellowship was taken from the National Resident Matching Program’s 2009 website34 and assumed the presence of such a fellowship throughout all the years of the study. There were 43 such fellowships identified. Information regarding the presence of a Society of Surgical Oncology (SSO) fellowship was taken from this society’s website35 and assumed the presence of such a fellowship throughout all the years of the study. There were 11 such fellowships identified.
A teaching hospital is defined within the NIS as a hospital with residents in any specialty and meeting any of the following criteria: Accreditation Council for Graduate Medical Education (ACGME) residency training approval, membership in the Council of Teaching Hospitals, or a ratio of full-time equivalent interns and residents to beds of 0.25 or higher. Hospitals having a surgical residency were defined as a subgroup. Details of such a surgical residency program were obtained by combining information from the American Medical Association’s Fellowship and Residency Electronic Interactive Database Access and the listings of accredited programs on the ACGME webpage.36,37 There were 192 identified accredited general surgery residencies. The NIS divides hospitals into size tertiles based on bed size, adjusted for region and teaching status.38
Comorbidity scores were applied to each inpatient stay record, using the Deyo adaptation of the Charlson comorbidity index.39 This validated index allocates a score between 0 and 35, with a higher score indicating more comorbidity. The comorbidities examined include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, pulmonary disease, connective tissue disease, peptic ulcers, chronic liver disease, hemiplegia, renal disease, diabetes, malignancy, leukemia, metastatic cancer, and acquired immune deficiency syndrome.
Perioperative complications were added based on ICD-9-CM codes, in a similar manner to that described by Santry et al.39 The diagnosis of “any complication” was made if the “died during hospitalization” field = 1 or if any of the NIS’s 15 diagnosis fields contained one of the following complication or procedure codes: abdominal drainage procedure (5491), acute cerebrovascular accident (43100–43191, 4330–4339, 4340–43491), acute dialysis (3895), acute deep venous thrombosis (4538, 4539), acute myocardial infarction (4100–4109), acute pulmonary embolism (4151, 41511, 41519), acute renal failure (5841–5849), acute respiratory failure (51881), adhesiolysis (5451, 5459), anastomotic leak (9986), bacterial pneumonia (481, 485, 486, 4820–4829), cardiac complications (9971), central nervous system complications (99701–99703), dialysis catheter insertion (3995), foreign body removal (5492), intraoperative hemorrhage (99811), laparotomy (5412), mechanical ventilation (967, 9671, 9672, 9673), postoperative shock (9980), reclosure of abdomen (5461), respiratory tract complications (99973), small bowel obstruction (5600–5609), splenectomy (4143, 415), splenic injury (8650–8651), tracheostomy (311, 3129), transfusion (9904, 9909), urinary complications (9975), wound dehiscence (9983, 99831, 99832), wound infection (9985, 99851, 99859), and wound seroma (99813).
Statistics
SAS 9.2 (SAS Institute, Cary, NC, USA) was used to analyze the data. Logistic regression modeling was performed using generalized estimating equations and assuming a binomial distribution of the data. This allowed control for certain covariables; thus, risk-adjusted outcome measures were calculated. Repeated measure analysis was performed with the experimental unit being hospital identification number clusters. The model was solved for empirical standard error estimates, and p values were based on these estimates. A p value < 0.05 was considered significant. Subsequently, the estimates were exponentiated to calculate an odds ratio (OR) and 95% confidence intervals. One of the authors (JAG) holds a Ph.D. in Biostatistics.
Results
Trends in Care
A total of 11,614 esophagectomies were recorded in the NIS database for the study period; this was the cohort utilized for subsequent analysis. NIS weightings indicate this cohort that represents 57,676 total esophagectomies performed in the USA during the 9-year study period of 1998–2006. With a nationwide weighted total of 6,425 esophagectomies being performed in 1998 and 6,032 in 2006, it is evident that the annual number of esophagectomies did not increase over this timeframe, despite the increasing number of new diagnoses of esophageal malignancy1 (Table 1). At the beginning of the study period, approximately 40% of these operations were performed in teaching hospitals, a proportion which remained constant throughout the study period. The majority of operations were performed in the largest third of hospitals (Table 2). The indications for surgery and the type of operations have remained similar over the same interval (Table 1).
As illustrated in Table 3, high-volume centers for esophagectomy are variously described as performing at least 13 to 20 esophagectomies per year,12,17,25 and the number of surgical programs meeting these standards has remained stable over time. In 1998, 4.2% of hospitals performing esophagectomies completed 13 or more cases, and 1.2% of hospitals performing esophagectomies completed 20 of more cases. In 2002, these numbers were 7.5% and 2.5% and in 2006 were 12.4% and 5.8%.
Mortality Rates
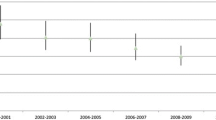
Concurrent with the stable hospital case volumes, the in-hospital mortality rate for esophagectomies taken as a group has steadily decreased throughout the study period (Fig. 1). The mortality rate of all esophagectomies performed in the USA in 1998 was 12.1%. By 2002, it was 9.0%, and by 2006, it had reached 7.0%. As noted in Table 1, approximately 40% of the operations performed were esophagogastrectomies. Improvements of in-hospital mortality were quite impressive in this subgroup, decreasing from 12.3% at beginning of the study period to 8.9% in 2002 and to 7.8% in 2006. Just fewer than 40% of the operations were partial esophagectomies; mortality rates for this subgroup also fell, from 10.7% in 1998 to 8.6% in 2002 and to 5.9% in 2006. Approximately 16% of operations were total esophagectomies, and here too, mortality rates improved markedly over the study period—15.2% in 1998, 9.8% in 2002, and 6.3% in 2006. The only operation which increased in mortality was “Esophagectomy, not otherwise specified”. The numbers performed were small, with 35, 34, and 40 procedures coded in 1998, 2002, and 2006, respectively. Corresponding mortality rates were 11.4%, 8.8%, and 15.0%. These trends in mortality rate occurred synchronously with a steady decrease in every year of the mean Charlson comorbidity scores, from 4.5062 in 1998 to 4.2311 in 2002 and to 3.7997 in 2006.
With all esophagectomies considered together, there was noticeable variation in mortality rates according to expected primary payer status or by self-described racial group (Table 4). The largest three expected payer groups were private including HMO, Medicare, and Medicaid; unadjusted mortality was 5.2%, 12.2%, and 11.3%, respectively. Of the three largest racial groups in which a racial identity was specified, the mortality rates were White 8.9%, Black 12.5%, and Hispanic 7.1%.
Interestingly, as seen in Fig. 1, anastomotic leak rates were quite constant throughout the study period, with little variance about the mean of 1.53 (±0.29).
Effect of Hospital Case Volume
Table 5 examines the independent effect of annual hospital case volume on complication rates, after controlling for the improvements in outcomes seen over the study period and for Charlson comorbidity scores. That is, the risk-adjusted effect of increasing annual case volume is reported. In contrast to previously published studies, artificial case volume groups were not applied and the models were solved for case volume as a continuous variable. An odds ratio <1.0 signifies an inverse correlation between case volume and the complication under review. The odds ratios tend to be very close to 1.0 because the ratios represent the effect of increasing the annual volume by a single case. That is, the effect of each and every case on outcomes is reported. Nearly all analyzed complication categories trended toward an inverse correlation with case volume, with any complication, myocardial infarction, respiratory tract complications, bacterial pneumonia, acute respiratory failure, acute renal failure, postoperative shock, blood transfusion requirement, and splenectomy rates achieving statistically significant improvement. No complication was associated with increasing case volume.
Results which have been tabulated reflect modeling for the linear effect of the variables only in order to simplify presentation. For a more detailed examination of the effects of case volume specifically on mortality rates, modeling was also performed adjusting for year, case volume, and Charlson comorbidity score and additionally the quadratic of case volume. This examines the effect of very high case volume on mortality. When used as predictors in a logistic regression model, both the case volume (p < 0.0001) and the quadratic (p < 0.0001) achieved statistical significance, with the predicted trends plotted in Fig. 2. The improvement in mortality rate observed with increasing hospital case volume seems to level out at approximately 30–40 cases per year and then slowly increases after about 80–100 cases per year.
Effect of Training Programs
Table 6 examines the independent effect of fellowship programs on outcomes of esophagectomies. Forty-three hospitals submitting data to the NIS and offering National Residence Matching Program (NRMP)-affiliated thoracic surgery fellowship programs were identified. Examining the independent effect of the presence of a thoracic surgery fellowship, after controlling for yearly variations, annual case volume, and Charlson comorbidity score, it is seen that the rate of any complication was significantly better, as were rates of bacterial pneumonia and incidental splenectomy. Anastomotic leak rates were significantly worse in this group, being nearly double those in hospitals without a thoracic surgery fellowship program (OR 1.81808, 95% confidence interval [1.18347, 2.79297]). Eighty-nine NIS hospitals had Fellowship Council-accredited fellowship programs. Examination of the independent effect of a FC-accredited fellowship on esophagectomy outcomes, after controlling for yearly variations, annual case volume, and Charlson comorbidity score, revealed that anastomotic leak rate was significantly increased (OR 1.71926 [1.09136, 2.70843]). Eleven NIS hospitals offered a Society of Surgical Oncologists-administered fellowship program during the study period. Only a very small number of these institution performed esophagectomies ranging from one to three hospitals per year. In the years 1998, 1999, 2000, and 2004, one of these institutions also offered either a Fellowship Council-accredited fellowship or a thoracic surgery fellowship. There was no significant independent effect of an SSO-administered fellowship on any of the measured variables. There was no detrimental effect of any fellowship program on in-hospital mortality following esophagectomy.
When all of the above fellowship programs were considered together, again controlling for yearly variations, annual case volume, and Charlson comorbidity score, it was noted that the presence of any fellowship program was associated with a decrease in the rate of any complication (OR 0.81655 [0.70613, 0.94425]) and an increase in rates of anastomotic leak (OR 1.64538 [1.12423, 2.40811], myocardial infarction (OR 1.47069 [1.02836, 2.10329]), and requirement for postoperative tracheostomy (OR 1.37774 [1.09114, 1.73961]).
The effects of the presence of an ACGME-accredited general surgical residency program in hospitals submitting data to the NIS are shown in Table 7. There were clear benefits in rates of any complication (OR 0.85656 [0.75270, 0.97482]), in-hospital mortality (OR 0.73408 [0.60460, 0.89128]), acute renal failure, acute respiratory failure (OR 0.77169 [0.63680, 0.93518]), and postoperative bacterial pneumonia (OR 0.70775 [0.60034, 0.83437]). The first column shows the independent effect of a surgical residency program, after controlling for yearly variations, annual case volume, and Charlson comorbidity score. The second column shows the effect of a surgical residency program after controlling for any fellowship in addition to the other controlled variables.
Discussion
With the incidence of esophageal adenocarcinoma in the USA increasing, demand for esophagectomies will persist for the foreseeable future. Several studies have reported an association between increasing hospital esophagectomy volumes and improved outcomes, and these data have been often cited by proponents of centralization of care. However, many of these studies have ignored case mix and comorbidity profiles. There has also been confusion in the studies between mortality rates attributed to institutions and those associated with individual surgeons, especially in hospitals where more than one division performs these operations.40 A further confounder of volume–outcome studies is the categorization of institutions into either low- or high-volume centers based on arbitrary case thresholds.41 Finally, interpreting such results is difficult when poorly described or suboptimal statistical methodology is utilized.42
Despite accruing evidence of the beneficial effects of case volume on cancer surgery outcomes since the end of the twentieth century,6,26,43 the percentage of esophagectomies being performed in higher-volume hospitals has not increased significantly over the study period. This single fact may explain the discrepancy between the best reported mortality rates and the latest US esophagectomy mortality rate of over 7%. Surgeons and patients discussing informed consent for esophagectomy outside high-volume centers should consider that one in every 14 patients undergoing esophagectomy in this country will die in-hospital.
Although it is encouraging that the mortality rate for esophagectomy has diminished by 60% over recent years, there is no clear association with the movement toward regionalization. Mortality improvement may be partly explained by the decreasing comorbidities of the patient population described above. There have been parallel improvements in perioperative care44,45 as well as staging and selection.46,47 Unfortunately, limitations of the NIS database prevent analysis of the effect of tumor stage on outcomes.
Case volume requirements have been determined by various organizations, such as the Leapfrog Group. To meet the standards of this group, at least 13 esophagectomies must be performed by an institution per year. According to the newest Leapfrog criteria, certain nonesophagectomy operations can also be counted toward esophagectomy, such as total gastrectomy and radical gastrectomy.11 As described above, by excluding operations performed for primary gastric diagnoses such as gastric malignancy or gastric ulcer disease, we have minimized the possibility of inclusion of any cases other than esophageal resection in our study group. Thus, the cohort we reviewed is equally sensitive and more specific for esophagectomy than that used by other groups. The most striking feature of these data is the beneficial effect evident for each and every increment in annual hospital case volume. Nearly every measured complication was seen to significantly improve with increasing annual volume, at least to volumes seen in nonoutlier hospitals. This has now been demonstrated in a very large administrative database, without recourse to artificial case volume groups. It appears that there may be a reversal of these positive volume–outcome associations when hospital volume exceeds 100 cases per year, with some evidence for rising mortality rates. However, the sample size of these very-high-volume hospitals is very small, which limits interpretation of this interesting and never previously reported finding. We plan further investigation to determine whether this effect is true or perhaps a consequence of case mix or other uncaptured variables.
It is becoming more evident that volume criteria are not the sole determinant of outcome.48,49 Even with equally experienced surgeons in a high-volume hospital, a variable that differs widely between institutions is the composition of the other members of the surgical team. No previous study has evaluated the effect of fellowship programs or general surgical residencies on outcomes after esophageal resection. If hospital case volume is used as a surrogate for the experience and capabilities of the perioperative team, particular scrutiny should be given to the effect of training programs, which involve multiple and variably rotating trainees in perioperative care of patients and which may sacrifice case volume for educational focus and academic inquiry.
We have identified an overall independent beneficial effect of a fellowship program in hospitals performing esophagectomies. If any fellowship program exists (thoracic, FC or SSO) at a particular hospital, the total numbers of complications decrease, though there is no way to verify from the NIS data whether the esophagectomies were performed by fellowship-affiliated surgeons. This limitation is probably more relevant with the Fellowship Council programs than the SSO or thoracic surgery programs, since the former places emphasis on minimally invasive gastrointestinal surgery and not necessarily surgery for malignancy in the chest. Of much more interest is the apparent increase in the serious adverse events of anastomotic leak, myocardial infarction, and tracheostomy associated with fellowship programs. While we intend to examine this further in future studies, our current hypothesis for the association between anastomotic leaks and fellowships is that, compared with private practice and resident training programs, the fellowship model puts trainees in the position of operating surgeon at crucial stages of an esophagectomy procedure. This complication is not associated with a greater death rate, perhaps as a consequence of better detection and management in these fellowship sponsoring hospitals, but clearly this is an area requiring further investigation.
Outcomes do not clearly stratify along surgical specialties. In this study, hospitals with fellowship programs administered by the Fellowship Council and the SSO had very similar outcomes, whereas a few outcomes, namely rates of incidental splenectomy and rates of bacterial pneumonia, were comparatively better in thoracic surgery fellowship program hospitals. Bias may have been introduced by the small sample size of SSO training hospitals, a result of nonreporting of many of such programs to the NIS. That said, surgeons identifying themselves as thoracic surgeons have been shown to have improved outcomes over those identifying as general surgeons,50 especially in low-volume centers.
In contradistinction to fellows, residents are usually supervised to a far greater degree during operations. This supervision has been thought to be the major means for ensuring safe outcomes in a teaching environment.31 In this study, we have shown that an ACGME-accredited general surgical residency program independently improves many of the measured complications, including rates of any complications, in-hospital mortality, acute renal failure, acute respiratory failure, and bacterial pneumonia. It has previously been reported that high-volume centers may minimize the effect of complications by earlier detection and more appropriate management.25 It is possible that the factor which enables earlier detection of problems is the presence of a strong residency program. The authors hypothesize that a larger house staff permits more frequent physician–patient contact and earlier management of adverse events. Higher deep venous thrombosis rate is reported, and this might be due to either longer operation time with resident training, or because of improved detection by residents in the postoperative period, a consequence of the aforementioned increases in contact with the patient.
Limitations exist in searches of administrative databases related to the accuracy of data entry by institutional coders. The accuracy of coding has previously been reported as suboptimal,51 though the detection of the presence of a particular diagnosis (as performed in this study) has been validated.52 It is conceivable that the programs with an active surgical residency might have better entry into the medical record of complications, with disproportionate capture of these measured outcomes in this group.53 Also, many hospitals are not represented in the NIS cohort, including some of the higher-volume esophagectomy centers in the USA. While attempts have been made to control for this statistically, a larger sample will always provide more accurate representation of the population as a whole. Finally, length of stay was considered by the authors as a variable dependent on the number and severity of complications and so was not used as a control variable in the mathematical modeling. It is, however, conceivable that length of stay is at least partly independent insomuch as the longer a patient remains in hospital the more time is available to capture complications for inclusion in the NIS.
Conclusion
The current 7% esophagectomy mortality rate of hospitals reporting to the Nationwide Inpatient Sample has improved but without evidence for measurable centralization of cases within high-volume centers. This rate remains higher than that reported in most contemporary series. In this model, the hypothesized positive volume–outcome relationship of esophageal surgery has been validated without the use of arbitrarily assigned case volume categories. This volume-related improvement in mortality is seen to taper with approximately 30–40 annual cases and may reverse in the highest-volume centers. The performance of esophageal resections in training hospitals is safe and with no increase in either mortality or total morbidity, though fellowship training may be associated with a higher anastomotic leak rate.
References
Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998–2003. Int J Cancer 2008;123(6):1422–1428.
Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK (eds). SEER Cancer Statistics Review, 1975–2006. Bethesda: National Cancer Institute. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2005 Incidence and Mortality Web-Based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2009. Available at: www.cdc.gov/uscs.
Luft HS, Bunker JP, Enthoven AC et al. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301(25):1364–3649.
Miller JD, Jain MK, de Gara CJ et al. Effect of surgical experience on results of esophagectomy for esophageal carcinoma. J Surg Oncol 1997;65(1):20–21.
Gordon TA, Bowman HM, Tielsch JM et al. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg 1998;228(1):71–78.
Birkmeyer JD, Finlayson SR, Tosteson AN et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 1999;125(3):250–256.
Pal N, Axisa B, Yusof S et al. Volume and outcome for major upper GI surgery in England. J Gastrointest Surg 2008;12(2):353–357.
Alderson D. Scottish Intercollegiate Guidelines Network (SIGN) 87—the management of oesophageal and gastric cancer. Clin Oncol (R Coll Radiol) 2008;20(7):530–531.
National Comprehensive Cancer Network. Clinical practice guidelines in oncology: esophageal cancer. Fort Washington: National Comprehensive Cancer Network, 2009.
The Leapfrog Group. Evidence-Based Hospital Referral (EBHR) and Common Acute Conditions (CACs) Coding Specifications for Volume v5.1.1. Washington, DC: The Leapfrog Group, 2008.
The Leapgfrog Group Factsheet: Evidence-Based Hospital Referral http://www.leapfroggroup.org/media/file/FactSheet_EBHR.pdf. Accessed May 3, 2009.
Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery 2004;135(6):569–575.
Allareddy V, Konety BR. Specificity of procedure volume and in-hospital mortality association. Ann Surg 2007;246(1):135–139.
Christian CK, Gustafson ML, Betensky RA et al. The Leapfrog volume criteria may fall short in identifying high-quality surgical centers. Ann Surg 2003;238(4):447–455. discussion 455–7.
van Lanschot JJ, Hulscher JB, Buskens CJ et al. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91(8):1574–1578.
Dimick JB, Cowan JA Jr., Ailawadi G et al. National variation in operative mortality rates for esophageal resection and the need for quality improvement. Arch Surg 2003;138(12):1305–1309.
Birkmeyer JD, Siewers AE, Finlayson EV et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346(15):1128–1137.
Forshaw MJ, Gossage JA, Stephens J et al. Centralisation of oesophagogastric cancer services: can specialist units deliver? Ann R Coll Surg Engl 2006;88(6):566–570.
Migliore M, Choong CK, Lim E et al. A surgeon’s case volume of oesophagectomy for cancer strongly influences the operative mortality rate. Eur J Cardiothorac Surg 2007;32(2):375–380.
Yannopoulos P, Theodoridis P, Manes K. Esophagectomy without thoracotomy: 25 years of experience over 750 patients. Langenbecks Arch Surg 2009;394:611–616.
Decker G, Coosemans W, De Leyn P et al. Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg 2009;35(1):13–20. discussion 20–1.
Collins G, Johnson E, Kroshus T et al. Experience with minimally invasive esophagectomy. Surg Endosc 2006;20(2):298–301.
Law S, Wong KH, Kwok KF et al. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg 2004;240(5):791–800.
Metzger R, Bollschweiler E, Vallbohmer D et al. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus 2004;17(4):310–314.
Begg CB, Cramer LD, Hoskins WJ et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280(20):1747–1751.
Dimick JB, Cattaneo SM, Lipsett PA et al. Hospital volume is related to clinical and economic outcomes of esophageal resection in Maryland. Ann Thorac Surg 2001;72(2):334–339. discussion 339–41.
Patti MG, Corvera CU, Glasgow RE et al. A hospital’s annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg 1998;2(2):186–192.
Kuo EY, Chang Y, Wright CD. Impact of hospital volume on clinical and economic outcomes for esophagectomy. Ann Thorac Surg 2001;72(4):1118–11124.
Dimick JB, Wainess RM, Upchurch GR Jr et al. National trends in outcomes for esophageal resection. Ann Thorac Surg 2005;79(1):212–216. discussion 217–8.
Rohatgi A, Sutcliffe R, Forshaw MJ et al. Training in oesophageal surgery—the gold standard: a prospective study. Int J Surg 2008;6(3):230–233.
HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 1998–2006. Rockville: Agency for Healthcare Research and Quality. www.hcup-us.ahrq.gov/nisoverview.jsp.
The Fellowship Council website. Directory of Fellowships https://fellowshipcouncil.org/directory/browse2.php. Accessed September 11, 2008.
National Resident Matching Program. Website http://www.nrmp.org/fellow/match_name/thoracic/prev.html. Accessed April 29, 2009.
The Society of Surgical Oncology. Website http://surgonc.org/default.aspx?id=60#1. Access April 29, 2009.
American Medical Association website. FREIDA Online GME program search results. https://freida.ama-assn.org/Freida/user/programSearchSubmitDispatch.do. Accessed November 26, 2008.
Accreditation Council for Graduate Medical Education. website http://www.acgme.org/adspublic/. Access Novermber 26, 2008.
Healthcare Cost and Utilization Project (HCUP). 1998–2006 [database online]. Insert City of Publication Here see notes: Agency for Healthcare Research and Quality, Rockville, MD.
Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA 2005;294(15):1909–1917.
Scottish Intercollegiate Guidelines Network. Scottish Intercollegiate Guidelines Network (SIGN) 87—the management of oesophageal and gastric cancer: A national clinical guideline. Edinburgh: SIGN, 2006.
Kohn GP, Galanko JA, Overby DW et al. Volume–outcome relationships and other influences of outcome in bariatric surgery. Surg Endosc 2009;23(S1):149.
Urbach DR, Austin PC. Conventional models overestimate the statistical significance of volume–outcome associations, compared with multilevel models. J Clin Epidemiol 2005;58(4):391–400.
Birkmeyer JD, Finlayson EV, Birkmeyer CM. Volume standards for high-risk surgical procedures: potential benefits of the Leapfrog initiative. Surgery 2001;130(3):415–422.
Muller JM, Erasmi H, Stelzner M et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990;77(8):845–857.
Whooley BP, Law S, Murthy SC et al. Analysis of reduced death and complication rates after esophageal resection. Ann Surg 2001;233(3):338–344.
Gananadha S, Hazebroek EJ, Leibman S et al. The utility of FDG-PET in the preoperative staging of esophageal cancer. Dis Esophagus 2008;21(5):389–394.
Tomaszek S, Cassivi SD. Esophagectomy for the treatment of esophageal cancer. Gastroenterol Clin North Am 2009;38(1):169–181. x.
Wouters MW, Wijnhoven BP, Karim-Kos HE et al. High-volume versus low-volume for esophageal resections for cancer: the essential role of case-mix adjustments based on clinical data. Ann Surg Oncol 2008;15(1):80–87.
Rodgers M, Jobe BA, O’Rourke RW et al. Case volume as a predictor of inpatient mortality after esophagectomy. Arch Surg 2007;142(9):829–839.
Leigh Y, Goldacre M, McCulloch P. Surgical specialty, surgical unit volume and mortality after oesophageal cancer surgery. Eur J Surg Oncol 2009;35:820–825.
Quan H, Li B, Saunders LD et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008;43(4):1424–1441.
Lacasse Y, Montori VM, Maltais F. Administrative database: validity of recording vs. validity of diagnosis. J Clin Epidemiol 2006;59(1):104. author reply 104–5.
Kohn GP, Galanko JA, Overby DW et al. Recent trends in bariatric surgery case volume in the United States. Surgery 2009;146(2):375–380.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Geoffrey Paul Kohn, presenter (University of North Carolina at Chapel Hill, NC, USA).
Discussant
Dr. Jeff Peters (Rochester): This is about the esophagectomy outcome relationships that have been hammered home over the past few years. As you will see a little bit later today, the other side of that equation is the people that do not get resected, and there are a fair number of them. In fact, your observation that the mortality went down—it plateaued, I should say, at about 30 cases per year. Interestingly, this mirrors the number that is true in high resection prevalence institutions as well, and of course, that is much higher than the leapfrog criteria which is 13 and the five per year that have been talked about, and I think a more realistic number.
A couple of questions for you, perhaps one observation and a couple of questions. You interestingly showed that there is no change in the number of esophagectomies per year, and of course you started off we have the thesis that the prevalence of this cancer has increased dramatically over the last 10 or 15 years. Does that imply that we are not operating on the growth in this disease, or the relative proportion of patients that are coming to surgery is less?
You also showed that there was a shift in the number of high-volume hospitals that took care of these patients. So slowly over the decade, I think that you studied, more patients were taken care of in high-volume hospitals. You showed almost a 50% reduction in mortality, 12% to 7%, although you highlighted the 7% as still too high, which is true.
How much of that decrease—that 50% decrease in mortality—was due to that shift? Did you do that analysis?
Lastly, I just quibble with one of your conclusions. You said surgical residents are safe. You showed that resident hospitals were safe. You have no data on who actually did the operation. So, you might want to clarify that a little bit.
Closing Discussant
Dr. Geoffry Paul Kohn (University of North Carolina at Chapel Hill, NC): Thank you, Dr. Peters, for your comments. Addressing the first, the increasing prevalence of esophageal cancer is indeed a real phenomenon, though there is no doubt that the numbers of esophagectomies performed in this country have been relatively stable over the study period.
Data I presented showed the decreasing mean comorbidity score over the period. I think this probably highlights the improved patient selection criteria that we have. Unfortunately, these types of national administrative databases do not have provide any indication as to whether the patients have undergone neoadjuvant therapy. We also do not have very good staging information. However, with the decreasing comorbidity scores, I think we do show that we currently have better or at least more restrictive patient selection, and I think that is the reason that the total case numbers have not increased.
Regarding the cause for the decrease in mortality, we have demonstrated that up to somewhere around 80–100 annual cases, each and every esophagectomy performed in a specific center will improve in-hospital mortality. We did not specifically control for the number of hospitals in each case volume group, but we would expect the observed decrease in mortality to have resulted at least in part from a shift to higher-volume centers.
With regards to the residency point, I completely agree. Again, administrative databases can only determine hospitals in which fellowship programs or residency program exist. No data are available about who actually performed the operation. Your comment is valid and it applies both to the residency institutions and the fellowship institutions.
Our hypothesis, which we have actually started investigating further in a new study, is that the actual vital technical components of the procedure, for example, the construction of the anastomosis, are probably being performed more times by the fellow than by the residents in those institutions. A resident is also probably more strictly supervised by the attending staff. We do not have anything yet to back it up, but I think that is least a possible explanation about data.
Discussant
Dr. Tom Demeester (USC, Los Angeles (Los Angeles, CA)): Dr. Kohn, thank you for the opportunity to review the preprinted manuscript. It is well written and I compliment you for getting the prize for the best manuscript of the meeting.
Your study is based on administrative data with all the shortcomings that are associated with such a database. Yet, you have been able to use the data to help clarify some of the issues regarding esophagectomy for the treatment of esophageal cancer. I have four questions.
Your basic theme has been supportive of other prior investigations that greater hospital volume is related to better outcome. At the recent American Surgical Meeting, a paper by Birkmeyer’s group at Michigan suggests that hospitals with large volumes have better outcomes because they are more able to rescue patients from complications. Does your data provide any evidence that high-volume hospitals have better services to allow a better capacity to rescue patients with complications? For instance, surgical intensivists as opposed to medical intensivists, dedicated esophageal anesthesiologists, 24-h availability of interventional radiologists, 24-h operating room availability for surgical therapy of complications, and 24-h surgical endoscopy support to name a few.
My second question focuses on your observation that the survival associated with increasing volume improves to a point, up to about 100 cases. Do you conclude that a hospital will go beyond a safe limit if it exceeds 100 cases per year? In other words, there is an upper limit to the benefit of volume.
My third question regards your statement that overall mortality is going down from roughly 12% to 7%. In the manuscript, you did not show that the reduction was across the board. Was it only due to the effect of the improved mortality in the high-volume hospitals? What happened to mortality in those hospitals that did less than 13, between 13 and 20, and over 20?
The key part of the operation is the esophagogastric anastomosis. Was anastomotic breakdown and sepsis more common in hospitals with resident or fellow? I believe you stated, the leak rate was significantly higher, in fact 50% higher in hospitals with training programs. Further, tracheostomies were more common in teaching hospitals which may be a surrogate for a greater complication rate. If this is true, is it correct to conclude that house officers and teaching programs do not alter safety? Could you comment on this?
My last question deals with where are all these studies going? We continue to talk about high-volume hospitals have better outcomes. Will a point come when organized surgical societies of surgery will recommend criteria for hospitals in order to perform esophagectomies? That completes my questions. I enjoy reading the paper. It was very thought provoking.
Closing Discussant
Dr. Geoffrey Paul Kohn: I think your first and fourth questions are very closely related. The first one was about whether rescue of complications are better at high-volume hospitals and whether the anastomotic leak rates are a concern in fellowship and residency offering hospitals.
Dr. Birkmeyer’s group at that meeting did report that, while total complication rates can be similar in high-volume institutions as compared to lower-volume institutions, the outcomes are often superior, probably because of earlier detection and better management. I think that is exactly what our data show. We do show that higher anastomotic leak, and we do show higher risk of certain complications. Some of that might be selection bias because of more attention being paid by the residents in training hospitals, for example, to myocardial infarction. But we do have higher rates of leak, though it does not affect the mortality.
I think there is an improvement in the management of the complications at some of these big institutions. That is the main focus of our next paper that we are in the process of drafting—to look at the outcomes following the index complication.
The second question is, are we doing too many cases? We came into this with the hypothesis that the more cases you did, the better. We discovered that U-shaped curve and we thought that perhaps there was a problem with our analysis; perhaps, the high-volume institutions are choosing more difficult cases. The Charlson Index is a validated comorbidity score, but the specific validation for esophageal cancer has not been attempted. However, since our results have come out, I have had correspondence with surgeons at some of the larger volume institutions. It seems, anecdotally, that this U-shaped curve is a real phenomenon. I am told that when their institutions are ramping up case volume for the first 2 or 3 years, they are noticing a higher morbidity–mortality rate. They think it is probably due to an inability of the facility to accommodate the large increase in volume. It may also be a staffing or personnel issue. The increased mortality seems to settle down over a few years. This is an interesting phenomenon which has not previously been reported and requires further study.
With regard to your case volume groups question, I think ours is a very powerful model using logistic regression with no artificially allocated case volume groups. We show that, up to a point, each and every single esophagectomy does cause a benefit.
The last question, why are we doing this and what is the likely outcome of this? I think centralization of care is probably going to be forced on us from external regulators to a certain degree. This is already occurring for example in the UK. However, I think we have to be very wary of volume being the only criterion. I think the volume we are using is only a surrogate marker for quality. There are other effects on quality. Additionally, we have to be very aware that by moving cases to high-volume institutions, we are usually moving them to seats of surgical training, and therefore, we have to look at both the effect of and the effect on our educational training system for surgeons.
No grant support was received in the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Kohn, G.P., Galanko, J.A., Meyers, M.O. et al. National Trends in Esophageal Surgery—Are Outcomes as Good as We Believe?. J Gastrointest Surg 13, 1900–1912 (2009). https://doi.org/10.1007/s11605-009-1008-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-1008-2