Abstract
Introduction
The purpose of our study was twofold: (1) to determine the incidence, patient and tumor characteristics, and outcome of patients with gastrointestinal carcinoid tumors using the Surveillance, Epidemiology and End Results (SEER) database, and (2) to delineate the expression pattern of growth factor receptors (GFRs) in carcinoid tumors.
Materials and methods
The SEER database search provided information on patients diagnosed with carcinoid tumors from 1990 to 2002. Carcinoid tumor sections (n = 46) were stained for the GFRs: epidermal growth factor receptor, insulin-like growth factor receptor (IGFR), vascular endothelial growth factor receptor (VEGFR), and HER-2/neu.
Results
Over the 12-year analysis period, 18,180 patients were identified with carcinoid tumors of the foregut, midgut, and hindgut; the incidence of carcinoid tumors increased ∼2-fold during this time period. Of the patients with carcinoid tumors, there was a trend of increased expression of VEGFR and IGFR, particularly in the foregut and midgut carcinoids. Analysis of the SEER database confirms that the incidence of carcinoid tumors is increasing with an approximate doubling in the number of carcinoid cases from 1990 to 2002. Furthermore, an increase in VEGFR and IGFR expression suggests that GFR inhibitors may be effective adjuvant therapy for carcinoid cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinoid tumors are uncommon, slow-growing neuroendocrine neoplasms arising from the enterochromaffin cells of the gut.1 Although uncommon, carcinoids are increasing in incidence at a rate greater than other cancers.2 Due to the indolent nature of carcinoid cancers, these tumors are usually not detected until after the development of metastases or intestinal fibrosis.3 Patients with metastatic disease may present with carcinoid syndrome, a set of symptoms including flushing, diarrhea, bronchospasm, and hypotension, or with manifestations of peritumoral and distant fibrosis.4 Common amine and peptide products secreted from carcinoids include serotonin, chromogranin A, and neurotensin.5
Currently, surgical resection is the only treatment with the possibility of achieving a cure and remains the mainstay of treatment for all patients with primary carcinoid tumors.6 Although there are ongoing research and clinical trials aimed at increasing survival of patients with metastatic disease, there has been no significant increase in survival in the past decade due, in part, to the lack of response to standard chemotherapeutic treatments.7 Somatostatin analogues have commonly been used for symptomatic relief of carcinoid syndrome after the development of hepatic metastasis.8 We have previously shown that administration of the somatostatin analogue octreotide significantly decreases hepatic metastasis using an in vivo mouse model, suggesting an antiproliferative effect of octreotide.9 Current combination chemotherapeutic regimens with streptozocin, 5-fluorouracil, and doxorubicin are commonly used in the treatment algorithms of highly proliferating carcinoid tumors.10
The lack of in vitro and in vivo model systems for carcinoid tumors has limited our understanding of the progression of this disease. We are fortunate to have established the novel carcinoid cell line BON, derived from a pancreatic carcinoid metastasis.11 We have utilized the BON cell line to delineate signaling pathways regulating carcinoid cell growth and secretion.9,11 BON cells express growth factor receptors (GFRs), including epidermal growth factor receptor (EGFR) and HER-2/neu, that may contribute to the development and sequelae of carcinoid tumors.12,13 Recently, using the BON cell line, we developed a novel in vivo model of carcinoid syndrome which recapitulates many of the clinical sequelae noted in humans and determined that treatment with the vascular endothelial growth factor receptor (VEGF) inhibitor bevacizumab significantly inhibited tumor growth.14
Alterations in GFR expression have been linked to an increased risk of neoplastic transformation.15 Overexpression of HER-2/neu occurs in several cancers such as ovarian, stomach, breast, and aggressive forms of uterine cancer.16,17 With ligand binding, EGFR stimulates intrinsic intracellular protein-tyrosine kinase activity which results in autophosphorylation of tyrosine residues. Downstream signaling proteins then initiate several signal transduction cascades, including the MAPK, phosphoinositide 3-kinase (PI3K), and JNK pathways, which are involved in important functions such as DNA synthesis and cell proliferation.18,19 Insulin-like growth factor receptor (IGFR) is another receptor-tyrosine kinase that plays a critical role in cell survival and proliferation.20 IGFR binding to its ligand activates the same pathways as EGFR to promote cell proliferation and suppress apoptosis.21,22
In the current study, we analyzed carcinoid tumor incidence using The Surveillance, Epidemiology and End Results (SEER) registry database of the National Cancer Institute and compared this to our institutional incidence. Furthermore, we analyzed the expression of various GFRs known to be involved in cancer development, including VEGFR, EGFR, IGFR, and HER-2/neu in a set of carcinoid tumors from our institutional tumor bank as well as from commercial tissue arrays.
Materials and Methods
Materials
Rabbit monoclonal anti-chromogranin, anti-synaptophysin, anti-VEGFR, anti-IGFR, anti-EGFR, anti-platelet-derived growth factor receptor (PDGFR), and anti-HER-2/neu antibodies were purchased from Cell Signaling (Danvers, MA, USA). Carcinoid tissue arrays were purchased from Biomax (Rockville, MD, USA). Immunostaining was performed using a DAKO EnVision Kit (Carpinteria, CA, USA).
Study Design
The histopathology and clinical course of patients undergoing carcinoid resection from 1986 to 2006 at The University of Texas Medical Branch (UTMB) were retrospectively analyzed. UTMB Institutional Review Board approval was obtained for the collection of patient data, tissue acquisition, and subsequent use. A comprehensive search of the medical records was first performed using ICD-9 Common Procedure Terminology codes for “carcinoid,” “malignant carcinoid,” “carcinoid syndrome,” and “neuroendocrine tumor.” Histopathology reports were then obtained for all patients during the specified time period. Patients with a pathologically confirmed diagnosis of carcinoid (typical or atypical) were then entered into the UTMB Carcinoid Database. Demographic data (e.g., age, gender, race), tumor–node–metastases (TNM) stage, lymph node status, presence of distant metastasis, and presence or absence of synchronous lesions was collected for all patients. For tissue analysis, paraffin-embedded blocks of resected carcinoid tissue were obtained from 20 UTMB patients with carcinoid tumors. Blocks were sectioned for immunohistochemistry.
SEER Database
The National Cancer Institute’s SEER-9 program was used to collect national data on carcinoid incidence, patient age and gender distribution, tumor histology, TNM stage, lymph node status, and tumor size for the years 1990–2002. SEER data are compiled from population-based cancer data from nine cancer registries in geographically distinct areas of the USA (five states: Connecticut, Hawaii, Iowa, New Mexico, and Utah; four cities: Atlanta, Detroit, San Francisco, and Seattle).23 Population data for additional statistical analysis was obtained from the 2000 US Census Bureau (available at: www.census.gov).
Immunohistochemistry
Paraffin-embedded carcinoid blocks were sectioned (5 μm) and deparaffinized in xylene and rehydrated in a descending ethanol series. Immunostaining was performed using a DAKO EnVision Kit (Dako Corp) as we have described previously.24 Briefly, sections were incubated overnight at 4°C with monoclonal antibodies diluted 1:100 in 0.05 M Tris–HCL with 1% bovine serum albumin against anti-chromogranin A, anti-synaptophysin, anti-EGFR, anti-VEGFR, anti-IGFR, and anti-HER-2/neu antibodies (Cell Signaling). After three washes with Tris-buffered saline Tween-20 (TBST), sections were incubated for 30 min with secondary antibody labeled with peroxidase, then washed three times with TBST. Lastly, peroxidase substrate diaminobenzidinetetrahydrochloride was added for staining. All sections were counterstained with hematoxylin and observed by light microscopy. All specimens were reviewed by a pathologist in a blinded fashion.
Statistical Analysis
Differences in GFR expression were assessed using Pearson chi-square test. Comparison of UTMB and SEER data was tested using the Pearson chi-square test for GFRs, gender, race, and tumor stage. The median test was used for age, presence of positive lymph nodes, and synchronous lesions. Association between carcinoid location and data set (UTMB or SEER), controlling for gender or race, was assessed using the Cochran–Mantel–Haenszel test. Associations between carcinoid tumors and GFRs were assessed using the Pearson chi-square test for gender, tumor stage, presence of positive lymph nodes, presence of synchronous lesions, and using the median test for age and tumor size. All statistical computations were carried out using SAS statistical software (release 9.1; SAS Institute).
Results
UTMB Patient Demographics
Between January 1986 and December 2006, 44 patients had resections performed for gastrointestinal carcinoid tumors at UTMB. There was a total of 21 women (47.7%) and 23 men (52.3%), with a mean age of 59 years (range 34–77 years). The average age was 59.3 years with a standard deviation of 11.7 years. Caucasians represented 75% of all patients, followed by African Americans (13.6%) and Hispanics (11.4%). The most common site of occurrence was the foregut (45.4%), followed by midgut (39.2%) and hindgut (9.1%). When carcinoid tumors were further divided by organ system, the small intestine was the most common site (36.4%), followed by the respiratory tract (31.8%), colorectal/anal (9.1%), stomach (6.8%), pancreas (6.8%), and appendix (2.3%). Of patients with disseminated disease at the time of presentation, 6.8% had an unknown primary tumor. Lymph node metastases were present in 29 patients (65.9%), nine patients (20.5%) had node-negative disease, and six patients (13.6%) had unknown nodal status. The majority of patients (54.5%) presented to UTMB with disseminated distant disease, and 25.5% of patients had localized or regional disease. Tumor stage was unknown in 4.5% of patients.
Comparison of UTMB Patients with the SEER Database
Demographics for patients with carcinoid tumors diagnosed at UTMB and those recorded in the SEER database are presented in Table 1. A search of the SEER database identified 18,180 patients with carcinoid tumors during the study period. The incidence of carcinoid tumors in the foregut, midgut, and hindgut increased ∼2-fold during this time period. The two populations had a similar ratio of male and female patients. Median age was slightly higher in SEER patients (62 versus 59; p = 0.09). Racial distribution was also noted to be similar between the two populations. UTMB had a slightly higher percentage of African-American and Hispanic patients compared to the SEER database. The majority of SEER patients presented with localized disease (48%); 23% of patients presented with distant disease. In contrast, ∼54.5% of UTMB patients presented with distant disease.
Comparison of GFR Expression in Carcinoid Tumors
Specific immunohistochemistry staining for carcinoid tissues using chromogranin A and synaptophysin verified that the tissue sections were carcinoid tumors. Associations between outcome measures, tumor location (foregut, midgut, or hindgut), patient age, and sex were assessed using the multiple logistic regression model. Hindgut carcinoids were excluded from the multiple logistic regression model due to very small sample size (n = 4).
Of 46 samples stained for immunohistochemistry, all stained positive for synaptophysin. Association between carcinoid location and gender are summarized for UTMB or commercial arrays in Table 2. A significant association was not observed between gender and carcinoid location adjusting for UTMB versus tissue array (p = 0.64). This indicates that the ratio of men to women from each carcinoid location was similar. However, a significant association was observed between UTMB or tissue array and location adjusting for gender (p = 0.005). This suggests that the number of samples from each carcinoid location were significantly different between UTMB and array tissues when adjusted for gender. The majority of tissues from tissue arrays were from the foregut, whereas tissues from UTMB patients were similarly distributed between foregut and midgut tumors.
Mean patient age for each carcinoid location with number of samples and standard deviation was analyzed. There were no significant differences in patient age compared with carcinoid location (p = 0.38); however, there was a statistically significant age difference between foregut and midgut carcinoid tumors among men at UTMB. The mean ages were 48 and 68 with a standard deviation of 12 and 9 years for foregut and midgut tumors, respectively (p < 0.05).
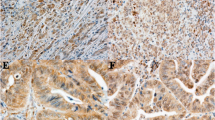
Representative tissue staining patterns from selected UTMB patients are shown in Fig. 1. Similar sections of carcinoid tumors with areas of normal tissue were chosen to illustrate changes in the expression of various proteins. These samples were representative of all specimens analyzed. Expression of CgA was observed in the selected foregut, midgut, and hindgut tissues. Strong EGFR expression was observed in lung carcinoids, whereas weak expression was observed in small-bowel sections. Lung, gastric, and small-bowel carcinoids all stained strongly positive for VEGFR expression. Weak VEGFR expression was also noted in hindgut carcinoids. Strong IGFR expression was found in lung, gastric, and small-bowel carcinoids, whereas weak expression was observed in hindgut carcinoids. HER-2/neu was expressed in all of the (Fig. 1) tissues except for hindgut carcinoids.
Expression of growth factor receptors in carcinoid cancer tissues. Immunohistochemical analysis of chromogranin A (CgA), epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), insulin-like growth factor receptor (IGFR), and HER-2/neu in representative carcinoid cancer tissues derived from the foregut, midgut, and hindgut (×4 magnification with ×10 inlay).
Comparison of receptor staining between UTMB patient samples and commercial tissue arrays is summarized in Table 3. Some carcinoid sections were unable to be stained due to tissue loss which is reflected in the number of stained tissues. Ninety percent of UTMB samples (18 of 20) stained positive for IGFR. On the other hand, 88% of array samples (22 of 25) were negative for IGFR expression. Twenty-nine percent of array samples (seven of 24) were HER-2/neu positive, while only 15% of UTMB samples (three of 20) stained positive for HER-2/neu . When all carcinoid tissues were categorized based on tissue location and receptor staining, VEGFR expression was fairly evenly distributed among foregut and midgut carcinoids, and all hindgut carcinoids exhibited positive expression (Table 4). IGFR expression was greater in midgut carcinoids compared to foregut carcinoids. Gender did not significantly influence the outcome of GFR expression (Table 5). Although not statistically significant (p = 0.10), men were ∼8 times less likely to have positive staining for EGFR. Patients less than 50 years of age were ∼9 times (p = 0.07) more likely to have positive staining for VEGFR than patients between 50 and 59 (Table 6). The odds ratio of exhibiting positive VEGFR staining in patients 60 years or older was almost identical to those between 50 and 59. Patients under age 50 years of age were ∼24 times more likely (p = 0.048) to have positive staining for IGFR than patients between the ages of 50 and 59. The odds ratio of patients 60 years or older demonstrating IGFR expression was almost identical to those between the ages of 50 and 59.
Discussion
In 1907, Siegfried Obendorfer, a German pathologist, coined the term “karzinoide” to describe a benign tumor resembling a carcinoma microscopically.25 In 1948, serotonin was identified from the Kulchitsky cell and later hypothesized to be the hormone responsible for carcinoid syndrome.26 Since this time, studies have attempted to determine targets for the treatment of carcinoid tumors. Recently, there has been growing interest in the use of GFR inhibitors in the treatment of carcinoid tumors. Endostatin, sunitinib, sorafenib, and bevacizumab are drugs that are under investigation and have shown promise.27–29 Although carcinoid tumors are mostly slow growing and indolent in nature, current chemotherapeutic options have not altered survival. Somatostatin analogues attenuate the symptoms of carcinoid syndrome, but their effects on tumor growth are controversial. Currently, platinum-based chemotherapy is not useful in these patients, and traditional single agents are only minimally effective in a small number of patients.30,31
Increased expression of GFRs has previously been demonstrated in neuroendocrine tumors and has been implicated as a possible mechanism for tumor development and progression. Carcinoid tumors are highly vascular in nature with many of these tumors exhibiting a marked desmoplastic reaction. Expression of VEGF has been demonstrated in both gastrointestinal and pulmonary carcinoids.32,33 Recently, overexpression of VEGF was found to promote the growth of human neuroendocrine tumors through the up-regulation of angiogenesis; bevacizumab significantly reduced tumor angiogenesis and impaired tumor growth in vivo.34 Similarly, our group found that treatment with bevacizumab significantly inhibited tumor growth using a novel in vivo metastasis model.14 Increased VEGF expression appears to correlate with metastases and decreased progression-free survival.35 In our current study, we demonstrated that carcinoid tumors from patients under 50 years of age were more likely to express VEGFR. These findings are intriguing and suggest that targeting VEGFR may represent a treatment option in a subset of patients with carcinoid tumors.
IGFR is implicated as an important component of growth factor signaling in neuroendocrine tumors.36 We found increased IGFR expression in UTMB patient tissues when compared to the commercial array tissues, which may be attributed to the increased number of midgut carcinoids in the UTMB patient population. Patients under 50 years of age were also more likely to have increased expression of IGFR. Exogenous IGF has been shown to activate mTOR and increase cellular proliferation in carcinoid cells.37 Due to the importance of IGFR signaling in carcinomas, it has become another possible target for kinase inhibitors. Our findings suggest that IGFR is important in carcinoid tumors, specifically in younger patients. In the future, this finding may lead to the investigation of patient-specific treatment therapies based on patient age. Although EGFR expression has been identified in carcinoid tumors of the gastrointestinal tract, we did not observe a significant trend in EGFR expression in our current study. Previously, EGFR and p-EGFR were found to be more highly expressed in small bowel carcinoid tumors compared with islet-cell tumors, and p-EGFR expression was associated with decreased survival among patients with pancreatic endocrine tumors.38 Even though previous studies have shown increased expression of PDGFR in carcinoid tumors, we did not detect PDGFR expression in carcinoid tumors from our patient population. In a phase-II study of imatinib, which is specific for the tyrosine kinase domain in PDGFR, there was no significant regression of carcinoid disease, but a significant number of patients with progressive disease did achieve disease stabilization.39 This suggests that although imatinib may not be a future first-line agent in the treatment of carcinoid tumors it may play a role in palliative treatment.
Slow-growing carcinoids are often indolent in nature, and like other cancers with a similar course, they are often discovered in advanced stages of the disease. Currently, complete operative resection is the only option for cure. Recent clinical trials evaluating the effectiveness of GFR inhibition show promise in disease stabilization, but more research in combination drug therapy is forthcoming. Our study highlights the importance of GFR signaling in carcinoid tumors, specifically VEGFR and IGFR, and provides evidence to support current investigations into the inhibition of these GFRs as a novel treatment strategy for carcinoids. Evaluation of the SEER database demonstrated a twofold increase in the number of carcinoids over the last decade. During that time, the most common site of occurrence was in the foregut followed by hindgut and midgut locations. When divided by gastrointestinal organ system, the colorectal location intestine was the most common site of occurrence. Although there has not been a significant change in survival, new information about the occurrence of GFRs in carcinoid tumors will provide novel treatment options in the future.
References
Kulke MH. Clinical presentation and management of carcinoid tumors. Hematol Oncol Clin North Am 2007;21:433–455; vii–viii.
Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–959.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072.
Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5–21.
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61–72.
Pinchot SN, Pitt SC, Sippel RS, Kunnimalaiyaan M, Chen H. Novel targets for the treatment and palliation of gastrointestinal neuroendocrine tumors. Curr Opin Investig Drugs 2008;9:576–582.
Modlin IM, Moss SF, Chung DC, Jensen RT, Snyderwine E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J Natl Cancer Inst 2008;100:1282–1289.
Srirajaskanthan R, Toumpanakis C, Meyer T, Caplin ME. Future therapies for management of metastatic gastroenteropancreatic neuroendocrine tumours. Aliment Pharmacol Ther 2009;29:1143–1154.
Evers BM, Townsend CM Jr., Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC, Thompson JC. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology 1991;101:303–311.
Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist 2008;13:1255–1269.
Evers BM, Ishizuka J, Townsend CM Jr., Thompson JC. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann N Y Acad Sci 1994;733:393–406.
Stilling GA, Zhang H, Ruebel KH, Leontovich AA, Jin L, Tanizaki Y, Zhang S, Erickson LA, Hobday T, Lloyd RV. Characterization of the functional and growth properties of cell lines established from ileal and rectal carcinoid tumors. Endocr Pathol 2007;18:223–232.
Gugger M, Burckhardt E, Kappeler A, Hirsiger H, Laissue JA, Mazzucchelli L. Quantitative expansion of structural genomic alterations in the spectrum of neuroendocrine lung carcinomas. J Pathol 2002;196:408–415.
Jackson LN, Chen LA, Larson SD, Silva SR, Rychahou PG, Boor PJ, Li J, Defreitas G, Stafford WL, Townsend CM Jr., Evers BM. Development and characterization of a novel in vivo model of carcinoid syndrome. Clin Cancer Res 2009;15:2747–2755.
Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest 2007;117:2051–2058.
Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int J Gynaecol Obstet 2008;102:128–131.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127–137.
Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol 2005;1:2005–2010.
Sharma PS, Sharma R, Tyagi T. Receptor tyrosine kinase inhibitors as potent weapons in war against cancers. Curr Pharm Des 2009;15:758–776.
Galvan V, Logvinova A, Sperandio S, Ichijo H, Bredesen DE. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1). J Biol Chem 2003;278:13325–13332.
Warshamana-Greene GS, Litz J, Buchdunger E, Garcia-Echeverria C, Hofmann F, Krystal GW. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res 2005;11:1563–1571.
Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, Knowlden JM, Williams S, Wakeling AE, Nicholson RI. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer 2004;11:793–814.
Program S. SEER program public-use data (1973–2003).Available at: http://seer.cancer.gov. Accessed: August 2007.
Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg 2006;243:833–842. discussion 843–834.
Obendorfer S. Karzinoide tumoren des dunndarms. Frankf Zschr Pathol 1907;1:426–430.
Erspamer V, Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 1952;169:800–801.
Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, Li JZ, Baum CM, Fuchs CS. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008;26:3403–3410.
Zitzmann K, De Toni EN, Brand S, Goke B, Meinecke J, Spottl G, Meyer HH, Auernhammer CJ. The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology 2007;85:54–60.
Yao JC, Hoff PM. Molecular targeted therapy for neuroendocrine tumors. Hematol Oncol Clin North Am 2007;21:575–581. x.
Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, Yao JC. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762–4771.
Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005;23:4897–4904.
Terris B, Scoazec JY, Rubbia L, Bregeaud L, Pepper MS, Ruszniewski P, Belghiti J, Flejou J, Degott C. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology 1998;32:133–138.
Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Oser SM, Khan MA, Jones RT, Harris CC. Vascular endothelial growth factor and nitric oxide synthase expression in human lung cancer and the relation to p53. Br J Cancer 1998;78:233–239.
Zhang J, Jia Z, Li Q, Wang L, Rashid A, Zhu Z, Evans DB, Vauthey JN, Xie K, Yao JC. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007;109:1478–1486.
Phan AT, Wang L, Xie K et al. Association of VEGF expression with poor prognosis among patients with low-grade neuroendocrine carcinoma. Annual Meeting of American Society of Clinical Oncology. Atlanta: American Society of Clinical Oncology, 2006.
Nilsson O, Wangberg B, Theodorsson E, Skottner A, Ahlman H. Presence of IGF-I in human midgut carcinoid tumours—an autocrine regulator of carcinoid tumour growth? Int J Cancer 1992;51:195–203.
von Wichert G, Jehle PM, Hoeflich A, Koschnick S, Dralle H, Wolf E, Wiedenmann B, Boehm BO, Adler G, Seufferlein T. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res 2000;60:4573–4581.
Papouchado B, Erickson LA, Rohlinger AL, Hobday TJ, Erlichman C, Ames MM, Lloyd RV. Epidermal growth factor receptor and activated epidermal growth factor receptor expression in gastrointestinal carcinoids and pancreatic endocrine carcinomas. Mod Pathol 2005;18:1329–1335.
Carr K, Yao JC, Rashid A et al. A phase II trial of imatinib in patients with advanced carcinoid tumor. Journal of Clinical Oncology 2004;22:4124.
Acknowledgments
This work was supported by grants RO1 CA104748, RO1 DK48498, PO1DK35608, R01 CA125454 (to BPZ) and T32DK07639 from the National Institutes of Health. KB is a recipient of a Jeane B. Kempner Scholar Award. The authors thank Karen Martin for manuscript preparation and Tatsuo Uchida for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kanika A. Bowen presented at the Society for Surgery of the Alimentary Tract on June 2, 2009, Chicago, IL.
Rights and permissions
About this article
Cite this article
Bowen, K.A., Silva, S.R., Johnson, J.N. et al. An Analysis of Trends and Growth Factor Receptor Expression of GI Carcinoid Tumors. J Gastrointest Surg 13, 1773–1780 (2009). https://doi.org/10.1007/s11605-009-0958-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-0958-8