Abstract
Background
The appropriate surgical intervention for sigmoidal esophagus in the setting of achalasia remains controversial. The objective of this study is to review our experience with minimally invasive myotomy (MIM) and minimally invasive esophagectomy (MIE) in the treatment of these patients.
Methods
We reviewed the records of 30 patients (19 men, 11 women); mean age 59.1 years (range 25–83 years) who underwent MIM (n = 24) or MIE (n = 6). Primary variables included perioperative and long-term outcomes. Univariate and multivariate analyses were performed to identify clinical variables predictive of myotomy failure.
Results
The operative mortality was zero and median hospital stay was 2 days (MIM) and 7 days (MIE). On follow-up (mean 30.5 months), nine (37.5%) patients undergoing primary MIM had failure requiring redo myotomy (n = 1) or esophagectomy (n = 8). Univariate analysis showed that previous myotomy and duration of symptoms were significant predictors of failure of MIM, with patient age trending toward significance. Multivariate analysis showed age and longer symptom duration to be significant.
Conclusions
MIM affords symptomatic improvement in many patients. Age and symptom duration may be preoperative indicators of MIM failure. MIE offers similar symptom relief but is associated with a longer hospital stay. Further prospective studies are required to define the optimum treatment algorithm in the management of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
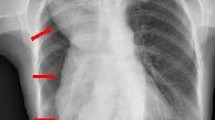
Achalasia is an idiopathic motility disorder of the esophagus characterized by aperistalsis of the esophageal body and failure of complete relaxation of the lower esophageal sphincter (LES) during deglutition.1 Treatment options are palliative in nature and are directed at decreasing the lower esophageal sphincter pressure (LESP) to promote enhanced esophageal emptying.2 Though Heller myotomy with partial fundoplication can achieve excellent long-term outcomes in 80–90% of patients with achalasia,3,4 the optimal therapy for patients with sigmoidal esophageal changes remains controversial (Fig. 1).
Several authors advocate myotomy as the primary treatment in patients with sigmoidal esophagus, reserving esophagectomy for myotomy failure and persistent symptoms.5,6 Others recommend primary esophagectomy, citing concerns regarding the capacity of a dilated, sigmoidal esophagus to empty efficiently even with a myotomy.7–9 The aim of the current study is to review our experience with minimally invasive myotomy (MIM) and minimally invasive esophagectomy (MIE) in the treatment of patients with achalasia and sigmoidal esophagus.
Materials and Methods
Patients
Approval for this study was provided by the Institutional Review Board of the University of Pittsburgh. We performed a retrospective review of 250 patients undergoing minimally invasive Heller myotomy or MIE for achalasia at the University of Pittsburgh from 1992 to 2007. Sigmoidal esophageal changes were identified in 19 male and 11 female patients with a mean age of 59.1 years (range 25–83 years) (Table 1).
Preoperative Evaluation and Therapy
The diagnosis of achalasia was confirmed by barium esophagram demonstrating the classic appearance of achalasia (proximal esophageal dilation with a distal “bird’s beak”) and esophageal manometry (aperistalsis; LES with incomplete relaxation) when possible. In addition, each patient demonstrated sigmoidal esophageal changes by barium esophagram in varying geometrical configurations and with varying degrees of associated esophageal dilation. Preoperative manometry data is available in only 11 patients. Manometry was not required or was unable to be performed in the remaining patients due to difficulty in positioning the probe within the tortuous esophagus or to patient intolerance. All patients had a preoperative esophagogastroduodenoscopy to confirm the absence of other esophageal pathology prior to surgery. The most common presenting symptom was persistent or progressive dysphagia (95%), followed by regurgitation. Dysphagia scores were assessed preoperatively and postoperatively utilizing the following scale: 1 = no dysphagia; 2 = difficulty with hard solids; 3 = difficulty with soft solids 4 = difficulty with liquids; 5 = cannot swallow saliva. Mean preoperative dysphagia score was 3.0. Mean duration of symptoms for the entire patient cohort was 18.7 years. Endoscopic therapy (balloon dilation, botulinum toxin injection, or both) was performed in 19 out of 24 (79.2%) patients prior to Heller myotomy and in six out of six (100%) patients prior to MIE. Prior myotomy had been performed in three (10%) patients—two in the myotomy group and one in the esophagectomy group.
Operative Technique
A minimally invasive approach was performed in all patients. Minimally invasive esophagomyotomy (±partial fundoplication) was performed in 24 patients as described previously.10 A partial fundoplication was performed in 22 out of 24 (91.7%) of the patients undergoing myotomy. In 17 (70.8%) patients, a posterior (Toupet) fundoplication was performed. An anterior (Dor) wrap was performed in five (20.8%) patients. Two patients (8.3%) underwent myotomy alone (Table 2). MIE was performed in six patients, as described previously.11
Postoperative Course
For MIM, patients were typically extubated in the operating room at the conclusion of the case. A barium swallow was performed on the first postoperative day and, if satisfactory, a clear liquid diet was initiated. Patients were typically discharged on the second postoperative day. Our current protocol is to evaluate the patients at 2 weeks for the first postoperative visit. Follow-up at 6 months, 1 year, and yearly thereafter is performed with repeat barium swallows. Manometry and esophageal transit studies are optional, based on surgeon and patient preference.
After MIE, patients remain nil per os with nasogastric tube decompression. Tube feeds are initiated at a low rate on postoperative day 3. A barium swallow is performed on postoperative day 5, after which a clear liquid diet is instituted. Patients are discharged home on a clear liquid diet and cycled night-time tube feeds. Patients are evaluated at 2 weeks, and their diet is advanced. The feeding tube is removed during their second postoperative visit if the diet is being tolerated well. Patients are then followed up yearly with repeat barium swallows.
Follow-Up
Follow-up data was successfully acquired in all patients. The primary postoperative outcome variables included length of stay, morbidity, mortality, and need for reoperation. Myotomy failure was defined as patients with no improvement in dysphagia score or those requiring reoperation (redo myotomy or esophagectomy). Postoperative dysphagia scores were assessed at each clinic or hospital visit and compared with preoperative values. Mean follow-up for all patients (MIM and MIE) was 25.3 months.
Statistical Analysis
Data were summarized with descriptive statistics (mean, standard deviation, median, and range for continuous variables, frequency, and percentage for categorical variables). A log rank test was used to assess the association between time to failure and age, duration of symptoms, previous myotomy, prior endoscopic treatment, and preoperative LESP. Univariate Cox regression model was used to assess the association between time to failure and age, duration of symptoms, and preoperative LESP as continuous variables, respectively. Multivariate Cox regression model was used to assess the association between time to failure and age, duration of symptom, and previous myotomy simultaneously.
Results
Perioperative Outcomes
Demographics and preoperative data are detailed in Table 1. Patients undergoing primary MIE were younger, had a higher rate of preoperative interventions, a lower LESP, and a higher incidence of “end-stage” sigmoidal megaesophagus on barium esophagram. None of these features attained statistical significance. Duration of symptoms and mean preoperative dysphagia scores were similar between the two groups. There were no perioperative deaths. MIM was associated with a similar improvement in symptoms compared with MIE, but had a significantly shorter length of hospital stay. There were trends towards less morbidity and reduced need for postoperative endoscopic interventions, though these did not attain statistical significance (Table 2). Complications were few and are detailed in Table 3. Pneumonia was the most common complication among the MIM group (8.3%). Complications in the MIE group included a contained anastomotic leak treated with opening of the cervical wound, a hemothorax necessitating drainage by video-assisted thoracoscopic surgery, and a pleural effusion.
Surgical Management of Sigmoidal Esophagus
From 1992 to 2007, 30 patients with sigmoidal esophageal changes in the setting of achalasia underwent surgical intervention. Among these, minimally invasive Heller myotomy was performed in 24 patients (Fig. 2). Partial fundoplication was performed in 22 (91.7%) of these patients (17 Toupet, five Dor). The remaining two patients underwent myotomy alone. Six patients received primary MIE. The principal criterion for primary esophagectomy was an end-stage sigmoidal esophagus in fit patients where long-term functionality of the esophagus was in doubt (Fig. 3). Among the 24 patients undergoing MIM, 15 patients (62.5%) had durable relief of dysphagia and required no further surgical intervention. Seven patients (29.2%) developed recurrent dysphagia and/or regurgitation, requiring subsequent esophagectomy. One patient required takedown of the partial wrap and extension of the myotomy, with improvement in her symptoms. One patient had no durable improvement after MIM and is considered a failure of therapy, but refused further surgical intervention and has been managed with periodic dilations (Fig. 2).
Analysis of Myotomy Failures
During follow-up (mean 30.5 months; range 0.3–105.4 months), nine patients (37.5%) undergoing MIM were considered clinical failures due to the need for subsequent operative intervention (n = 8) or lack of symptomatic improvement (n = 1). Mean time to myotomy failure was 18.4 months. Interestingly, there was no apparent correlation between preoperative radiographic findings and outcomes in either group. Similarly, there was no apparent correlation with surgical approach and rate of failure following myotomy. There were two failures with the Dor approach (40%), six with the Toupet approach (35.3%), and one with myotomy alone (50%).
A comparison of the clinical features between successful and failed myotomy is shown in Table 4. All patients who failed myotomy had prior endoscopic interventions. Both patients with a history of previous myotomy undergoing a redo myotomy failed and required subsequent esophagectomy. Myotomy failure was associated with a trend toward lower preoperative LESP (19.5 versus 33.8 mmHg) and longer duration of symptoms (24.8 versus 13.1 years) when compared with patients with successful outcomes after MIM (Table 4). Univariate analysis to assess the association between myotomy failure and age, duration of symptoms, prior myotomy, preoperative LESP, and prior endoscopic therapy was performed. Univariate analysis showed that previous myotomy and duration of symptoms were significant predictors of failure of MIM, with patient age demonstrating a strong trend toward significance. Multivariate analysis identified patient age and symptom duration as independent predictors of myotomy failure (Table 5).
Discussion
MIM has been established as a highly effective treatment modality in the management of patients with achalasia, with a greater than 80–90% long-term success rate.12,13 The development of sigmoidal esophageal changes in the setting of achalasia presents a unique set of challenges in surgical management. As achalasia progresses, the esophagus can progressively dilate and acquire tortuous undulations due to persistent obstruction to forward flow, retained foodstuff, and pressurization from swallowed air.8 These anatomic features further impair esophageal emptying and promote food retention and/or impaction, resulting in recurring dysphagia as well as regurgitation. The progressive nature of these findings has led some to believe that myotomy alone is unlikely to succeed long-term in patients with dilated, sigmoidal esophageal changes where a functionless esophagus fails to empty efficiently even after myotomy, promoting the risk of retention esophagitis, regurgitation, and carcinoma.9,14 From this perspective, esophagectomy provides a definitive “curative” solution for advanced or refractory achalasia with excellent relief of symptoms in the majority of patients.9,15,16
Concern is routinely raised regarding the morbidity and mortality of esophagectomy for benign disease. In experienced centers, mortality rates range from 1% to 5%.17 MIE is a particularly useful technique in the setting of advanced achalasia. Several technical concerns encountered during open esophagectomy for achalasia include difficulty encircling the dilated esophagus, deviation of the esophagus into the right chest, enlarged aortoesophageal arteries, and the adherence of the exposed esophageal submucosa to the adjacent aorta subsequent to myotomy.9 The enhanced visualization achieved with a laparoscopic/thoracoscopic approach can augment the successful management of each of these issues with acceptable morbidity and low mortality (1.4%).11
Several studies have shown, however, that esophageal resection may not be necessary in all patients with a sigmoid esophagus. Patti and colleagues evaluated the outcomes of seven patients with sigmoidal esophageal changes treated primarily with laparoscopic Heller myotomy and Dor fundoplication.5 They were able to achieve good–excellent results in 100% of the patients with complete resolution or significant improvement in dysphagia, regurgitation, and chest pain. None of the patients in their study required further operative intervention. Mineo and coworkers evaluated 14 patients with achalasic sigmoid esophagus treated with an open or minimally invasive Heller myotomy and Dor fundoplication by the same surgeon.6 In this series, ten out of 14 patients (71.4%) achieved good–excellent results with similar morbidities and hospital stays after the operation compared to those patients without a dilated esophagus. In addition, a substantial reduction in postoperative LESP and esophageal width was noted secondary to the relief of distal obstruction. Importantly, quality of life measures (SF-36) improved in all measured domains.
Currently, there are no randomized data available to definitively establish who should undergo primary myotomy or esophagectomy in the setting of advanced achalasia with sigmoid esophagus. The Practice Parameters Committee of the American College of Gastroenterology currently recommends graded pneumatic dilatation or laparoscopic myotomy as primary therapy for patients with achalasia (including early sigmoidal changes) and propose esophagectomy for those patients with megaesophagus (>8 cm) and those with low LESP with persistent symptoms.18 In addition to sigmoid esophagus, several preoperative features have been postulated to impact negatively upon the results of myotomy including low preoperative LESP,19,20 prior endoscopic therapy,21,22 and longer duration of symptoms.23 In the current series, each of these variables was seen more commonly in patients who failed primary myotomy (Table 4). Multivariate analysis confirmed age and duration of symptoms as independent risk factors for myotomy failure (Table 5).
Despite this, the majority (15 out of 24; 62.5%) of patients with sigmoidal esophagus in the present study achieved durable improvement in dysphagia and regurgitation, requiring no further operative intervention (redo myotomy or esophagectomy) at a mean follow-up of 30.5 months. In addition, myotomy was associated with reduced length of stay and a trend toward decreased morbidity compared with esophagectomy (Table 2). Similar to the observations of Mineo and associates,6 the relief of distal obstruction not only improved patient symptoms, but was associated with improved anatomic features (decreased width and curvature, improved emptying) of the esophagus on barium esophagram in several cases (Fig. 4).
Radiographic improvement after Heller myotomy and relief of distal esophageal obstruction (a, c preoperative; b, d postoperative). a Sigmoidal esophagus prior to Heller myotomy. b Improved emptying of sigmoidal esophagus immediately following Heller myotomy. c Significant dilation and sigmoidal changes prior to Heller myotomy. d Decreased distension and sigmoidal angulation, as well as improved emptying, 3.5 years after Heller myotomy.
Conclusion
Minimally invasive Heller myotomy can be performed safely in patients with sigmoidal esophageal changes and can be successful in many patients with this condition. Significant symptomatic improvement can be achieved in approximately two thirds of these patients, without need for further operative intervention. Younger patients with longer duration of symptoms are at higher risk for myotomy failure, and consideration should be given to primary esophagectomy in this setting. Failure of myotomy in the setting of sigmoidal esophageal changes is likely multifactorial in nature, however, and the decision to perform primary myotomy or esophagectomy should thus be individualized based on patient characteristics as well as surgeon experience and judgment. Further prospective studies with longer-term follow-up are required to define the optimal treatment algorithm in these patients.
References
Lendrum FC. Anatomic features of the cardiac orifice of the stomach with special reference to cardiospasm. Arch Intern Med 1937;59:474–511.
Spiess AE, Kahrilas PJ. Treating achalasia: from whalebone to laparoscope. JAMA 1998;280:638–642.
Richards WO, Torquati A, Holzman MD, Khaitan L, Byrne D, Lutfi R, Sharp KW. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective, randomized, double-blind clinical trail. Ann Surg 2004;240:405–415.
Patti MG, Fisichella PM, Perretta S, Galvani C, Gorodner MV, Robinson T, Way LW. Impact of minimally-invasive surgery on the treatment of esophageal achalasia: a decade of change. J Am Coll Surg 2003;196:698–705.
Patti MG, Feo CV, Diener U, Tamburini A, Arcerito M, Way LW. Laparoscopic Heller myotomy relieves dysphagia when the esophagus is dilated. Surg Endosc 1999;13:843–847.
Mineo TC, Pompeo E. Long-term outcome of Heller myotomy in achalasic sigmoid esophagus. J Thorac Cardiovasc Surg 2004;128:402–407.
Pinotti HW, Cecconello I, Mariano da Rocha J, Zilberstein B. Resection for achalasia of the esophagus. Hepatogastroenterology 1991;38:470–473.
Peters JH, Kauer WKH, Crookes PF, Ireland AP, Brenner CG, DeMeester TR. Esophageal resection with colon interposition for end-stage achalasia. Arch Surg 1995;130:632–637.
Devaney EJ, Lannettoni MD, Orringer MB, Marshall B. Esophagectomy for achalasia: patient selection and clinical experience. Ann Thorac Surg 2001;72:854–858.
Luketich JD, Fernando HC, Christie NA, Buenaventura PO, Keenan RJ, Ikramuddin S, Schauer PR. Outcomes after minimally-invasive esophagomyotomy. Ann Thorac Surg 2001;72:1909–1913.
Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS, Litle VR, Schauer PR, Close JM, Fernando HC. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238(4):486–494.
Patti MG, Pellegrini CA, Horgan S, Arcerito M, Omelanczuk P, Tamburini A, Diener U, Eubanks TR, Way LW. Minimally-invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg 1999;230(4):587–594.
Rosemurgy A, Villadolid D, Thometz D, Kalipersad C, Rakita S, Albrink M, Johnson M, Boyce W. Laparoscopic Heller myotomy provides durable relief from achalasia and salvages failures after BoTox or dilation. Ann Surg 2005;241:725–735.
Peracchia A, Segalin A, Bardini R, Ruol A, Bonavina L, Baessato M. Esophageal carcinoma and achalasia: prevalence, incidence and results of treatment. Hepatogastroenterology 1991;38:514–516.
Banbury MK, Rice TW, Goldblum JR, Clark SB, Baker ME, Richter JE, Rybicki LA, Blackstone EH. Esophagectomy with gastric reconstruction for achalasia. J Thorac Cardiovasc Surg 1999;117:1077–1085.
Miller DL, Allen MS, Trastek VF, Deschamps C, Pairolero PC. Esophageal resection for recurrent achalasia. Ann Thorac Surg 1995;60:922–926.
Schuchert MJ, Luketich JD, Fernando HC. Complications of minimally-invasive esophagectomy. Semin Thorac Cardiovasc Surg 2004;16(2):133–141.
Vaezi MF, Richter JE. Diagnosis and management of achalasia. Am J Gastroenterol 1999;94(12):3406–3412.
Arain MA, Peters JH, Tambankar AP, Portale G, Almogy G, DeMeester SR, Crookes PF, Hagan JA, Bremner CG, DeMeester TR. Pre-operative lower esophageal sphincter pressure affects outcome of laparoscopic esophageal myotomy for achalasia. J Gastrointest Surg 2004;8(3):328–334.
Torquati A, Richards WO, Holzman MD, Sharp KW. Laparoscopic myotomy for achalasia: predictors of successful outcome after 200 cases. Ann Surg 2006;243:587–593.
Smith CD, Stival A, Howell DL, Swafford V. Endoscopic therapy for achalasia before Heller Myotomy results in worse outcomes than Heller myotomy alone. Ann Surg 2006;243(5):579–586.
Portale G, Costantini M, Rizzetto C, Guirroli E, Ceolin M, Salvador R, Ancona E, Zaninotto G. Long-term outcome of laparoscopic Heller–Dor surgery for esophageal achalasia: possible detrimental role of previous endoscopic treatment. J Gastrointest Surg 2005;9(9):1332–1339.
Schuchert MJ, Luketich JD, Landreneau RJ, Kilic A, Gooding WE, Alvelo-Rivera M, Christie NA, Gilbert S, Pennathur A. Minimally-invasive esophagomyotomy in 200 patients: factors influencing post-operative outcomes. Ann Thorac Surg 2008;85:1729–1734.
Acknowledgements
The authors wish to acknowledge the important contributions of Diane Sabilla, Kathy Lovas, Theresa Krupka, and Darla Justus in database organization and management.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussion
969. Minimally Invasive Surgical Treatment of Sigmoid Esophagus in Achalasia. Paper presented by Matthew J. Schuchert, M.D., Pittsburgh, PA. E-mail: schuchertmj@upmc.edu
Discussion by Lee L. Swanstrom, M.D., Oregon
E-mail: lswanstrom@aol.com
Dr. L. Swanstrom (Portland, OR):
I would like to represent both some of my own questions as well as some comments by Blair Jobe, who was the original discussant and who is tied up at another session.
I would first like to compliment Dr. Schuchert and the folks from the University of Pittsburgh for their large series. It is one of the largest series that I presume will soon be in the literature and certainly represents their great experience in these very difficult cases. To recap, out of their 250 patients having minimally invasive surgery for achalasia, they had 30 who had a sigmoid esophagus, a very difficult end-stage finding. Of those, six had an esophagectomy as a primary treatment. There were several other salvage treatments of esophagectomy following myotomy. It should be noted that I think what is unusual with this is that the majority of these patients (25 out of 30) had previous treatment, which once again shows that this is an end-stage phenomenon.
Dr. Jobe commented that only 11 of these patients had preoperative manometry. He certainly stresses, and I concur, that preoperative motility testing is very valuable in these difficult cases. You have one-shot short of esophagectomy, so certainly every test that you can do would be good, including manometry and perhaps even pH testing if they have had previous myotomies or balloon dilatation to see if this could be related to GERD causing their failure.
Three questions were presented by Blair and then I have a couple of my own. One deals with manometry. How did you decide which approach to use as the primary therapy? In other words, what was your triage strategy in determining who went directly to esophagectomy versus myotomy? The second question is, please describe your technique for the myotomy. Was the myotomy carried down onto the stomach? Did you go to extra lengths carrying it proximally and distally? And how did you assess the completeness of the myotomy in the six failures that went on to esophagectomy? Three is, were any of the reinterventions after the primary therapy for GERD-related complications, particularly in those that did not have a fundoplication?
A couple of my own questions would be, do you advocate any techniques to correct the angulation of the distal esophagus? Do you perform an extended type II mediastinal dissection? And perhaps we should reconsider, especially if there were not GERD-related complications, not doing a fundoplication on these patients, because that, of course, adds a little bit of outflow resistance.
Thank you very much.
969. Minimally Invasive Surgical Treatment of Sigmoid Esophagus in Achalasia. Response by Matthew J. Schuchert, M.D., Pittsburgh, PA
Dr. Schuchert: Thank you very much, Dr. Swanstrom, for your insightful commentary and questions. We agree that preoperative manometry represents an important part of the work-up in patients in whom we are contemplating myotomy. Preoperative manometry was attempted in the majority of the patients undergoing myotomy in the current study. In cases where we could not get manometry, it was due to either difficulty with probe placement within a dilated, tortuous esophagus or due to patient refusal. We do agree that having all the information possible before making surgical decisions in these complex cases definitely is important and should be emphasized.
The principal determinant for deciding our approach, whether myotomy or esophagectomy, was somewhat subjective when you go back and review the charts and the thoughts of the individual surgeons. The most common reason was a dilated end-stage-appearing megaesophagus, where it was the opinion of the surgeon that is it was highly unlikely that a meaningful functional result could be achieved with myotomy alone. Esophagectomy was also considered in end-stage cases among younger patients with a longer expected life-span, who were deemed fit for esophagectomy. So those would be our main criteria, a burned-out mega esophagus and a younger, fit patient who might better tolerate primary esophagectomy. Going forward, what we have learned from the current analysis is that younger patients who have longer duration of symptoms (especially >15 years) may represent a high-risk group for long-term myotomy failure. Esophagectomy may be a reasonable option in these patients.
With regard to myotomy technique, after mobilizing the esophagogastric fat pad we extend our myotomy proximally at least 4–6 cm above the GE junction to reach a level of normal-appearing muscle. We pay particular attention to extending the myotomy onto the stomach by at least 1 to 2 cm, with very meticulous and careful dissection of the sling fibers of the proximal stomach. Intraoperatively we also perform endoscopy both before and after the myotomy to confirm visual release of the narrowed segment and abrogation of the associated “pop.” We routinely perform a partial fundoplication after completion of the myotomy.
The majority of failures following myotomy in this series were due to refractory dysphagia. With respect to GERD-related failures, there was one patient after primary myotomy who developed significant GERD with associated stricture, both subjectively and objectively confirmed, who ended up requiring subsequent esophagectomy.
We do feel that there is some importance in correcting the angulation of the esophagus intraoperatively, and we do spend a significant amount of time with our mediastinal dissection to try to straighten out the esophagus when possible. We feel that this approach may help to improve the dynamics of emptying, and may help to resolve the sink-trap effect that can be seen above the diaphragm in these patients.
Whether or not to add a fundoplication after myotomy in these patients can be debated given the severity of their disease. It is our practice to perform a partial fundoplication in these patients to minimize the risk of postoperative GERD symptoms, as highlighted in the prospective, randomized trial by Richards and associates (Ann Surg 2004;240:405–415).
969. Minimally Invasive Surgical Treatment of Sigmoid Esophagus in Achalasia. Paper presented by Matthew J. Schuchert, M.D., Pittsburgh, PA. E-mail: schuchertmj@upmc.edu
Discussion by Nathaniel J. Soper, M.D., Illinois
E-mail: nsoper@nmh.org
Dr. N. Soper (Chicago, IL):
That was a great talk. I will make one follow-up question to Lee, and that is, the patient who had the reflux-associated stricture and problems, had that patient had a fundoplication at the initial myotomy?
969. Minimally Invasive Surgical Treatment of Sigmoid Esophagus in Achalasia. Response by Matthew J. Schuchert, M.D., Pittsburgh, PA
Dr. Schuchert: Yes. What ended up happening was that patient had a partial fundoplication at the time of the initial operation. This was taken down and an attempted redo myotomy was performed which failed, and the patient ultimately went on to esophagectomy.
Dr. Soper: The other question is I believe there were seven patients who had the initial myotomy and then went on to esophagectomy. Was that subsequent esophagectomy made more difficult by the myotomy? In other words, is there much harm done by initially trying a myotomy in the majority of these patients, among whom some may have to ultimately go on to esophagectomy?
Dr. Schuchert: The mediastinal dissection, myotomy and wrap does create some additional scarring at the level of the hiatus, but in none of the cases did that dissection significantly influence our ability to do the esophagectomy. So I would say that prior attempts at myotomy can make the dissection more challenging, but do not appear to have a significant impact on outcomes with subsequent esophagectomy.
The Society for Surgery of the Alimentary Tract 48th Annual Meeting, Washington DC, May 19–23, 2007.
Rights and permissions
About this article
Cite this article
Schuchert, M.J., Luketich, J.D., Landreneau, R.J. et al. Minimally Invasive Surgical Treatment of Sigmoidal Esophagus in Achalasia. J Gastrointest Surg 13, 1029–1036 (2009). https://doi.org/10.1007/s11605-009-0843-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-0843-5