Abstract
Aim
The clinical features of postoperative bleeding from the ileal pouch–anal anastomosis(IPAA) vary and its management can be difficult. There is no published literature regarding pouch bleeding and its treatment.
Materials and Methods
Pouch bleeding was defined as the passage of blood or clots transanally or into the ileostomy bag with or without hypotension or a drop in hemoglobin within 30 days after surgery. Patients were identified from a prospectively maintained pouch database.
Results
Pouch bleeding developed in 47 (1.5%) patients out of 3,194 patients undergoing IPAA since 1983. Forty-two patients had inflammatory bowel disease, four had familial adenomatous polyposis, and one had colonic inertia. Sixty-six percent of bleeding occurred within 7 days postoperatively and 59.6% required transfusion; 72.3% patients developed transanal bleeding, nine from ileostomy and two from both. After initial fluid resuscitation, five patients were observed while 28 patients had pouch endoscopy and clot evacuation followed by cauterization or epinephrine(1:100,000) enemas, 27 of these had cessation within 24 h. Epinephrine enema was used as initial treatment in the remaining 12 patients. Overall success rate of epinephrine enema was 96%.
Conclusion
Postoperative pouch bleeding after IPAA is uncommon, and it usually requires nonsurgical intervention. Epinephrine enema appears to be successful in managing this complication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ileal pouch–anal anastomosis (IPAA) after proctocolectomy was first described by Parks et al.1 in the 1970s and has become the surgical procedure of choice ever since for patients with ulcerative colitis and familial adenomatous polyposis. The terminal segment of the ileum is utilized to construct a pouch as a reservoir for stool storage. Pouch configuration includes two (J-shaped), three (S-shaped), or four (W-shaped) loops of the small intestine.2 The J-pouch configuration has become the preferred pouch type for most colorectal surgeons.3 Continuity is then restored with a hand-sewn or stapled anastomosis after pouch construction. Although modifications and improvements have been made to the technique for pouch configuration,4 postoperative complications may occur even in experienced hands. Common complications include pouchitis, anastomotic leak, pelvic abscess, etc.5
Postoperative bleeding from the pouch is a less frequent complication after this procedure and is seldom described. A previous study from our institution reported 38 cases with post-IPAA bleeding from the pouch in a consecutive series of 1,005 patients undergoing pouch surgery.6 The details of clinical features were, however, not provided. The clinical features of postoperative pouch bleeding vary depending on severity and the management can be difficult. Furthermore, it may result in longer length of stay and increased rate of readmission. The diagnostic and therapeutic methods include endoscopy, observation, fluid resuscitation, and interventional managements. The limited information on outcomes of this particular complication prompted us to take on the current study. The aim of the study was to review our experience in its management.
Materials and Methods
Patients
The study was approved by Institutional Review Board at the Cleveland Clinic Foundation. Data of 3,194 patients undergoing restorative proctocolectomy and IPAA were recorded in a prospectively maintained pouch database since 1983. As of October 2007, 47 patients who had post-IPAA bleeding were identified. Retrospective chart review was performed to confirm all the data in the database including demographics, clinical parameters, and surgical technique. Review of records for data related to bleeding such as initial manifestation, symptoms, severity, bleeding sites, and management was performed. There were incomplete data pertaining to medication for two patients due to unavailability of pertinent chart volumes. These patients were included for other analyses.
Inclusion and Exclusion Criteria
Pouch bleeding was considered as a short-term complication and defined as the occurrence of passage of blood or clots transanally or into an ileostomy bag with or without hypotension or a drop in hemoglobin within 30 days after surgery. Patients who developed pouch bleeding more than 30 days after surgery were excluded.
Outcome Measurement
Primary outcome was defined as cessation of visible bleeding. Secondary outcome was readmission and death.
Surgical Technique
The IPAA was performed as previously described.7 After the left colon and right colon were mobilized and the splenic flexure and hepatic flexure were taken down, the terminal ileum was transected. The ileocolic vessels were then ligated, divided, and excised. A low ligation was carried out in the inferior mesenteric artery and vein, as well as the sigmoid branches. The rectum was mobilized down to the coccyx. When necessary, incisions were made in the anterior and posterior leaves of the mesentery overlying the superior mesenteric artery in order to get adequate reach. An approximately 20 cm J-pouch was then constructed with two firings of the ILA-100 stapler. The pouch was tested to ensure that it was airtight and watertight. The PI-30 was used to close off the tip of the “J” and this was reinforced with a running suture. Hemostasis inside the pouch was checked visually. A purse string suture was applied and the anvil inserted and deployed. The linear staple line was reinforced by some surgeons. A diverting ileostomy was made, usually 40–50 cm upstream of the pouch.
Statistical Analysis
Descriptive statistics were performed for all variables. These include the mean and standard deviation for continuous variables and frequencies for categorical factors. Statistical significance was tested using chi-squared or Fisher’s exact probability tests. Student’s t tests or Wilcoxon rank sum tests were used for continuous factors. Differences were statistically significant when the p value was less than 0.05 (two-sided). To further assess the risk factor of pouch bleeding, we compared the 47 patients to the rest of the patients in the pouch database for investigator selected variables.
Results
Pouch bleeding developed in 47 (1.5%) patients out of 3,194 patients undergoing IPAA since 1983. IPAA was performed in patients with inflammatory bowel disease, including ulcerative colitis (n = 25), indeterminate colitis favoring UC (n = 5), indeterminate colitis favoring CD (n = 1), indeterminate colitis (n = 9), Crohn’s disease (n = 2), familial adenomatous polyposis (n = 4), and colonic inertia (n = 1).
We compared the 47 patients to the rest of the patients in the pouch database for selected variables as shown in Table 1. There were no differences in gender distribution, pouch configuration, and anastomotic type between patients with and without pouch bleeding. The patients with pouch bleeding, however, were younger than the rest of the patients in the database.
Reinforcement of the linear staple line was performed after J-pouch formation in 17 (44.7%) patients with J-pouch. Sixty-six percent bleeding occurred within 7 days; 41.9% of these patients had postoperative anticoagulant use for thrombosis prophylaxis.
Thirty-four (72.3%) patients bled transanally, nine from ileostomy and two from both locations. Two patients had concurrent abdominal bleeding and two had anemic symptoms. The latter two were later found to be bleeding from the pouch at endoscopy.
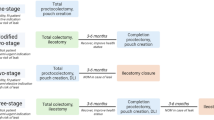
Among the two patients who had concurrent abdominal bleeding, one patient was reoperated on and the other died after being transferred to the intensive care unit due to concurrent intraabdominal bleeding. The management of the remaining 45 patients is shown in Fig. 1. Twenty-eight patients had pouch endoscopy and clot evacuation, 12 patients underwent initial epinephrine enema, and five patients underwent observation only. Of the 28 who underwent endoscopy with clot evacuation, 15 (53.6%) patients had active bleeding from the linear staple line which was cauterized. Generalized oozing was found in the remaining 13 (46.4%) cases and these patients were treated by saline with epinephrine (1:100,000) enemas. Of these 28 patients, 27 had cessation of bleeding within 24 h. One patient required 3 days of enema treatment before complete cessation. Of the 12 patients treated with epinephrine enema as initial treatment, one patient failed to respond and had endoscopy with cauterization of bleeding point.
Overall, 28 (59.6%) patients underwent blood transfusion. Twenty patients bled within 7 days after surgery. None of the patients required surgery for postoperative bleeding from the pouch. Two patients treated with enema had rebleeding 3 and 5 days, respectively, after initial treatment and required readmission. They were treated successfully with epinephrine enema.
Discussion
Total proctocolectomy and IPAA was introduced as an alternative to end ileostomy and continent ileostomy. This procedure preserves the natural route of defecation by using the patient’s own sphincters to maintain continence and has a relatively low reoperation rate for complications with a high patient satisfaction.6,8 Pouch-related complications including surgical and mechanical complications, inflammatory disorders, functional disorder, and systemic complications have been demonstrated.9 Perioperative pouch bleeding as a rare complication has not been specially addressed previously in the literature.
Because patients undergoing IPAA might have a diverting ileostomy, bleeding from the pouch could present as bleeding from the ileostomy bag. Therefore, the diagnosis of pouch bleeding is suspected when clinical signs of bright red blood per rectum or excessive bloody stoma output are noticed. The reasons for bleeding from the pouch are not clear. Technical failure in terms of inadequate hemostasis in the operative field or a misfired stapler could be causative, while patients’ underlying hematological disorders and postoperative anticoagulant use could be predisposing. Due to the rarity of the condition with small numbers of patients, we were unable to analyze these potential risk factors. From a technical aspect, a J-shaped ileal pouch has approximately a single 20-cm staple line10 and an S-shaped pouch has approximately three 15-cm hand-sewn suture lines.11 In our study, the distribution of pouch configuration (J versus S) was not significantly different between patients with and without pouch bleeding. Hence, we did not find the association between pouch configuration and bleeding.
When bleeding from the pouch develops in the postoperative period, a standardized algorithm for its management has not been previously defined. Based on the findings in our series of patients, pouch endoscopy can be used to diagnose as well as treat pouch bleeding. Endoscopy and clot evacuation followed by cauterization of a specific bleeding point might be the most effective way in managing this complication, which was associated with 100% success rate in our study. When presented with diffuse bleeding, treatment with epinephrine enema can instead be used. However, the use of endoscopy depends on the surgeon’s preference and the severity of the bleeding.
Epinephrine, because of its pharmacological properties of vasoconstriction, has been widely used in the management of intraluminal bleeding, such as upper gastrointestinal (GI) bleeding,12 nasal bleeding, and even lower GI bleeding when the bleeding site can be adequately reached.13 It can be used by local irrigation or local injection of diluted epinephrine. Local injection of epinephrine is the most popular therapeutic method in treating bleeding peptic ulcers.14 Of patients who were evaluated via endoscopy in this series, 46.4% could be successfully treated with an epinephrine enema. Twenty-five patients in total received local enema of 0.9% saline and epinephrine (1:200,000). The current study showed that it was associated with a high success rate of 96%. Hence, an epinephrine enema could be the initial treatment of choice considering the related cost, low risk of recurrent bleeding, and ease of instillation when compared with endoscopy and cauterization.
The limitation of this study is that the risk factors for the occurrence of pouch bleeding were not evaluated due to the sample size and the lack of availability of relevant data. Furthermore, the choice of initial treatment in this case series was mainly based on the surgeon’s preference. Therefore, it is difficult to define the superiority of one treatment over the other.
Conclusion
Postoperative pouch bleeding after IPAA usually requires intervention but can be managed nonsurgically. Pouch endoscopy with clot evacuation and cauterization of visible bleeding point followed by iced saline and saline with epinephrine enema appears to be effective in managing this complication.
References
Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. BMJ 1978;2:85–8.
Nicholls RJ. Restorative proctocolectomy with various types of reservoir. World J Surg 1987;11:751–62. doi:10.1007/BF01656598.
McHugh SM, Diamant NE, McLeod R, et al. S-pouches vs. J-pouches. A comparison of functional outcomes. Dis Colon Rectum 1987;30:671–77. doi:10.1007/BF02561686.
Utsunomiya J, Iwama T, Imajo M, Matsuo S, Sawai S, Yaegashi K, et al. Total colectomy, mucosal proctectomy, and ileoanal anastomosis. Dis Colon Rectum 1980;23:459–66.
Shen B, Fazio VW, Remzi FH, Lashner BA. Clinical approach to diseases of ileal pouch–anal anastomosis. Am J Gastroenterol 2005;100:2796–807. doi:10.1111/j.1572-0241.2005.00278.x.
Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, et al. Ileal pouch–anal anastomoses complications and function in 1005 patients. Ann Surg 1995;222:120–7. doi:10.1097/00000658-199508000-00003.
Ballantyne GH, Pemberton JH, Beart RW Jr, Wolff BG, Dozois RR. Ileal J pouch-anal anastomosis. Current technique. Dis Colon Rectum 1985;28:197–202. doi:10.1007/BF02554246.
Farouk R, Pemberton JH, Wolff BG, et al. Functional outcomes after ileal pouch–anal anastomosis for chronic ulcerative colitis. Ann Surg 2000;231:919–26. doi:10.1097/00000658-200006000-00017.
Shen B, Remzi FH, Lavery IC, Lashner BA, Fazio VW. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol 2008;6:145–58. doi:10.1016/j.cgh.2007.11.006.
Hughes JP, Bauer AR Jr, Bauer CM. Stapling techniques for easy construction of an ileal J-pouch. Am J Surg 1988;155:783–5. doi:10.1016/S0002-9610(88)80043-6.
Rothenberger DA, Vermeulen FD, Christenson CE, Balcos EG, Nemer FD, Goldberg SM, et al. Restorative proctocolectomy with ileal reservoir and ileoanal anastomosis. Am J Surg 1983;145:82–8. doi:10.1016/0002-9610(83)90171-X.
Lau JY, Chung S. Management of upper gastrointestinal haemorrhage. J Gastroenterol Hepatol 2000;15(Suppl):G8–12. doi:10.1046/j.1440-1746.2000.02258.x.
Kim YI, Marcon NE. Injection therapy for colonic diverticular bleeding. A case study. J Clin Gastroenterol 1993;17:46–8. doi:10.1097/00004836-199307000-00013.
Vergara M, Calvet X, Gisbert JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev 2007;18:CD005584.
Author information
Authors and Affiliations
Corresponding author
Additional information
This abstract was presented as a poster at the Digestive Disease Week 2008, San Diego.
Rights and permissions
About this article
Cite this article
Lian, L., Serclova, Z., Fazio, V.W. et al. Clinical Features and Management of Postoperative Pouch Bleeding after Ileal Pouch–Anal Anastomosis (IPAA). J Gastrointest Surg 12, 1991–1994 (2008). https://doi.org/10.1007/s11605-008-0611-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0611-y