Abstract
Achalasia, an esophageal motility disorder characterized by aperistalsis and failure of lower esophageal sphincter (LES) relaxation, is most effectively treated by surgical ablation of the LES. In this report, we describe our technique of laparoscopic extended Heller myotomy with Toupet partial posterior fundoplication. The technical details of this procedure include careful division of the longitudinal and circular muscle fibers of the LES anteriorly, including extension of the myotomy 3 cm distal to the esophagogastric junction onto the gastric cardia. The Toupet procedure, involving a posterior wrap of the gastric fundus which is secured to both edges of the myotomy as well as to the crura of the hiatus, is added to prevent post-myotomy gastroesophageal reflux. From a recently published report, mean dysphagia scores remained low (3 out of 10 severity on a visual analog scale) and symptoms of reflux were reported minimally in a series of 63 patients followed for a median of 45 months. This technique provides excellent and durable relief of dysphagia associated with achalasia while minimizing post-myotomy acid reflux symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Achalasia is a relatively rare but well-known esophageal motility disorder involving the failure of relaxation of the lower esophageal sphincter (LES) upon swallowing and the loss of esophageal peristalsis. While not specifically curable, treatment for achalasia is aimed at ablation of the LES in order to relieve the symptoms of dysphagia and regurgitation invariably produced by this disease. The first such therapy, described by Thomas Willis in 1674, involved rigid esophageal dilation with a sponge-tipped whalebone.1 Surgical treatment was introduced by Ernest Heller in 1913, consisting of an anterior and posterior myotomy across the LES performed via a thoracotomy.2 Much more recently, the laparoscopic technique has become the most widely used surgical approach to this disease, yielding excellent results which are generally superior to non-surgical therapy. The purpose of this report is to describe in detail how we perform the extended laparoscopic Heller myotomy with Toupet fundoplication for achalasia. Results from a recently published series with long-term follow-up are included to illustrate the success and durability of this particular technique.3
Technique
All patients are routinely evaluated with upper endoscopy, barium esophagography, and esophageal manometry to confirm the diagnosis and plan the procedure. The patient is prepared for surgery in the standard fashion, with orders to be NPO after midnight. For patients who present with a dilated esophagus, in particular those with esophageal dilatation greater than 4 cm and/or with a long or tortuous esophagus, we will discontinue solid food 4–5 days prior to the procedure and utilize a high-energy liquid diet. The idea is to clear the esophagus of solid debris as much as possible. A first-generation cephalosporin such as cefazolin is given as antibiotic prophylaxis prior to incision. The patient is placed under general endotracheal anesthesia and then positioned in low-lithotomy; a bean-bag underneath the patient is secured to the table in order to prevent slippage when the patient is subsequently placed in a steep reverse-Trendelenberg position. Alternatively, a pair of safety belts may be mounted on each side of the operating table and used to secure the patient’s thighs, in essence, forming a sling rather like a climbing harness. It is also helpful to turn the operating table at an approximately 30° angle to the long axis of the room, so that the laparoscopic tower and screen may be placed at the patient’s left shoulder leaving the head of the table free for the anesthesia team and for the surgeon in the event the need arises for instrumentation (endoscopy) of the esophagus.
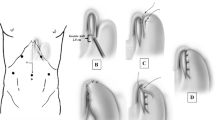
Pneumoperitoneum is obtained using a Veress needle technique, either in the left subcostal position or at the primary camera position, a point approximately 2 cm above and 1–2 cm to the left of the umbilicus, as desired. The remaining ports, five in all, are placed in a fashion identical to that used in a laparoscopic antireflux procedure (Fig. 1). It is our practice to use a 10-mm supraumbilical port for a 10-mm, 30° laparoscope, (a good quality 5 mm laparoscope can be used instead) 10 mm ports in the right and left subcostal positions respectively, a 10 mm right flank port, and a 5 mm left flank port. The right flank port may be omitted in patients with relatively small livers if one chooses a Nathason retractor which can be placed through a 4–5-mm epigastric opening without a port. The left lateral segment of the liver is then retracted with a paddle-style liver retractor through the right flank port (or the Nathanson through the epigastric entry site). Either retractor is secured using a table-mounted “iron intern” self-retracting device.
A point is chosen along the greater curvature of the stomach approximately 1/3 of the way distal to the esophagogastric junction, and the short gastric vessels are divided using an ultrasonic coagulator proximally to mobilize the fundus, thus avoiding tension on the antireflux procedure performed subsequent to the myotomy. The hiatus is then dissected and the esophagus is exposed within the distal mediastinum. It is not necessary to dissect the esophagus circumferentially in the mediastinum, only the anterior aspect where the myotomy will be made. The dissection is carried up far enough to permit the performance of the myotomy to 8 cm proximal to the level of the esophagogastric junction. A 1/2-in. Penrose drain placed around the esophagogastric junction is helpful in providing retraction to complete this dissection, and brings the distal esophagus more easily into the operative field, thus limiting the amount of work that needs to be done above the hiatus. The anterior (left) vagus nerve is identified and carefully preserved, and the epigastric fatpad is dissected away to facilitate precise identification of the esophago-gastric junction and the distal extension of the myotomy onto the cardia. In most patients, there is no need to close the hiatus. In fact, we prefer to leave it open as we do not wish to risk compromising the lumen of the esophagus. Thus, when we anticipate performing a partial posterior fundoplication (Toupet type), we dissect both crura free from adjacent tissues but leave the hiatus open and slightly larger than when we started the operation given that we have been dissecting the esophagus up above and have produced some lateral stretching. If a large hiatus hernia is identified, present in approximately 20% of patients with achalasia, the crura may need to be closed, using interrupted sutures posterior to the esophagus. Care must be taken to avoid tightening the hiatus too much, allowing for a small window posterior to the esophagus such that when the dilator is subsequently placed the hiatus is not tightly apposed to the circumference of the esophagus.
A lighted 52-French esophageal dilator is next passed transorally by the anesthesiologist with the tip positioned well into the gastric body. This provides a platform for performance of the myotomy, and aids in identification of the submucosal layer. Notably, it is important to have an anesthesiologist who is very familiar with this surgical procedure and thus experienced and comfortable with placement of the dilator, to best avoid esophageal perforation. Otherwise, it is beneficial for a member of the surgical team to perform this important step. A 10-mm laparoscopic Babcock clamp is used to spread the anterior esophagus and/or the stomach wall over the Babcock clamp, providing tension on the muscularis and thus facilitating division of the muscle fibers. With that platform in place, we then expose and review the anatomy, identify the location of the previously dissected anterior vagus and, precisely, the gastroesophageal junction (GEJ). We then measure 3 cm below the GEJ, and at a point close to the lesser curvature (usually 2–2.5 cm to the left of the lesser curvature), we start the myotomy over the bougie and move upwards in a straight line towards the GEJ. This is always the most difficult part of the procedure, and it is useful to do it first. It is challenging in this area to clearly identify the muscular planes and to find the mucosa, and it is helpful to divide longer areas (1 cm or so) on the serosa and initial muscular layers and then gently “find” your way through the rest of the muscularis until the mucosa is seen. The deepest part of the muscularis of the stomach in this area, the oblique fibers that are descending from the angle of His down to the lesser curvature, do not easily separate away from the mucosa and a lot of gentle work must be done as the myotomy is enlarged. We usually do the entire 3 cm to the GE junction and then gently re-do the area that has been myotomized until we have good mucosal exposure and completely separated edges. Electrocautery is used rarely and with very short bursts in the area near the mucosa. The myotomy is then continued up through the GEJ and into the body of the esophagus by gently dividing the muscular fibers with either a hook electrocautery or the ultrasonic coagulator to expose the submucosa of the esophagus. The esophageal myotomy is much simpler, as the layers are well delineated (except in patients who received botulinum toxin preoperatively) and the mucosa is thicker with a submucosal plane that allows relative ease of dissection. Both the outer longitudinal and inner circular muscle layers must be identified and divided (Fig. 2). Extension of the myotomy for a distance of 3 cm distally onto the gastric cardia and about 6–8 cm proximally on the esophagus is the goal. Most bleeding encountered during this step of the procedure can be controlled with time and/or the application of pressure using a closed blunt grasper. In our experience, bleeding subsides when it is coming from the submucosal vessels in all instances, sometimes with the application of pressure, or by holding on to the bleeding vessel gently with the grasper. We do not recommend using electrocautery on the mucosa. The vessels that tend to be more worrisome are always within the wall of the esophagus and start bleeding as the muscularis, or the adventitia is divided (the latter is more common in large esophagi and the vessels are superficial to the muscularis). These vessels can and should be controlled either with electrocautery, making sure that the electrocautery is pressed against the muscle and not in contact with the mucosa, or, should the bleeding be more substantial, with an ultrasonic coagulator, a clip (with a tiny piece of muscle attached) or a suture. If a perforation of the mucosa should occur, it is typically easily identified and can be repaired using interrupted intracorporeal stitches with fine (4–0) absorbable suture.
Once the myotomy is deemed to be adequate in terms of length, it can be checked using flexible fiberoptic endoscopy, taking great care when approaching the myotomy with the endoscope. The myotomized region should easily open with air insufflation from the scope, and there should be no narrow or tight areas from the esophagus to the stomach. It is sometimes necessary to extend the myotomy at this point based on localization of a residual high-pressure zone. This technique also helps to identify any previously-unrecognized perforation.
The final step is the performance of the antireflux procedure. Our preference is for the Toupet partial posterior fundoplication. This is begun by passing the tip of the gastric fundus posterior to the esophagogastric junction, and securing it with interrupted sutures of 2–0 silk to the right crus of the diaphragm. The fundic tip is then sutured to the right edge of the myotomy, using three sutures. Similarly, the proximal aspect of the fundus is sutured to the left edge of the myotomy, and also to the left crus (Fig. 3). A Dor fundoplication is routinely chosen in patients in whom there has been a perforation of the mucosa so as to buttress the mucosal closure. The procedure is now complete, the ports are all removed, and the port sites closed.
Patients are started on a clear liquid diet after transfer from the post-anesthesia care unit, and a soft diet is begun the following morning. Patients are typically discharged home on the first postoperative day.
Results
Between 1998 and 2003, 63 patients underwent the laparoscopic extended Heller myotomy with Toupet fundoplication as described above, and these results have been published recently.3 Mean dysphagia frequency, assessed preoperatively by five-point questionnaire administered to all patients (0 = “no symptoms,” 1 = “once per month,” 2 = “once per week,” 3 = “daily,” and 4 = “multiple times per day”) was 3.8 ± 0.7. There were no significant perioperative complications, and median hospital stay was 1.8 ± 0.6 days. With a median follow-up of 45 months, mean dysphagia frequency was significantly reduced to 1.7 ± 1.4 and mean dysphagia severity scores were 3.1 ± 2.6 on a 0–10 visual analog scale. Other symptoms, including heartburn, were minimal. Three patients required subsequent dilations, between 36 and 56 months after surgery, and no patient required a reoperation. A subset of 31 patients was followed up for a median of 63 months, with similar scores for postoperative dysphagia (3.7 ± 3.0) and other symptoms.3
Discussion
The technique described above proves to be a very effective and durable treatment for achalasia, while minimizing the amount of esophageal acid reflux associated with division of the lower esophageal sphincter. In particular, both the length of the myotomy onto the gastric cardia and the performance and choice of an antireflux procedure are important in achieving these results.
Extended Myotomy
The debate concerning the length of the myotomy onto the gastric cardia has evolved over time, and centers around the balance between maximal relief of dysphagia and the prevention of postoperative reflux. The argument for a shorter myotomy proposes that by maintaining some of the “clasp and sling” fibers of the gastric cardia, a component of the antireflux barrier is preserved. Ellis et al., describing their technique of modified Heller myotomy via a left thoracotomy, as it was commonly performed at the time, advocated that the LES be divided only a few millimeters onto the cardia, citing an incidence of clinically significant reflux in only 3% of their patients after the procedure, with good to excellent results in 84%.4 The first description of a minimally invasive approach to the Heller myotomy emulated this technique using a thoracoscopic approach. Notably, three of 17 patients required an early second operation for extension of the myotomy onto the cardia.5 A subsequent comparison between 35 patients undergoing the thoracoscopic approach and 133 patients undergoing a laparoscopic Heller myotomy, in which it is easier to extend the myotomy further onto the cardia, yielded good to excellent relief of dysphagia in 85% of the thoracoscopic group compared to 93% of patients approached laparoscopically.6 Based upon these results and subsequent observations, the laparoscopic technique was further modified at our institution to extend the myotomy a full 3 cm onto the cardia. When compared with a shorter myotomy (1.5 cm onto the cardia) and Dor fundoplication, this extended myotomy has resulted in significantly lower postoperative dysphagia scores and markedly less reintervention.7
Antireflux Procedure
The issue of whether or not to perform an antireflux procedure in conjunction with the Heller myotomy has been a longstanding controversy. The incidence of reflux after myotomy performed without some type of antireflux procedure is reported to be as high as 100%. Some authors have expressed concern over the creation of a barrier in the face of an aperistaltic esophagus.8 However, in a 2004 randomized trial of Heller myotomy with or without Dor partial anterior fundoplasty, Richards et al. demonstrated equivalent postoperative dysphagia scores, while the Heller–Dor group had significantly lower mean acid exposure times compared to the Heller-alone group at 6 months follow-up with 24-h pH monitoring (0.4% vs. 4.9%, p = 0.001). Of the 43 patients enrolled in the trial, only 9% of the Heller–Dor group exhibited pathologic reflux, compared to 47.6% of the Heller-alone patients.9
In our experience, the addition of an antireflux procedure, whether Dor anterior fundoplasty or Toupet partial posterior fundoplication leads to a relatively low incidence of reflux-related complaints while providing good to excellent relief of dysphagia in the majority of patients.3,7,10 In some patients with a very wide distal esophagus (usually seen in conjunction with a dolico-megaesophagus, redundant length and loss of the axis of the esophagus) it is not possible to perform a Toupet (or a Dor) without narrowing the size of the esophagus. In these patients, we prefer to dissect the distal esophagus and simply suture the esophageal wall to the right and left crura to try and restore its axis as much as possible. For the average patient, our preference is for the Toupet fundoplication as described above, as we feel it provides better control of reflux than the Dor fundoplasty. However, this is yet to be definitively demonstrated when it is combined with Heller myotomy. Another likely advantage of the Toupet procedure includes a “stenting” effect of the myotomy which may help to prevent scarring and reapproximation of the divided muscle edges. Some authors have also described good results with a total fundoplication performed in conjunction with Heller myotomy. For example, Rossetti et al. reported relief of dysphagia in 92% of 195 patients, with no measurable pathologic acid reflux in a subset of 75 patients who had undergone postoperative pH monitoring.11 Nonetheless, the majority of surgeons performing Heller myotomy continue to employ a partial fundoplication procedure when an antireflux procedure is included.
Conclusion
The laparoscopic extended Heller myotomy with Toupet fundoplication is an effective and definitive procedure for the relief of dysphagia in patients with achalasia. Important technical considerations in this procedure include division of all longitudinal and circular muscle fibers in the high-pressure zone of the LES, and extension of the myotomy distally to 3 cm below the esophagogastric junction onto the cardia. The addition of the Toupet partial posterior fundoplication is relatively effective in the control of gastroesophageal reflux which is otherwise expected after ablation of the LES, and does not result in an increase in postoperative dysphagia.
References
Willis T. Pharmaceutice rationalis sive diatribe de medicamentorum operationibus in human corpore. London, England: Hagae Comitis, 1674.
Heller E. Extramukose kardioplastic beim chronischen kardiospasmus mit dilatation des oesophagus. Mitt Grenzeb Med Chir 1913;27:141–9.
Wright AS, Williams CW, Pellegrini CA, Oelschlager BK. Long-term outcomes confirm the superior efficacy of extended Heller myotomy with Toupet fundoplication for achalasia. Surg Endosc 2007;21(5):713–8. doi:10.1007/s00464-006-9165-9.
Ellis FH Jr, Gibb SP, Crozier RE. Esophagomyotomy for achalasia of the esophagus. Ann Surg 1980;192(2):157–61. doi:10.1097/00000658-198008000-00004.
Pellegrini C, Wetter LA, Patti M et al. Thoracoscopic esophagomyotomy. Initial experience with a new approach for the treatment of achalasia. Ann Surg 1992;216(3):291–6. discussion 296–9doi:10.1097/00000658-199209000-00008.
Patti MG, Pellegrini CA, Horgan S et al. Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg 1999;230(4):587–93. discussion 593–4doi:10.1097/00000658-199910000-00014.
Oelschlager BK, Chang L, Pellegrini CA. Improved outcome after extended gastric myotomy for achalasia. Arch Surg 2003;138(5):490–5. discussion 495–7doi:10.1001/archsurg.138.5.490.
Richards WO, Clements RH, Wang PC et al. Prevalence of gastroesophageal reflux after laparoscopic Heller myotomy. Surg Endosc 1999;13(10):1010–4. doi:10.1007/s004649901158.
Richards WO, Torquati A, Holzman MD et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg 2004;240:405–15. doi:10.1097/01.sla.0000136940.32255.51.
Tatum RP, Kahrilas PJ, Manka M, Joehl RJ. Operative manometry and endoscopy during laparoscopic Heller myotomy. An initial experience. Surg Endosc 1999;13(10):1015–20. doi:10.1007/s004649901159.
Rossetti G, Brusciano L, Amato G et al. A total fundoplication is not an obstacle to esophageal emptying after Heller myotomy for achalasia: results of a long-term follow up. Ann Surg 2005;241(4):614–21. doi:10.1097/01.sla.0000157271.69192.96.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tatum, R.P., Pellegrini, C.A. How I Do It: Laparoscopic Heller Myotomy with Toupet Fundoplication for Achalasia. J Gastrointest Surg 13, 1120–1124 (2009). https://doi.org/10.1007/s11605-008-0585-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0585-9