Abstract
Objectives
We investigated the effect of suramin on tumor growth and spread in an immunocompetent, orthotopic rat model of pancreatic cancer and analyzed the tumor vasculature by intravital microscopy.
Methods and Methods
In vitro, rat ductal pancreatic cancer cells (DSL-6A) were incubated with suramin (10–800 µg/ml), and cell proliferation was assessed. In vivo, DSL-6A tumors were induced in the pancreas of Lewis rats. Animals received suramin (60 mg/kg, weekly i.p.) or the vehicle (controls). Treatment started after 3 days. Intravital microscopy after 1, 4, and 8 weeks quantified diameter, density, and permeability of tumor vessels. Primary tumor volume, local infiltration, and metastatic spread were determined at autopsy. Microvessel density was analyzed by immunohistochemistry.
Results
In vitro, proliferation was inhibited by suramin up to 95%. In vivo, all controls developed extensive tumor growth and spread. No tumor was detectable in half of the suramin-treated animals after 8 weeks; tumor dissemination was almost completely depressed. Suramin therapy resulted in a complete regression of tumor macrovessels and a significant reduction of microvessel density.
Conclusion
Suramin significantly reduces primary tumor growth and dissemination in a clinically relevant rat model of pancreatic cancer and seems to play an important role for the inhibition of tumor angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenocarcinoma of the pancreas is the fifth leading cause of cancer-related death in Western countries. The poor overall 5-year survival rate of less than 5% is due to the tumors propensity toward aggressive tumor growth, early metastasis, and its resistance to cytotoxic agents and radiation. More than 80% of patients are diagnosed with pancreatic cancer at a locally advanced or metastatic stage, which excludes a curative surgical resection.1 Therefore, novel therapeutic strategies are required to improve the prognosis of patients with pancreatic cancer.
Angiogenesis, the development of vascular supply by sprouting from existing vessels, is a critical step for tumor growth and appears to impact prognosis. Control of angiogenesis with pharmacological drugs represents an alternative approach to the management of solid malignancies. The complex process of angiogenesis involves many growth factors [including vascular endothelial growth factor (VEGF)],2 extracellular matrix molecules, enzymes, and several cell types in vivo.3,4 Anti-angiogenic agents decrease tumor growth and metastatic dissemination of numerous solid tumor types.
One potential anti-angiogenic agent is suramin. This polysulfonated napthylurea derivative, originally developed in 19165 to treat trypanosomiasis, has been extensively evaluated over the past 15 years as an anticancer agent. On the molecular level, suramin is able to bind to several growth factors such as fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factors alpha and beta (TGF), insulin-like growth factor I (IGFI), and influences growth factor-receptor interactions.5–8 Suramin can also interfere in processes involved in cellular adhesion and migration and with different signal transduction pathways, and in addition, it has been shown to be a strong inhibitor of angiogenesis.9 Previous studies from our group have shown that suramin reduces tumor growth and neoangiogenesis in a T-cell-deficient nude mouse model of pancreatic cancer.10
To improve the existing knowledge on the therapeutic activity of suramin and to characterize in more detail its anti-angiogenic potential in pancreatic cancer, we tested this drug in vitro by evaluating the effects on proliferation and cell viability of a ductal rat pancreatic cancer cell line. Furthermore, we studied the therapeutic and anti-angiogenic potential of suramin in a clinically relevant, fully immunocompetent, orthotopic rat model of pancreatic cancer. We determined parameters like microvessel density, vessel diameter, and vessel permeability by intravital microscopy with a novel computer-assisted image analysis system for quantitative assessment of microcirculation after 1, 4, and 8 weeks of tumor implantation. Tumor growth and metastatic behavior were analyzed at the autopsy of each animal, and microvessel density of the whole tumor was determined by immunohistochemistry.
Materials and Methods
Cell Line and Culture Conditions
The rat pancreatic adenocarcinoma cell line of ductal origin DSL/6A was obtained from the European Collection of Cell Cultures (Salisbury, UK). The cells were cultured in Waymouth’s medium (Invitrogen, Karlsruhe, Germany), supplemented with 10% heat-inactivated fetal bovine serum (FBS-Gold, PAA, Cölbe, Germany), penicillin G (100 U/ml), streptomycin (100 µg/ml; PAA), and amphothericin B (0.25 mg/ml). DSL/6A cells were incubated at 37°C in humified air with 5% CO2. The medium was replaced twice a week, and cells were maintained by serial passaging after treatment with 0.1% trypsin.
Drug
Suramin was a generous gift from Bayer AG (Leverkusen, Germany), as a sodium salt and stored at room temperature. For in vitro assays and intraperitoneal injection, suramin was first dissolved in 0.9% NaCl (pH 7.5). Further dilutions for in vitro studies were made with Waymouth’s medium and filtered before use.
In Vitro Assessment of Cell Proliferation and Viability
To examine the effect of suramin on in vitro cell proliferation, 2 × l05 cells from the DSL-6A cell line were seeded in six-well culture plates in 2 ml of the respective cell culture medium. The medium was changed the next day (day 1), and suramin was added in the following concentrations: 10, 100, 200, and 800 µg/ml. After 72 h (day 4), the cells were trypsinized and counted in a standard hemocytometer. Cell viability was assessed by a colorimetric dye reduction assay with monotetrazolium (MTT, Boehringer, Mannheim, Germany) according to the manufacturer’s instructions. Briefly, cells were seeded in 96-well plates at a density of 5 × 103 cells in 0.2 ml of the respective medium. Medium was changed the next day (day 1), and suramin was added as described above. After 72 h (day 4), 10 µl of MTT (5 mg/ml) solution, and after additional 4 h, 100 µl of 10% SDS were added to the cells. The plates were allowed to stand overnight (37°C, 5% CO2). The change in absorbance measured at 550 nm with an enzyme-linked immunosorbent assay reader (Biotek Instruments Inc., Burlington, VT, USA) has been shown to strongly correlate with the number of viable cells. All experiments were generated in triplicates and repeated three times.
Orthotopic Rat Model of Pancreatic Cancer
As previously described for an orthotopic nude mouse and rat model of pancreatic cancer,11,12 we used the same transplantation technique in this study. Four-week-old male Lewis rats were obtained from Charles River Laboratories (Charles River, Sulzfeld, Germany). Donor rats were anesthetized with isoflurane (Forene, Abbott, Wiesbaden, Germany) inhalation. Ten million cells of the DSL/6A cell line were injected subcutaneously into the animals’ flanks. The animals were killed by a lethal dose of isoflurane inhalation and opening of the thorax after 8 weeks, when the subcutaneous tumors had reached a size of 1 cm in largest diameter. The donor tumors were harvested and minced by a scalpel (no. 11) into small (1 mm3) fragments. Tumor recipient Lewis rats were anesthetized with isoflurane, followed by intraperitoneal injection of xylazinhydrochloride (Rompun, 12 mg/kg BW; Bayer, Leverkusen, Germany) and Esketaminhydrochloride (Ketanest S, 40 mg/kg BW; Parke-Davis/Pfizer, Karlsruhe, Germany). The animals’ abdomens were opened by a midline incision, and the pancreatic tail with the spleen was gently exteriorized. Five small tissue pockets were prepared in the pancreatic parenchyma as an implantation bed with a microscissor (RS-5610 VANNAS; Roboz, Rockville, MD, USA). One donor tumor fragment was placed into each pancreatic tissue pocket in such a way that the neoplastic tissue was completely surrounded by pancreatic parenchyma. The pancreas was relocated into the abdominal cavity, which was then closed in two layers with 3-0 absorbable suture (Vicryl, Ethicon, Germany). For pain relief, a subcutaneous injection of Carprofen (Rimadyl, 4 mg/kg BW; Pfizer) was given after surgery.
In Vivo Treatment with Suramin
The animals were allocated randomly into a treatment group and a control group, and intravital microscopy was done 1, 4, and 8 weeks after tumor induction (12 rats per group and time point). The dosage of suramin administration was chosen according to the references in the literature and the manufacturers’ recommendation and had been tested in previous studies.10,13 Treatment with suramin (60 mg/kg weekly i.p.) or the vehicle (0.9% saline) was started 3 days after orthotopic tumor implantation. Suramin was administered by intraperitoneal injections twice per week in the first 2 weeks and once a week subsequently. The rats were monitored daily to evaluate their clinical conditions.
Intravital Microscopy
Intravital microscopy of the pancreas was studied as previously described.14–18 Briefly, the animals were anesthetized with Rompun and Ketamine as described above. Polyethylene catheters (inside diameter, 0.5 mm; B.Braun, Germany) were inserted into the right jugular vein and the left carotid artery for monitoring heart rate, blood pressure, and injection of substances needed for intravital microscopy. The animals were placed on a heated operating table and relaparotomized through a small midline incision. The spleen and the tail of the pancreas with the growing tumors were mobilized and exteriorized, placed in an immersion chamber with Ringer’s lactate maintained at 37°C, and positioned under a fluorescence microscope (Leitz, Wetzlar, Germany) with a heat protection and excitation filter (450 to 490 nm) connected to a video recorder. After exposure of the pancreas, 1 ml/kg body weight 0.02% rhodamine 6G (Sigma-Aldrich, Deisenhofen, Germany) was injected intraarterial, and after a 5-min stabilization period, five randomly chosen regions on the tumor site were recorded for off-line analysis of vessel density and vessel diameter. Capillary permeability was determined after an intraarterial injection of 0.2 ml of 5% FITC-Dextran (molecular weight, 150,000; ICN, Aurora, OH, USA). Heart rate and arterial pressure were continuously monitored during intravital microscopy. Only the data from animals with stable cardiovascular conditions were included in the analysis of the microcirculatory parameters to avoid bias possibly resulting from systemic cardiovascular derangement. Exclusion criteria were mean arterial pressure <80 mmHg, PO2 <80 mm Hg, PCO2 >50 mmHg, and pH <7.3 or >7.5.
Image Analysis
All images were analyzed offline using the software CAP-Image (Zeintl, Heidelberg, Germany).19 This computer-assisted video frame analysis system for dynamic capillaroscopy allows the off-line analysis of a variety of microcirculatory parameters and calculates vessel density, vessel diameter, and capillary permeability from the changes in perivascular density caused by extravasation of the fluorescent-labeled dextran over a defined observation period. Details of the equipment, techniques, and methods of calculating the microcirculatory parameters have been described elsewhere.18
Quantification of Tumor Growth and Spread
All animals underwent autopsy after intravital microscopy. The perpendicular diameters of the primary orthotopic tumor were measured with calipers, and the volume was calculated using the following formula: volume = length × width × depth/2. A dissemination score was used to assess local tumor infiltration and distant metastasis.11,12 Local infiltration was determined at the following sites: spleen, stomach, liver (hilus), kidney, retroperitoneum, diaphragm, mesentery loops, and abdominal wall. Isolated tumor nodules with no anatomic connection to the primary tumor were considered distant metastases. The sites of evaluation included liver, kidney, spleen, lung, diaphragm, mesentery, retroperitoneum, mediastinum, and the suture line. Tumor dissemination was quantified as follows: Each manifestation of tumor infiltration or metastatis was counted with one point. Additional points were awarded for massive local infiltration (e.g., including more than half of the circumference of the spleen), multiple metastatic nodules (more than one in parenchymal organs; more than ten in diaphragm, mesentery, and retroperitoneum), and metastatic nodules >50 mm3. Clinical consequences of tumor growth were incorporated into this scoring system: formation of ascites (two points if volume >5 ml), development of jaundice, ileus, and cachexia. The primary tumor and all sites of potential infiltration or metastasis were harvested, fixed in 4% formaldehyde, and embedded in paraffin. Then, 3-µm thick tissue sections were obtained and stained with hematoxylin and eosin for microscopic examination. The sections were reviewed to confirm the findings of the macroscopic dissemination score.
Microvessel Density
Anti-CD31 was used as an endothelial marker to highlight intratumoral microvessels. Immunohistochemical staining was performed on paraffin-embedded tissue of the collected primary tumor tissue. Three-micrometer-thick sections were cut, using a rotation microtom (Leica, RM2125RT). The sections were deparaffinized in xylene and rehydrated in graded alcohols and distilled water. After antigen retrieval with 0.01% ethylenediaminetetraacetic acid, pH 8.0, endogenous peroxidase activity was blocked with 1% hydrogen peroxide in distilled water for 25 min followed by washing with distilled water and finally phosphate-buffered saline (PBS) + 0.1% Tween for 5 min. To bind nonspecific antigens, the sections were incubated with 1× Power Block (BioGenex, San Ramon, CA, USA) for 5 min. The primary antibody was a purified anti rabbit CD-31 (PECAM) and was purchased from Santa Cruz Biotechnology, (Santa Cruz, CA, USA). Antibody dilution was 1:150 in PBS for 30 min at 37°C. As a negative control, sections were incubated with PBS instead of the primary antibody. This was followed by incubation with biotinylated anti-rabbit immunoglobulin G (1:200, Santa Cruz) for 30 min at 37°C and after washing with PBS + Tween by peroxidase-conjugated avidin-biotin complexes (KPL, Gaithersburg, MD) and 3,3′-diaminobenzidine (Sigma, DE). The sections were then counterstained with Mayer’s hematoxylin, upgraded alcohols, mounted and analyzed by standard light microscopy. Microvessel density was quantified as described by Weidner.20 Areas of highest neovascularization were found by scanning the sections at a magnification of ×100; individual microvessel counts were made on ten fields at ×200 magnification (≈0.74 mm2 per field).
Statistical Analysis
All results are presented as mean ± standard error of the mean (SEM). Continuous normally distributed variables were analyzed by the Student’s t test. Discontinuous variables (dissemination score, microvessel density) were analyzed by the Mann–Whitney rank sum test. A p value < 0.05 was considered as statistically significant.
Results
Effect of Suramin on Proliferation and Cell Viability In Vitro
The effect of suramin on the proliferation and viability of the rat pancreatic cell line DSL-6A was studied over a time period of 72 h. The chemotherapeutic agent was applied to the cells using four different concentrations: 10, 100, 200, and 800 µg/ml. Figure 1 shows proliferation and viability changes during drug treatment. Suramin inhibited the proliferation of the ductal pancreatic cancer cell line DSL-6A in a dose-dependent manner. The highest concentration of suramin reduced cell proliferation to less than 10% in this cell line. Loss of cell viability was not detected by low concentrations of suramin, but viability was reduced at high concentrations up to 60% (Fig. 1b)
Effect of Suramin on Tumor Growth and Spread
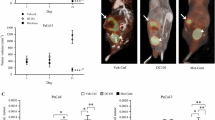
All control animals developed extensive tumor growth (9,118 ± 3,011 mm3), local infiltration, and distant metastasis. In contrast, there was no tumor detectable in half of the suramin-treated animals after 8 weeks; the other animals harbored small tumors (56 ± 38 mm3; p < 0.001; Fig. 2a). Tumor dissemination in treated animals was almost completely depressed after 8 weeks (0.4 ± 0.2 points vs 10.3 ± 2.8 points in control animals; p < 0.05; Fig. 2b).
Primary tumors were derived from the rat ductal pancreatic cancer cell line DSL-6A. a The volumes of the orthotopic primary tumors in controls and animals treated with Suramin were assessed after 1, 4, and 8 weeks (n = 12 per group and time point; *p < 0.05). b Local infiltration and metastatic spread in controls (n = 8) and animals treated with Suramin were evaluated after 1, 4, and 8 weeks and summarized in a dissemination score (n = 12 per group and time point; *p < 0.05).
Effect of Suramin on Tumor Macro- and Microvasculature
Pancreatic carcinomas of control animals displayed irregular blood vessels, which are characteristic for tumor macrovasculature (Fig. 3). Vessel permeability (Fig. 4) was significantly higher in suramin-treated animals after 1 and 4 weeks in comparison to controls (113.8 ± 3.2 grayscale points vs 105.6 ± 1.4 points after 1 week, and 122.6 ± 2.8 grayscale points vs 112.8 ± 2.4 points after 4 weeks, p < 0.05, respectively; 100 grayscale points are defined by normal pancreatic tissue).
Vessel diameter (Fig. 5) and vessel density (Fig. 6) measured by intravital microscopy showed no significant differences in suramin-treated animals vs control animals after 1 and 4 weeks (vessel diameter: 20.1 ± 3.2 vs 30.9 ± 4.6 µm after 1 week, and 29.8 ± 5.1 vs 28.4 ± 4.0 µm after 4 weeks; vessel-density: 82.4 ± 5.8 vs 72.4 ± 7.9/cm after 1 week, and 83.6 ± 7.0 vs 80.3 ± 5.1/cm after 4 weeks).
Eight weeks of suramin therapy resulted in a complete regression of tumor macrovessels. Permeability, diameter, and density of tumor blood vessels (Figs. 4, 5, and 6) could therefore not be determined at this time point by intravital microscopy.
Microvessel density, as quantified by immunohistochemistry, revealed no differences between suramin treatment and control groups after 1 and 4 weeks, whereas 8 weeks of suramin treatment led to a significant reduction of microvessels in the remaining small tumors (30.2 ± 13.4/0.74 vs 89.4 ± 5.4/0.74 mm2; Fig. 7).
Discussion
Suramin is a drug with a long history. Initially developed to treat sleeping sickness and onchoceriasis, the drug exhibited anti-tumor activity first in the treatment of patients with HIV-associated lymphomas and Kaposi’s sarcoma. This raised the possibility of using suramin in the therapy of solid tumors. The ability of suramin to decrease proliferation rates in vitro has been demonstrated in several types of cancer cells, including cells derived from stomach cancer, esophageal cancer, breast cancer, and non-small-cell lung cancer.21–24 The anti-angiogenic effect of suramin has been analyzed both in vitro and in vivo and documented in the chick chorioallantoic membrane assay and in a bFGF-induced model with gel sponges subcutaneously implanted in mice.25,26 Our group showed recently the inhibitory effect of suramin on tumor growth, metastasis, and angiogenesis in a orthotopic nude mouse model of pancreatic cancer.10 The present study is the first to visualize the effect of suramin on tumor blood vessels in a clinically relevant, fully immunocompotent model of pancreatic cancer in rats by intravital microscopy. Our in vitro results demonstrated the inhibitory action of suramin on the rat pancreatic cancer cell line DSL-6A (Fig. 1). Proliferation was decreased dose-dependently by suramin and cell viability was influenced at high doses of suramin treatment. These results indicate that suramin acts in a cytostatic, rather than in a cytotoxic manner.
The effects of suramin were further evaluated in an orthotopic immunocompetent rat model of pancreatic cancer, which was established in our laboratory.12 The in vivo results showed that suramin had an influence on primary tumor growth, metastasis, and microvessel density in tumor bearing rats. The volumes of DSL-6A tumors in the treated groups were significantly smaller than those of the control animals after 4 and 8 weeks, and in half of the suramin-treated animals, there was no tumor detectable after 8 weeks. Dissemination in treated animals was almost completely depressed after 8 weeks. Intravital microscopy showed that control animals displayed irregular tumor blood vessels. It has been shown that tumor blood vessels have multiple abnormalities, like sprouting, proliferation, and remodeling, resulting from the bizarre environment in which they grow.27 Treatment of tumors with angiogenesis inhibitors can stop new vessel growth, cause regression of some vessels, and normalize others. Suramin therapy resulted in a complete regression of tumor macrovessels and a significant reduction of microvessel density. This results confirm that suramin inhibits angiogenesis by affecting tumor blood vessels. It is known that suramin interacts with a number of peptide growth factors, such as PDGF and bFGF,8,28 and acts as a functional VEGF-antagonist by binding to VEGF receptor-2 (KDR).9 We recently reported the inhibitiory effect of suramin on the VEGF-level in human pancreatic cancer cells, and these results strongly argue that tumor vessel permeability was significantly higher in treated animals vs control animals.
We did not note any apparent side effects of suramin such as a change in food intake or activity in our study. As a surrogate marker of toxicity, animal weights were observed throughout the in vivo study and were not found to be different at autopsy in any group. Other investigators claim that the clinical use of suramin is limited by its toxicity, which is mainly characterized by the development of a polyneuropathy.25 As a consequence of suramin’s toxicity, a new generation of suramin analogs is currently being investigated and seems to be a promising approach to circumvent toxic side effects while preserving the advantages of suramin’s anti-tumor activities.29,30 A reasonable alternative to suramin analogs is the application of suramin in low concentrations. Recently, it has been reported that low-dose administration of suramin as a chemosensitizer was able to improve the effects of chemotherapy in a mouse model of human breast cancer without enhancing host toxicity. However, this concept of a combination therapy has yet to be investigated in pancreatic cancer.
Conclusion
The present report demonstrated an inhibitory effect of suramin on proliferation and viability of the rat pancreatic cancer cell line DSL-6A in vitro. In a clinical relevant immunocompetent orthotopic rat model of pancreatic cancer, therapy with suramin resulted in a decrease of tumor size and metastatic spread. In addition, we assumed an anti-angiogenic effect of suramin as verified by the reduction of microvessel density in primary tumors of animals. In summary, our results strongly argue for further investigation of suramin as a part of novel treatment strategies for human pancreatic cancer.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics 2007, CA: a. Cancer J Clin 2007;57:43–66.
Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–1027.
McNamara DA, Harmey JH, Walsh TN, Redmond HP, Bouchier-Hayes DJ. Significance of angiogenesis in cancer therapy. Br J Surg 1998;85:1044–55. (erratum appears in Br J Surg 1998 Oct;85(10):1449).
Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol 2001;61:253–270.
Kaur M, Reed E, Sartor O, Dahut W, Figg WD. Suramin’s development: What did we learn? Invest New Drugs 2002;20:209–219.
Coffey RJ Jr, Leof EB, Shipley GD, Moses HL. Suramin inhibition of growth factor receptor binding and mitogenicity in AKR-2B cells. J Cell Physiol 1987;132:143–148.
Kathir KM, Kumar TK, Yu C. Understanding the mechanism of the antimitogenic activity of suramin. Biochemistry 2006;45:899–906.
La Rocca RV, Stein CA, Danesi R, Myers CE. Suramin, a novel antitumor compound. J Steroid Biochem Mol Biol 1990;37:893–898.
Waltenberger J, Mayr U, Frank H, Hombach V. Suramin is a potent inhibitor of vascular endothelial growth factor. A contribution to the molecular basis of its antiangiogenic action. J Mol Cell Cardiol 1996;28:1523–1529.
Bhargava S, Hotz B, Hines OJ, Reber HA, Buhr HJ, Hotz HG. Suramin inhibits not only tumor growth and metastasis but also angiogenesis in experimental pancreatic cancer. J Gastrointest Surg 2007;11:171–178.
Hotz HG, Reber HA, Hotz B, Yu T, Foitzik T, Buhr HJ, Cortina G, Hines OJ. An orthotopic nude mouse model for evaluating pathophysiology and therapy of pancreatic cancer. Pancreas 2003;26:e89–98.
Hotz HG, Reber HA, Hotz B, Foitzik T, Buhr HJ, Cortina G, Hines OJ. An improved clinical model of orthotopic pancreatic cancer in immunocompetent Lewis rats. Pancreas 2001;22:113–121.
Walz TM, Abdiu A, Wingren S, Smeds S, Larsson SE, Wasteson A. Suramin inhibits growth of human osteosarcoma xenografts in nude mice. Cancer Res 1991;51:3585–3589.
Mithofer K, Schmidt J, Gebhard MM, Buhr HJ, Herfarth C, Klar E. Measurement of blood flow in pancreatic exchange capillaries with FITC-labeled erythrocytes. Microvasc Res 1995;49:33–48.
Hotz HG, Schmidt J, Ryschich EW, Foitzik T, Buhr HJ, Warshaw AL, Herfarth C, Klar E. Isovolemic hemodilution with dextran prevents contrast medium induced impairment of pancreatic microcirculation in necrotizing pancreatitis of the rat. Am J Surg 1995;169:161–166. (see comment).
Dewhirst MW, Shan S, Cao Y, Moeller B, Yuan F, Li CY. Intravital fluorescence facilitates measurement of multiple physiologic functions and gene expression in tumors of live animals. Dis Markers 2002;18:293–311.
Matheson PJ, Garrison RN. Intravital intestinal videomicroscopy: techniques and experiences. Microsurgery 2005;25:247–257.
Halin C, Rodrigo Mora J, Sumen C, von Andrian UH. In vivo imaging of lymphocyte trafficking. Annu Rev Cell Dev Biol 2005;21:581–603.
Klyscz T, Junger M, Jung F, Zeintl H. Cap Image-ein neuartiges computerunterstutztes Videobildanalysesystem fur die dynamische Kapillarmikroskopie. Biomedizinische Technik 1997;42:168–175.
Weidner N. Tumoural vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol 1998;184:119–122. (comment).
Choe G, Kim WH, Park JG, Kim YI. Effect of suramin on differentiation of human stomach cancer cell lines. J Korean Med Sci 1997;12:433–442.
Shin R, Naomoto Y, Kamikawa Y, Tanaka N, Orita K. Effect of suramin on human esophageal cancer cells in vitro and in vivo. Scand J Gastroenterol 1997;32:824–828.
Song S, Yu B, Wei Y, Wientjes MG, Au JL. Low-dose suramin enhanced paclitaxel activity in chemotherapy-naive and paclitaxel-pretreated human breast xenograft tumors. Clin Cancer Res 2004;10:6058–6065.
Lu Z, Wientjes TS, Au JL. Nontoxic suramin treatments enhance docetaxel activity in chemotherapy-pretreated non-small cell lung xenograft tumors. Pharm Res 2005;22:1069–1078.
Garcia-Schurmann JM, Schulze H, Haupt G, Pastor J, Allolio B, Senge T. Suramin treatment in hormone- and chemotherapy-refractory prostate cancer. Urology 1999;53:535–541.
Gagliardi A, Hadd H, Collins DC. Inhibition of angiogenesis by suramin. Cancer Res 1992;52:5073–5075.
Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 2005;15:102–111.
Pesenti E, Sola F, Mongelli N, Grandi M, Spreafico F. Suramin prevents neovascularisation and tumour growth through blocking of basic fibroblast growth factor activity. Br J Cancer 1992;66:367–372.
Meyers MO, Gagliardi AR, Flattmann GJ, Su JL, Wang YZ, Woltering EA. Suramin analogs inhibit human angiogenesis in vitro. J Surg Res 2000;91:130–134.
Marchetti D, Reiland J, Erwin B, Roy M. Inhibition of heparanase activity and heparanase-induced angiogenesis by suramin analogues. Int J Cancer 2003;104:167–174.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hotz, B., Buhr, H.J. & Hotz, H.G. Intravital Microscopic Characterization of Suramin Effects in an Orthotopic Immunocompetent Rat Model of Pancreatic Cancer. J Gastrointest Surg 12, 900–906 (2008). https://doi.org/10.1007/s11605-008-0507-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0507-x