Abstract
Angiopoietin-2 (Ang-2) and vascular endothelial growth factor (VEGF) contribute to gastric cancer aggressiveness by up-regulating the expression of proteases. We evaluated the expression and the prognostic significance of angiogenic factors and proteases in 148 patients with R0-resected gastric cancer. Expression of VEGF, Ang-2, cyclooxygenase-2 (COX-2), urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1, matrix metalloproteinases (MMP)-1 and -9 were assayed by immunohistochemistry. After a mean of 63 ± 4 months, 81 out of 148 patients had died due to disease. The probability of being free of recurrence was 62, 48, and 42% at 2, 5, and 10 years, respectively. Single bivariate analysis identified VEGF, Ang-2, COX-2, PAI-1, and MMP-9 expression, along with several clinicopathological parameters (grade of curability, lymph node ratio, pTNM, pT, pN), as variables associated with both decreased disease-specific survival and recurrence. On multivariate analysis, after adjusting for significant clinical covariables, positive VEGF immunostaining was the primary prognostic factor, and no other tumor marker variable could add any significant improvement for the prediction, for both disease-specific survival (p = 0.001; HR, 3.27; 95% CI, 1.76 to 6.10) and tumor recurrence (p = 0.002; HR, 2.81; 95% CI, 1.48 to 5.35). Our study suggests that VEGF alone may be clinically useful for establishing therapeutic decisions in gastric cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the second leading cause of death from cancer worldwide, being responsible for 10% of all cancer-related deaths.1 The overall negative outcome for this neoplasia in western countries has not significantly improved over the last decades, with a 5-year survival rate estimated at 10–30%.2 Despite new adjuvant therapies, surgical resection still remains the only potentially curative treatment for this condition.3,4 Identification of prognostic and predictive factors that reflect the biology of GC (tumor spread and metastasis) is important for refining our assessment of prognosis and the selection of patients who may benefit from adjuvant systemic therapy.5
Angiogenesis, the formation of new blood vessels that develops from preexisting blood vessels, is a fundamental process in tumor growth and metastasis,6,7 and the vascular endothelial growth factor (VEGF) has been identified as the most potent and specific promoter of tumor angiogenesis, being secreted by almost all solid cancers.8 Among other pro-angiogenic factors, angiopoietin-2 (Ang-2) is a destabilization factor, rendering vasculature more amenable to sprouting under the influence of VEGF.9 Inhibition of VEGF and Ang-2 suppress angiogenesis and tumor growth in in vivo models.10,11 Moreover, proteolytic degradation of the basement membrane surrounding vascular endothelial cells with remodeling of the extracellular matrix (ECM) can allow endothelial cells to migrate and invade the surrounding stroma. The matrix metalloproteinases (MMPs) and the urokinase-type plasminogen activator (uPA) system are strongly implicated in this process.12
In gastric cancer, a positive correlation between VEGF expression and lymphatic invasion, lymph node metastasis, venous invasion, and patient outcome has been described by several groups;13–16 however, the association between Ang-2 and patient prognosis remains less well studied.17,18 We have previously reported that VEGF expression had an independent prognostic value with respect to tumor recurrence and overall survival in curatively resected gastric cancer patients.16 Several studies assessing protein expression have found that increase in the plasminogen activator (PA) components uPA and PAI-1 are associated with either aggressive tumor characteristics or a poor prognosis in gastric cancer.19,20 A recent in vitro study by Etoh et al.17 demonstrated that Ang-2 derived from Ang-2-transfected MKN-7 gastric cancer cells in the presence of VEGF up-regulated the expression of uPA, MMP-1, and MMP-9 in endothelial cells. Although previous studies have demonstrated up-regulation in the expression of these angiogenic factors and proteases in gastric cancer, most of these clinical studies analyzed only a few factors simultaneously, the study groups were frequently limited in number, and were heterogeneous (R0 vs R1–R2), and the follow-up of patients was usually short (less than 30 months). Taking all these clinical and experimental data together, it is unclear which of these molecular parameters is the most relevant to patient outcome.
In the present study, we therefore examined the expression of the pro-angiogenic factors VEGF, Ang-2, COX-2, and the proteases uPA, PAI-1, MMP-1, and MMP-9 in a large series of patients with homogeneous management (all were R0) and with extended follow-up (>5 years) and have correlated the immunohistochemical findings and clinicopathologic parameters with patient survival.
Patients and Methods
Study Population
We studied 148 patients with histologically verified primary gastric adenocarcinoma who underwent a curative (R0) resection between 1984 and 1999 at the Hospital Clínic, Barcelona, Spain. None of the patients entered into the study had evidence of distant metastases or had received neoadjuvant therapy. The study protocol was approved by the Ethics Committee of the Hospital Clinic.
Immunohistochemical expression of angiogenic factors (VEGF, Ang-2, COX-2) and proteases such as uPA, PAI-1, MMP-1, MMP-9, and microvessel density (MVD) in the gastric tumor was assessed. Sixteen epidemiological (age, gender), therapeutic (extent of gastrectomy, extent of lymphadenectomy, grade of curability, adjuvant therapy), and tumor-related (presence of signet-ring cell type, Lauren’s classification, degree of differentiation, lymphatic invasion, microvascular invasion, perineural invasion, ratio of involved to resected lymph nodes, pT, pN, and pTNM stage) variables were also evaluated.
The surgical procedure included a complete resection of the primary tumor and its lymphatic drainage. Based on the decision of the surgeon, 48 (32%) patients had a D1 lymphadenectomy, including the first-level lymph nodes (paracardial, major and minor curvature, supra-, and infrapyloric), and 100 (68%) patients had a D2 lymphadenectomy, in which the second-level nodes (left gastric artery, hepatic artery, celiac trunk, splenic hilum, and splenic artery) were also excised. The spleen and tail of the pancreas were resected only when required because of tumor invasion. To detect free abdominal tumor cells, analysis of abdominal fluid obtained by irrigation of the abdominal cavity immediately after laparotomy was routinely performed.
Tumors were classified according to the 2002 tumor-node-metastasis (TNM) system of the American Joint Committee on Cancer (AJCC).21 After histological examination of the resected specimens, the operation was classified as R0 resection if the microscopical evidence indicated complete tumor removal, with no involvement of distant lymph nodes or distant metastases, and no malignant cells in the abdominal-washing fluid. The curability grade, defined by the Japanese Gastric Cancer Association,22 divides the curative resection patients into two groups, A and B. Group A patients (no evidence of residual disease with high probability of cure) had tumor stage T1 or T2; N0 treated with lymphadenectomy D1 or D2 or N1 treated with D2; M0, no malignant cells in the abdominal-washing fluid; and margins of resection > 10 mm. Group B patients also had no evidence of residual disease, but had D1 lymphadenectomy in the presence of N1 or had margin resection < 10 mm). Group C patients, with residual disease, were not included in the study.
Follow-Up
Postoperative chemotherapy (mitomycin-C, 10 mg/m2, intravenously on day 1 and Tegafur, 400 mg/12 h, orally, for a 6-week cycle, until four cycles were completed) was administered in 75 (51%) patients in the context of investigational protocols.23 To investigate time to recurrence and disease-specific survival, two of the investigators evaluated all patients in a prospective manner every 3 months during the first 2 years and every 6 months thereafter. Histological confirmation of tumor recurrence was sought in all cases. Whenever follow-up was not complete, patients or their families were contacted by telephone, and death certificates were obtained from the Civil Register of the Barcelona Council. The final follow-up date was December 15, 2005.
Immunohistochemical Staining
Paraffin-embedded tissue blocks of formalin-fixed surgically resected samples were processed for conventional histological study and for immunohistochemical analysis. We used the automated immunohistochemical system TechMate 500 + Dako with the EnVision system (Dako). Briefly, 4-μm-thick sections were deparaffinised and hydrated through graded alcohol to water. Peroxidase was blocked for 7.5 min in ChemMate peroxidase-blocking solution (Dako S2023). Then, the slides were incubated with the primary antibodies for 30 min and washed in ChemMate buffer solution (Dako K5006). The peroxidase-labeled polymer, anti-rabbit (Dako K4011) or anti-mouse (Dako K4007) was then applied for 30 min. After washing in ChemMate buffer solution, the slides were incubated with the diaminobenzidine substrate chromogen solution (Dako K3468), washed in water, counterstained with hematoxylin, washed, dehydrated, cleared, and mounted in micromount (Surgipath 01730). Details of primary antibodies and antigen-retrieval techniques used in this investigation are given in Table 1.
Assessment of Immunohistochemical Staining
Expression of VEGF, Ang-2, COX-2, uPA, PAI-1, MMP-1, and MMP-9 was based on the intensity of staining and was assessed in the malignant epithelial cells (Fig. 1). Staining of endothelial, fibroblastic, or other stromal cells was not considered. All these factors were analyzed in the invasive front of the tumor away from the tumor center. Smooth muscle cells were used as positive internal controls for VEGF immunoreactivity,24 and endothelial cells of tumor-associated vessels were positive controls for Ang-2.25 The degree of expression of VEGF was classified into one of three categories according to the percentage of immunoreactive cells over the total number of cells counted: score 0: carcinoma cells were stained less intensely than normal smooth muscle; score 1: <30% of carcinoma cells were stained, or carcinoma cells staining intensity was similar to normal smooth muscle, and score 2: >30% of carcinoma cells were stained more intensely than normal smooth muscle. Sections with scores 1 and 2 were considered positive.16,24 The degree of expression of Ang-2 was graded as score 0 (no immunostaining in tumor cells or less intense to that seen in control), score 1 (staining equivalent), score 2 (more stained than control), or score 3 (intense staining easily seen under low power on a microscope), regardless of the number of cells stained.18,25 In statistical analysis, Ang-2 scores were handled in two groups (negative: 0–1; positive: 2–3). Based on a preliminary study on 15 cases where we assessed the staining pattern of COX-2, uPA, and PAI-1 in the normal gastric epithelium and tumor areas, normal and benign gastric epithelia adjacent to the tumor were considered positive control for these factors. Immunostaining with all three antibodies was assessed in the cytoplasm of tumor cells. The degree of expression for C0X-2, uPA, and PAI-1 was graded as negative (no immunostaining in tumor cells or staining equivalent or less intense to that seen in nonmalignant epithelium) or positive (more stained than control), regardless of the number of cells stained.19,26 The degree of expression of MMP-1 and MMP-9 was estimated, as described by other authors,27 by semiquantitative evaluation into three groups according to the percentage of immunoreactive cells over the total number cells counted: score 0, if <10% of cells stained; score 1, if 10–25% were immunoreactive; score 2, if 26–50% were immunoreactive, and score 3 if >51% were immunoreactive. Sections with score ≥2 were considered positive.
Representative examples of a VEGF, b Ang-2, c COX-2, d uPA, e PAI-1, f MMP-1, and g MMP-9 immunostaining in gastric adenocarcinoma of intestinal type. Positive VEGF immunoreactivity is detected in the cytoplasm of cancer cells in the invasive front of invasion (a). Strong cytoplasmic immunostaining of Ang-2 in tumor cells within the malignant gland (b). Intense COX-2 immunoreactivity is observed in the perinuclear region and cytoplasm of the malignant cells (c). Moderate and weak immunostaining for uPA and PAI-1, respectively, is present in the cytoplasm of tumor cells (d–e). Strong granulose-type cytoplasmic MMP-1 staining is detected in the luminal part of tumor cells within the malignant gland (f). Strong cytoplasmic and cell membranous staining for MMP-9 is seen in the tumor cells (g). Original magnifications: ×40 (a) and ×100 (b–g).
Microvessel Density
For microvessel density (MVD) evaluation, quantitative vessel counts were performed by the method described by Weidner and assessed by international consensus.28 The entire tumor sections were systematically scanned at ×40 magnification to find the areas of most intense neovascularization or hot spots. These were identified as having the highest density of brown staining, CD34-positive cells, or cell clusters. For each slide, the most vascular areas within the tumor mass were chosen. A ×250 field in these areas was counted, and the average counts of the fields were recorded. If multiple vascular hot spots were present, counts were performed in each hot spot. Microvessels were defined as a discrete CD34-positive endothelial cell aggregate, with or without definable lumina.
The specimens were evaluated independently by two experienced investigators (AV and J.P M), and staining degree was assessed without knowledge of the clinical data of the individual patient at the time of the review. Conflicts in scores were resolved by consensus.
Statistical Analysis
Disease-specific survival and tumor recurrence were the main end points for the single bivariate and multivariate analysis of prognostic factors. Disease-specific survival was calculated from the date of surgery until death due to the cancer, whereas time to recurrence was established from the date of surgery to the date of recurrence (including either locoregional relapse or distant metastases).
For single bivariate analyses of disease-specific survival and tumor recurrence, Kaplan–Meier curves were plotted and then compared using log-rank statistics. For continuous variables (i.e., age and MVD), the cut-off level chosen was their median value. Multivariate analyses were performed in a forward stepwise fashion by the Cox proportional hazards model, including those variables with a p value ≤ 0.1 in the single bivariate analysis and adjusting by clinical variables with prognostic significance.
Differences were considered significant when p values were less than 0.05. All the calculations were performed by using the statistical SPSS package for Windows (version 11.05; SPSS Inc., Chicago, IL, USA).
Results
A total of 148 patients were observed prospectively for an average of 63 ± 4 months. Patient characteristics and treatment parameters are described in Table 2. Five patients were lost to follow-up. A total of 93 patients died: 81 due to malignant disease and 12 without evidence of tumor. Eighty-one recurrences were seen, 36 of which presented as peritoneal or distant metastases, and 45 as local and regional recurrences.
The immunohistochemical detection levels of the angiogenic markers evaluated in the primary tumor is listed in Table 3.
Prognostic Factors of Tumor Recurrence
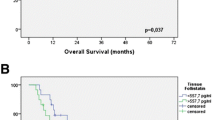
At the end of follow-up, the estimated mean time to recurrence was 52 ± 4 months (range, 9–252 months), the probability of being free of recurrence was 62, 48, and 42% at 2, 5, and 10 years, respectively (Fig. 2a). There were significant associations between tumor recurrence and tumor VEGF, Ang-2, COX-2, PAI-1, and MMP-9 expression in the single bivariate analysis (Table 4). Other significant variables affecting tumor recurrence in the bivariate analysis were grade of curability (p = 0.001), degree of differentiation (p = 0.026), ratio of lymph nodes (p = 0.001), pT stage (p = 0.001), pN stage (p = 0.001), and pTNM stage (p = 0.001).
Multivariate analysis of tumor recurrence showed VEGF expression (p = 0.001), grade of curability (p = 0.004), ratio of lymph nodes (p = 0.041), and extent of lymphadenectomy (p = 0.002) to have significant prognostic value. The Cox regression model, adjusted to the clinical variables, identified VEGF expression (p = 0.002; HR, 2.81; 95% CI, 1.48 to 5.35) as the primary prognostic factor, and no other tumor marker variable could add any significant improvement for the prediction.
Kaplan–Meier estimates of the probability of being free of recurrence after stratifying the patients according to VEGF expression in the primary tumor is represented in Fig. 2c.
Prognostic Factors of Disease-Specific Survival
After a mean follow-up of 63 ± 4 months (range, 9–252 months), 81 (55%) patients had died as a consequence of cancer progression, the probability of disease-specific survival being 66, 51, and 39%, at 2-, 5-, and 10 years, respectively (Fig. 2b). Single bivariate analysis revealed VEGF (p = 0.003), Ang-2 (p = 0.001), COX-2 (p = 0.014), PAI-1 (p = 0.024), and MMP-9 (p = 0.006) expression, along with extent of lymphadenectomy (p = 0.001), Lauren’s classification (p = 0.006), lymphatic invasion (p = 0.001), ratio of involved-to-resected lymph nodes (p = 0.001), grade of curability (p = 0.001), pT stage (p = 0.001), pN stage (p = 0.001), pTNM stage (p = 0.001), and adjuvant therapy (p = 0.006), as significant factors influencing disease-specific survival.
Multivariate analysis for disease-specific survival showed VEGF expression (p = 0.016), Ang-2 (p = 0.026), and PAI-1 expression (p = 0.020), grade of curability (p = 0.020), ratio of lymph nodes (p = 0.028), and extent of lymphadenectomy (p = 0.025) to have significant prognostic value. The Cox regression model, adjusted to the clinical variables, identified VEGF expression (p = 0.001; HR, 3.27; 95% CI, 1.76 to 6.10) as the primary prognostic factor, and no other tumor marker variable could add any significant improvement for the prediction. Kaplan–Meier analysis of disease-specific survival after stratifying patients according to VEGF expression in the primary tumor is depicted in Fig. 2d.
Discussion
The current TNM staging system of GC based on conventional pathologic features is still inadequate for the prognostic characterization because patients with identical clinical or pathological stages may differ widely in their clinical evolution. The assessment of tumor angiogenesis could provide supplementary prognostic information in patients with GC, identifying a subgroup with highly aggressive tumors and high likelihood of disease recurrence and death. An indirect way to measure angiogenic activity in cancers is to evaluate the expression of angiogenic factors in tumor tissue. We undertook the present immunohistochemical study, one of the largest to date, to simultaneously assess the expression of VEGF, Ang-2, COX-2, uPA, PAI-1, MMP-1, and MMP-9, and to determine which of these angiogenic factors was most closely correlated to GC recurrence and survival. This investigation demonstrated the prognostic value of VEGF, Ang-2, COX-2, PAI-1, and MMP-9 expression in GC patients undergoing a curative resection. All these factors were associated with decreased time to recurrence and disease-specific survival in Kaplan–Meier analysis. However, the Cox regression model, adjusted to the clinical variables, demonstrated that positive VEGF immunostaining was the only angiogenic marker with independent prognostic significance for poor clinical outcome.
Our report has potential limitations, namely, that the immunohistochemical study was conducted retrospectively and that 75 out of 148 patients (51%) received adjuvant chemotherapy. Clinical data, however, were collected prospectively, and immunohistochemical assessments were carried out in a blinded fashion using a methodology previously reported by others.
In many cancers, tumor VEGF expression was found to be a significant marker for tumor recurrence or reduced survival independent of conventional clinicopathological variables.29 Four studies from Japan and our previous study identified VEGF as the strongest predictor of survival in GC by multivariate analysis.14,16,30–32 However, it was still controversial which factor among those related to the process of angiogenesis was most important in the progression of GC. In the present study, we directly compared more angiogenic factors than had been previously evaluated in a large group of patients, and found, by multivariate analysis, that only VEGF was the primary prognostic factor. We had a large number of patients, most with earlier stages (64% stages I–II) and a longer follow-up (>5 years) than previous reports. Interestingly, 64% of our patients had stages I–II, so positive VEGF immunostaining was able to discriminate, even in these stages, patients with potential unfavourable outcomes who may benefit from a closer follow-up or their inclusion in protocols of adjuvant chemotherapy. VEGF immunostaining was present in a significantly greater percentage of gastric cancer patients than any other individual marker. This observation suggests that VEGF might be a final common pathway for other angiogenesis factors, but our data do not allow us to confirm or reject this hypothesis.
Preclinical studies of agents that selectively target VEGF and its receptors in GC have shown significant antitumor effects, confirming that this ligand/receptor system is a valid target for gastric cancer therapy.33 Future areas of development may include the addition of newer chemotherapeutic agents combined with targeted therapies such as the anti-VEGF agents (bevacizumab) in patients with predicted poor outcome based on tumor VEGF assessment.34
The mechanism of Ang-2 expression and its regulation in GC are mostly unknown. Increased Ang-2 mRNA levels have been detected in GC compared with normal tissue, and patients with increased levels of Ang-2 mRNA showed more frequent vascular involvement and more advanced stages of disease than those with low Ang-2-expression.17,18,35 Recently, Etoh et al.,17 using a coculture assay of endothelial cells (ECs) and Ang-2- transfected MKN-7 GC cells, demonstrated enhanced expression of uPA, PAI-1 and metalloproteinases (MMP-1, MMP-9) in ECs by Ang-2 derived from transfectants in the presence of exogenous VEGF. They concluded that overexpressed Ang-2, together with VEGF, might promote angiogenesis in GC. With regard to prognosis and in agreement with the results of Etoh et al.,17 our study shows that the prognosis of patients with Ang-2 expression is shorter, but in multivariate analysis, Ang-2 expression was not an independent prognostic factor.
Over the past two decades, numerous studies have confirmed an association between COX-2 overexpression and tumor progression and increased angiogenesis in several solid malignancies.8 Significant associations between COX-2 immunoreactivity and gastric cancer with respect to depth of tumor invasion, tumor grade, and lymph node involvement have been described.36–38 An impact of COX-2 expression on survival has been found in some, but not all studies.26,36 In our single bivariate analysis, COX-2 immunoreactivity was associated with decreased cancer-specific survival. However, contrary to the findings of Mrena et al.,38 we failed to demonstrate high COX-2 as an independent prognostic factor in GC, either in early (stages I–II) and in advanced stages (III–IV).
It was originally believed that uPA promoted cancer dissemination simply by degrading the ECM, thus allowing invasion and metastasis. It is now clear that uPA has additional activities stimulating angiogenesis, mitogenesis, cell migration, and cell adhesion involved in cancer spreading.39 Because uPA is directly involved in metastasis, it is an ideal candidate for investigation as a prognostic factor. In fact, high uPA concentrations have been shown to correlate with aggressive disease in patients with breast, esophageal, gastric, colorectal, and endometrial cancers.40
Heiss et al.19 demonstrated the prognostic impact of uPA, uPA-R, and PAI-1 expression, determined by immunohistochemistry, in 139 patients with curatively resected GC. uPA and especially PAI-1 were inversely correlated with recurrence-free survival. In multivariate analysis, PAI-1 was a strong independent prognostic factor. Similarly, in a series of 76 GC patients41 in whom uPA and PAI-1 tumor concentrations were measured by ELISA, these markers were inversely correlated with recurrence-free and overall survival, but only PAI-1 was an independent prognostic factor in multivariate analysis. Kaneko et al.20 evaluated immuhistochemically the expression of uPA and PAI-1 in 101 GC patients. The rates of positive expression in cancer cells of uPA and PAI-1 were 22.8 and 36.6%, respectively. Expression of uPA and PAI-1 in tumor cells was significantly associated with poor differentiation and vascular invasion. Furthermore, multivariate analysis identified uPA expression as an independent prognostic factor. Our survival analysis demonstrated that patients with PAI-1 expression had a significantly lower survival rate than those without it. However, in our study, the expression rates of uPA and PAI-1 by immunohistochemistry, 9 and 15%, respectively, were lower than those observed in the studies by Heiss et al. and Kaneko et al.19,20. It should be emphasized that assessment of tumor expression of uPA and PAI-1 by immunohistochemistry (IHC) can be misleading because these proteins are synthesized and expressed in varying proportions by both tumor and stromal cells. Such heterogeneity is difficult to quantify using IHC. It is also unclear whether it is their (relative) levels in the stroma or in the tumor cells themselves that is the most relevant to patient outcome42,43
In summary, we found that VEGF expression in primary tumor tissue is significantly associated with a worse prognosis in GC patients after curative surgical resection. Prognostic information based on VEGF expression, unlike multiple other tumor markers that we have studied, was independent of classic clinico-pathological parameters such as primary tumor extent and degree of lymph node involvement. Our results may suggest the potential value of VEGF assessment to identify patients at high risk for tumor recurrence and for whom adjuvant systemic therapy might be recommended. Future studies, particularly clinical trials involving anti-angiogenic agents and standard chemotherapeutic regimens, will be required to demonstrate the ultimate clinical relevance of VEGF expression in the management of patients with GC.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. 2002. CA Cancer J Clin 2005;55:74–108.
Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005;241:27–39.
Maehara Y, Kakeji Y, Koga T, Emi Y, Baba H, Akazawa K, Sugimachi K. Therapeutic value of lymph node dissection and the clinical outcome for patients with gastric cancer. Surgery 2002;131:S85–S91.
Cunningham D, Allum W, Stenning S, Thompson JN, Van de Velde CJH, Nicolson M, Scarffe H, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley, BVerma M, Weeden S, Chua YJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20.
Riley RD, Abrams KR, Sutton AJ, Lambert PC, Jones DR, Heney D, Burchill SA. Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer 2003;88:1191–1198.
Folkman J. Angiogenesis in cancer, vascular rheumatoid and other diseases. Nature Med 1995;1:27–31.
Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005;438:932–936.
Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–1027.
Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol 2004;204:1–10.
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993;362:841–844.
Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, Hawkins N, Rosenfeld R, Davy E, Graham K, Jacobsen F, Stevenson S, Ho J, Chen Q, Hartmann T, Michaels M, Kelley M, Li L, Sitney K, Martin F, Sun JR, Zhang N, Lu J, Estrada J, Kumar R, Coxon A, Kaufman S, Pretorius J, Scully S, Cattley R, Payton M, Coats S, Nguyen L, Desilva B, Ndifor A, Hayward I, Radinsky R, Boone T, Kendall R. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 2004;6:507–516.
Bergers G, Coussens LM. Extrinsic regulators of epithelial tumor progression: metalloproteinases. Curr Opin Genet Dev 2000;10:120–127.
Baba M, Konno H, Maruo Y, Tanaka T, Kanai T, Matsumoto K, Matsura M, Nishino N, Maruyama K, Nakamura S, Baba S. Relationship of p53 and vascular endothelial growth factor expression of clinicopathological factors in human scirrhous gastric cancer. Eur Surg Res 1998;30:130–137.
Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996;77:858–863.
Maehara Y, Kabashima A, Koga T, Tokunaga E, Takeuchi H, Kakeji Y, Sugimachi K. Vascular invasion and potential for tumor angiogenesis and metastasis in gastric carcinoma. Surgery 2000;128:408–416.
Fondevila C, Metges JP, Fuster J, Grau JJ, Palacin A, Castells A, Volant A, Pera M. p53 and VEGF expression are independent predictors of tumour recurrence and survival following curative resection of gastric cancer. Br J Cancer 2004;90:206–215.
Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res 2001;61:2145–2153.
Sun XD, Liu XE, Wu JM, Cai XJ, Mou YP, Li JD. Expression and significance of angiopoietin-2 in gastric cancer. World J Gastroenterol 2004;10:1382–1385.
Heiss MM, Babic R, Allgayer H, Gruetzner KU, Jauch KW, Loehrs U, Schildberg FW. Tumor-associated proteolysis and prognosis: new functional risk factors in gastric cancer defined by the urokinase-type plasminogen activator system. J Clin Oncol 1995;13:2084–2093.
Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci 2003;94:43–49.
Greene FL, Page DL, Fleming ID, Fritz A, Balch CH, Haller DG, Morrow M, eds. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer-Verlag, 2002.
Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma-2nd English Edition. Gastric Cancer 1998;1:10–24.
Grau JJ, Estape J, Fuster J, Filella X, Visa J, Teres J, Soler G, Albiol S, Garcia-Valdecasas JC, Grande L, Bombi JA, Bordas JM, Alcobendas F. Randomized trial of adjuvant chemotherapy with mitomycin plus ftorafur versus mitomycin alone in resected locally advanced gastric cancer. J Clin Oncol 1998;16:1036–1039.
Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer 1997;79:206–213.
Nakayama T, Yoshizaki A, Kawahara N, Ohtsuru A, Wen CY, Fukuda E, Nakashima M, Sekine I. Expression of Tie-1 and 2 receptors, and angiopoietin-1, 2 and 4 in gastric carcinoma; immunohistochemical analyses and correlation with clinicopathological factors. Histopathology 2004;44:232–239.
Tatsuguchi A, Matsui K, Shinji Y, Gudis K, Tsukui T, Kishida T, Fukuda Y, Sugisaki Y, Tokunaga A, Tajiri T, Sakamoto C. Cyclooxygenase-2 expression correlates with angiogenesis and apoptosis in gastric cancer tissue. Hum Pathol 2004;35:488–495.
Zhang S, Li L, Lin JY, Lin H. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol 2003;9:899–904.
Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Belien JA, de Waal RM, Van Marck E, Magnani E, Weidner N, Harris AL, Dirix LY. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 2002;38:1564–1579.
Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol 2001;19:1207–1225.
Maeda K, Kang SM, Onoda N, Ogawa M, Kato Y, Sawada T, Chung KH. Vascular endothelial growth factor expression in preoperative biopsy specimens correlates with disease recurrence in patients with early gastric carcinoma. Cancer 1999;86:566–571.
Saito H, Tsujitani S, Kondo A, Ikegushi M, Maeta M, Kaibara N. Expression of vascular endothelial growth factor correlates with hematogenous recurrence in gastric carcinoma. Surgery 1999;125:195–201.
Ichikura T, Tomimatsu S, Ohkura E, Mochizuki H. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol 2001;78:132–137.
McCarty MF, Wey J, Stoeltzing O, Liu W, Fan F, Bucana C, Mansfield PF, Ryan AJ, Ellis LM. ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor with additional activity against epidermal growth factor receptor tyrosine kinase, inhibits orthotopic growth and angiogenesis of gastric cancer. Mol Cancer Ther 2004;3:1041–1048.
Shah MA, Ramanathan RK, Ilson D, Randazzo J, Schwartz GK, Tse A, Tse A, D’Adamo D, Levner A, Capanu M, Kelsen DP. Final results of a multicenter phase II study of irinotecan (CPT), cisplatin (CIS), and bevacizumab (BEV) in patients with metastatic gastric or gastroesophageal (GEJ) adenocarcinoma (NCI #6447). J Clin Oncol 2006;24:183s.
Wang J, Wu K, Zhang D, Tang H, Xie H, Hong L, Pan Y, Lan M, Hu S, Ning X, Fan D. Expressions and clinical significances of angiopoietin-1, -2 and Tie2 in human gastric cancer. Biochem Biophys Res Commun 2005;337:386–393.
Chen CN, Sung CT, Lin MT, Lee PH, Chang KJ. Clinicopathologic association of cyclooxygenase 1 and cyclooxygenase 2 expression in gastric adenocarcinoma. Ann Surg 2001;233:183–188.
Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, Kim SJ. Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol 2003;37:28–33.
Mrena J, Wiksten JP, Thiel A, Kokkola A, Pohjola L, Lundin J, Ristimaki A, Haglund C. Cyclooxygenase-2 is an independent prognostic factor in gastric cancer and its expression is regulated by the messenger RNA stability factor HuR. Clin Cancer Res 2005;11:7362–7368.
Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 1997;72:1–22.
Duffy MJ, Maguire TM, McDermott EW, O’Higgins N. Urokinase plasminogen activator: a prognostic marker in multiple types of cancer. J Surg Oncol 1999;71:130–135.
Nekarda H, Schmitt M, Ulm K, Wenninger A, Vogelsang H, Becker K, Roder JD, Fink U, Siewert JR. Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res 1994;54:2900–2907.
Okusa Y, Ichikura T, Mochizuki H. Prognostic impact of stromal cell-derived urokinase-type plasminogen activator in gastric carcinoma. Cancer 1999;85:1033–1038.
Okusa Y, Ichikura T, Mochizuki H, Shinomiya N. Urokinase type plasminogen activator and its receptor regulate the invasive potential of gastric cancer cell lines. Int J Oncol 2000;17:1001–1005.
Acknowledgments
We thank Llorenç Badiella from the Department of Statistics, Universitat Autonoma de Barcelona, for expert help in the statistical analysis and Dr. Alan Cameron and Dr. Joaquim Bellmunt for editorial comments.
This work was supported in part by research grants from the Fundació “la Caixa” (LC 02/126-00, from the Instituto de Salud Carlos III (RC03/02 and RC03/10), and from Fundación Científica de la Asociación Española Contra el Cáncer (Junta Provincial de Albacete).
Author information
Authors and Affiliations
Corresponding author
Additional information
Óscar Vidal, Antonio Soriano-Izquierdo, contributed equally to this work.
Rights and permissions
About this article
Cite this article
Vidal, Ó., Soriano-Izquierdo, A., Pera, M. et al. Positive VEGF Immunostaining Independently Predicts Poor Prognosis in Curatively Resected Gastric Cancer Patients: Results of a Study Assessing a Panel of Angiogenic Markers. J Gastrointest Surg 12, 1005–1014 (2008). https://doi.org/10.1007/s11605-007-0336-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-007-0336-3