Abstract
Purpose
To investigate the contribution of preoperative apparent diffusion coefficient (ADC) values in the differential diagnosis of pediatric posterior fossa tumors.
Methods
Forty-two pediatric patients (mean age 7.76 ± 4.58 years) with intra-axial tumors in the infra-tentorial region underwent magnetic resonance imaging. ADC measurement was performed using regions of interest, obtained from the solid component of the mass lesions. ADC ratios were calculated by dividing the ADC values from the mass lesions by the ADC values from normal cerebellar parenchyma. Lesions were categorized as juvenile pilocytic astrocytoma (JPA), ependymoma and medulloblastoma based on histopathological diagnosis. ADC values of the lesions and histopathological diagnoses were statistically correlated.
Results
Histopathological diagnosis showed that 14 lesions were JPA, 10 were ependymoma; 18 were medulloblastoma. Both ADC values and ADC ratios were significantly correlated with tumor types (p <0.05). Astrocytoma was distinguished from ependymoma with sensitivity 85.7% and specificity 90% using an ADC ratio ≥1.7 and medulloblastoma was distinguished from ependymoma with sensitivity 100% and specificity 88.89% using an ADC ratio ≤1.18.
Conclusion
Preoperative ADC values could differentiate the main histological subtypes of pediatric posterior fossa tumors with high sensitivity and specificity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central nervous system (CNS) tumors constitute the second most frequent tumor group in childhood [1, 2]. Fifty percent of pediatric intracranial tumors occur at the posterior fossa and more than 90% comprise three tumor groups: juvenile pilocytic astrocytoma (JPA), ependymoma, medulloblastoma, and atypical teratoid rhabdoid tumor (AT/RT) [3].
Definitive diagnosis for pediatric brain tumors are histopathologically often made by postoperative biopsy, and rarely by preoperative biopsy. If the correlation between the diagnosis based on medical imaging and the diagnosis based on histopathology is increased, medical imaging could contribute to better treatment choice and possibly a better survival rate [4]. Conventional brain magnetic resonance imaging (MRI) is the most important diagnostic imaging methodology in evaluation of posterior fossa tumors and exhibits some special radiological findings which can be used in the differential diagnosis. These are typical inferior vermis localization of medulloblastomas; hypointense or isointense signal characteristics of tumors with respect to gray matter signal on T2-weighted images; pilocytic astrocytoma having cystic and mural nodules, and its high signal on T2-weighted images; extent of ependymoma to foramen magnum and out of fourth ventricle; and lastly a more heterogeneous form of AT/RT compared to other tumors. However, these radiological findings are not always available and thus may not allow the differentiation of all tumoral lesions and their true diagnoses [4,5,6,7,8].
Diffusion weighted (DW)-MRI and apparent diffusion coefficient (ADC) could contribute to the diagnosis of pediatric cerebellar tumors, based on cellularity [5,6,7,8]. In this single-centered and retrospective study, ADC values of cerebellar tumors in a pediatric age group were quantitatively evaluated and their contribution to preoperative definitive diagnosis was investigated.
Materials and methods
Patient selection
A total of 90 pediatric patients who underwent MRI between January 2007 and May 2015 and were diagnosed with mass lesions at infratentorial and intra-axial locations were included in this study. The study was approved by the local ethics committee and is compliant with the Helsinki Declaration. The mean age of the patients (23 male, 19 female) was 7.76 ± 4.58 years and the age ranged from 1 to 18 years. Histopathological diagnoses of posterior fossa tumors were performed based on pathology reports by a pediatric oncologist who was blinded to radiological images. The tumors were classified into three groups: JPA, ependymoma and medulloblastoma. The same doctor recorded the age and gender of the patients.
Magnetic resonance imaging
MR imaging of all patients was acquired on 1.5-T clinical MR scanners (Magnetom Aera, Siemens, Erlangen, Germany and Philips Intera, the Netherlands) using a standard head coil. Conventional MRI protocol included pre- and post-contrast T1-weighted, T2-weighted, fluid attenuated inversion recovery and DW-MRI sequences. Diffusion-weighted MR images were acquired using single-shot spin-echo echo-planar-imaging sequences with parameters: echo time (TE) = 83 ms and repetition time (TR) = 3500 ms, slice thickness (ST) = 5 mm, slice gap = 0.5 mm, field-of-view (FOV) = 230 × 230 mm, acquisition matrix = 128 × 99, b = 0–1000 s/mm2. ADC maps were automatically generated by the MR scanner, and DW-MR images and ADC maps were transferred to a workstation (Syngo MR D13, Siemens, Healthcare) for further analysis.
Analysis of MR images
MR images and ADC maps of the patients were evaluated by two radiologists in consensus who were blinded to histopathological diagnoses. One radiologist had 9 years of experience after radiology residency and the other was in his last year of radiology residency. Region of interest (ROI) measurements were obtained from the solid component of the tumoral lesions with the lowest signal on ADC maps, excluding necrotic and hemorrhagic areas. ROIs were ~1 cm2 and obtained from three consecutive slices, and the average of the measured values was calculated. The calculated mean was divided by the measurement value obtained from normal appearing cerebellar parenchyma.
Statistical analysis
The data were evaluated using IBM Statistics 22.0 (IBM Corp., Armonk, New York, USA) software. A Shapiro–Wilk test and Q–Q graphics were used to test the normality of the data. Homogeneity of the variances was studied using a Levene test. Descriptive statistics were reported as mean ± standard deviation (\(\bar{x} \pm sd\)), minimum–maximum values. Group comparisons were made using one-way variance analysis and multiple comparisons were made using the Tukey test. ROC analysis was performed to determine the ADC ratios to differentiate cerebellar tumors. The relationship among categorical variables was studied using the chi-square test and a value of p < 0.05 was considered significant.
Results
The following cases were excluded from the study: 4 cases with infratentorial extra-axial mass, 14 cases with brain stem glioma, 14 cases with no access to histopathological diagnosis due to the fact that their surgery was not done in our hospital, 2 cases with artifacts on MR images, 3 cases with no ADC maps, and 1 case with AT/RT. A total of 42 cases with cerebellar mass lesions were included in the study.
Histopathological diagnosis of 42 lesions showed that 14 lesions (32.6%) were JPA, 10 (23.3%) were ependymoma; and 18 (41.9%) were medulloblastoma. The mean age of all patients was 7.69 ± 4.66 years (19 female, range 1–18 years). The mean ages for JPA, ependymoma and medulloblastoma were 8.35 ± 5.31, 8 ± 4.74 and 7.38 ± 4.14, respectively. We did not determine any statistically significant relationship between age and tumor types (p > 0.05).
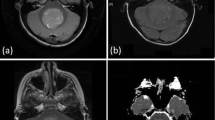
Mean ADC values for JPA, ependymoma, and medulloblastoma were 1427.5 ± 274.62 × 10−6, 1035 ± 217.07 × 10−6, and 707.16 ± 213.47 × 10−6 mm2/s, respectively (Fig. 1a–d). Statistically significant correlation between ADC values and tumor types were obtained (p < 0.05) (Table 1). Group comparisons indicated that there were statistically significant differences obtained among all tumor types (p < 0.005).
A 3-year-old girl with juvenile pilocytic astrocytoma (JPA). a Axial T2-weighted image shows a marked hyperintense regular outer-edged infratentorial mass lesion which extends from cerebellar vermis to fourth ventricle and compresses the fourth ventricle from posterior. b Axial T1-weighted image shows that the lesion is hypointense. c Post-contrast axial T1-weighted image shows a solid, homogenously enhancing mass lesion. d ADC image shows the ROI of lesion and cerebellum (ADC ratio: 2.12)
ADC ratios were obtained by dividing mean ADC values of lesions by ADC values of normal cerebellum parenchyma (Fig. 2a–d). The mean ADC ratios for JPA, ependymoma, and medulloblastoma were 1.95 ± 0.30, 1.50 ± 0.20, and 1.02 ± 0.30, respectively (Fig. 3a–d). The correlation between mean ADC ratio and tumor types were statistically significant (p < 0.05) (Table 2).
An 11-year-old boy with ependymoma. a Axial T2-weighted image shows a heterogeneous hyperintense regular outer edged infratentorial mass lesion that is filling the fourth ventricle. b Axial T1-weighted image shows the lesion is hypointense with respect to gray matter. c Post-contrast axial T1-weighted image shows the lesion is homogenously enhancing. e ADC image shows the ROI of lesion and cerebellum (ADC ratio: 1.45)
A 17-year-old girl with medulloblastoma. a Axial T2-weighted image shows a slightly hyperintense infratentorial mass lesion that is filling the fourth ventricle. b Axial T1-weighted image shows that the lesion is hypointense with respect to gray matter. c Post-contrast axial T1-weighted image shows the heterogeneously enhancing mass lesion. d ADC image shows the ROI of lesion and cerebellum (ADC ratio: 0.79)
Astrocytoma was distinguished from ependymoma with sensitivity 85.7% and specificity 90% using an ADC ratio ≥1.7 and medulloblastoma was distinguished from ependymoma with sensitivity 100% and specificity 88.89% using an ADC ratio ≤1.18 (Table 3).
Discussion
In this study, we investigated the contribution of ADC ratio of cerebellar-located pediatric tumors to normal appearing cerebellar parenchyma in preoperative diagnosis. ADC ratio thresholds of ≤1.18, 1.18–1.7, ≥1.7 allowed preoperative diagnosis of medulloblastoma, ependymoma, and JPA with high specificity and sensitivity. DWI is an MRI technique with contrast based on microscopic translational (Brownian) motion. It allows tissue characterization and tumor grastudied 140 patientsding when conventional sequences (T1- and T2-weighted) cannot [9, 10]. ADC values show the absolute value of the measured diffusion amount. On an ADC map, a low ADC value indicated restricted diffusion while a high ADC value indicated increased diffusion. JPA consists of low density cells and Rosenthal fibrils; the extracellular space is wider and the cellularity is less than in other posterior fossa tumors. Thus, they cause less restricted diffusion compared to extracellular compartments [11]. Medulloblastomas are composed of high density cells having high nucleus/cytoplasm ratio, thus having less extracellular matrix. As a result, increased diffusion restriction is observed [12, 13]. On the other hand, ependymoma has intermediate cellularity [14, 15].
There are studies in the literature that used ADC values in diagnosis and differentiation of all tumor types. Yamasaki et al. [16] studied all brain tumor types in 275 adult patients and showed that ependymomas have ADC >1.0 × 10−3 mm2/s and primitive neuroectodermal tumors have ADC <1.0 × 10−3 mm2/s. Guvain et al. [17] studied low-grade gliomas, embryonal tumors (medulloblastoma, AT/RT) and non-embryonal high-grade tumors (glioma, germ cell tumor, ependymoma) in 12 pediatric patients and showed that mean ADC values were 1.33 ± 0.21 10−3 mm2/s, 0.72 ± 0.20 10−3 mm2/s, and 1.22 ± 0.09 10−3 mm2/s, respectively.
Thus, low-grade glial tumors and embryonal tumors were differentiated from each other; however, non-embryonal tumors were shown to have similar ADC values as the other two groups. Rumboldts et al. [7] studied posterior fossa tumors (17 astrocytoma, 5 ependymoma, 8 medulloblastoma, and 2 AT/RT) in 32 patients and measured ADC values for astrocytoma and medulloblastoma (>1.4 × 10−3 and <0.9 × 10−3 mm2/s, respectively), and diagnosed their cases with 100% sensitivity.
Jaremko et al. [6] studied JPA, ependymoma, medulloblastoma, and AT/RT in a group of 40 patients and reported that a threshold ADC value of 0.8 × 10−3 mm2/s can be used to differentiate 88% of JPA from medulloblastoma, whereas the ADC values obtained for ependymoma and medulloblastoma coincided. However, we report in our study that (Table 3) although ADC values for JPA, ependymoma and embryonal tumors sometimes coincided when maximum and minimum values are considered, they differed significantly when mean ADC values were considered.
There are studies in the literature that tried to differentiate cerebellar tumors using the ratio of ADC values of cerebellar-located tumors to normal cerebellar parenchyma. Rumboldt et al. [7] determined the ADC ratios for JPA, ependymoma, and medulloblastoma as 2.11 ± 0.36, 1.39 ± 0.18, and 0.84 ± 0.14, respectively, and did not observe overlap in ADC ratios. Gimi et al. [5] studied 79 patients (31 JPA, 27 medulloblastoma, 14 ependymoma, 7 AT/RT) and measured the ADC ratios as 2.30, 1.58, 0.97, and 0.83 for astrocytoma, ependymoma, medulloblastoma, and AT/RT. They differentiated astrocytoma from ependymoma with 92% sensitivity and 79% specificity using an ADC ratio ≥ 1.7, and differentiated embryonal tumors (medulloblastoma and AT/RT) from ependymoma with 93% sensitivity and 88% specificity using an ADC ratio ≤1.2.
Koral et al. [8] studied 140 patients (68 embryonal tumors; 58 medulloblastomas, 10 AT/RT; 51 astrocytomas, 21 ependymomas) and could differentiate astrocytoma from ependymoma with 78% sensitivity and 78% specificity using an ADC ratio ≥1.8 and could differentiate embryonal tumors from ependymoma with 87% sensitivity and 83% specificity using an ADC ratio ≤1.2. They diagnosed 92% of embryonal tumors, 84% of astrocytomas, and 71.42% of ependymomas. In our study, the mean ADC ratios were found to be 1.95 ± 0.30, 1.50 ± 0.20, and 1.02 ± 0.30 for JPA, ependymoma, and medulloblastoma. Thus, we differentiated astrocytoma from ependymoma with 85.7% sensitivity and 90% specificity using an ADC ratio ≥1.7 and differentiated medulloblastoma from ependymoma with 100% sensitivity and 88.89% specificity using an ADC ratio ≤1.18.
There are some limitations in this study. First, this is a retrospective study. Two MR scanners with the same field strength were used to acquire the images. However, we used the same optimized protocol, so ADC maps were considered standard. Second, we had only one patient with an AT/RT diagnosis: for this reason the case was excluded from the study. Finally, the patient cohort was relatively small: further studies with larger patient populations are needed in the future.
In conclusion, ADC ratios can be used in the differential diagnosis of JPA, ependymoma and medulloblastoma, which are the most frequent pediatric posterior fossa tumors in pediatric patients, with high specificity and sensitivity.
References
Walker PA, Perilongo G, Punt JAG, Taylor RE. Brain and spinal tumors of childhood. London: Arnold; 2004.
Legler JM, Gloeckler Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS, et al. Brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382–90.
Gold E. Epidemiology of brain tumors. Rev Cancer Epidemiol. 1982;1:245.
Panigrahy A, Bluml S. Neuroimaging of pediatric brain tumors: from basic to advanced magnetic resonance imaging (MRI). J Child Neurol. 2009;24(11):1343–65.
Gimi B, Cederberg K, Derinkuyu B, Gargan L, Koral KM, Bowers DC, Koral K. Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad Radiol. 2012;19(7):794–800.
Jaremko JL, Jans LB, Coleman LT, Ditchfield MR. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. AJNR Am J Neuroradiol. 2010;31(9):1613–6.
Rumboldt Z, Camacho DL, Lake D, Welsh CT, Castillo M. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR Am J Neuroradiol. 2006;27(6):1362–9.
Koral K, Alford R, Choudhury N, Mossa-Basha M, Gargan L. Applicability of apparent diffusion coefficient ratios in preoperative diagnosis of common pediatric cerebellar tumors across two institutions. Neuroradiology. 2014;56(9):781–8.
Maier SE, Sun Y, Mulkern RV. Diffusion imaging of brain tumors. NMR Biomed. 2010;23(7):849–64.
Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, Yamada R. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001;22(6):1081–8.
Graham DI, Lantos PL. Greenfield’s neuropathology. 7th ed. London: Hodder Arnold; 2002.
Giangaspero F, Eberhart CG, Haapasalo H, Pietsch T, Wiestler OD, Ellison DW. Medulloblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4th ed. Lyon: IACR; 2007. p. 132–40.
Scheithauer BW, Hawkins C, Tihan T, VandenBerg SR, Burger PC. Pilocytic astrocytoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. Lyon: IACR; 2007. p. 14–21.
Koral K, Mathis D, Gimi B, Gargan L, Weprin B, Bowers DC, Margraf L. Common pediatric cerebellar tumors: correlation between cell densities and apparent diffusion coefficient metrics. Radiology. 2013;268(2):532–7.
McLendon RE, Wiestler OD, Kros JM, Korshunov A, Ng H-K. Ependymoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4th ed. Lyon: IACR; 2007. p. 74–8.
Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–91.
Gauvain KM, McKinstry RC, Mukherjee P, et al. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. AJR Am J Roentgenol. 2001;177:449–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This work was approved by the local institutional review board.
About this article
Cite this article
Zitouni, S., Koc, G., Doganay, S. et al. Apparent diffusion coefficient in differentiation of pediatric posterior fossa tumors. Jpn J Radiol 35, 448–453 (2017). https://doi.org/10.1007/s11604-017-0652-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-017-0652-9